Comparison of Various Indices in Identifying Insulin Resistance and Diabetes in Chronic Spinal Cord Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants, Physical Characteristics, and Body Composition
2.2. Fasting Blood Plasma and Intravenous Glucose Tolerance Test
2.3. Calculation of the Insulin Resistance/Sensitivity Indices
2.4. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FPG | Fasting plasma glucose |
| CV | Coefficient of variance |
| DR | Discriminatory ratio |
| EHIC | Euglycemic hyperinsulinemic clamp |
| HbA1C | Hemoglobin A1C |
| HOMA | Homeostatic Model Assessment of Insulin Resistance |
| HOMA2 | Homeostatic Model Assessment 2 of Insulin Resistance |
| IVGTT | Intravenous glucose tolerance test |
| OGTT | Oral glucose tolerance test |
| QUICKI | Quantitative Insulin-sensitivity Check Index |
| SCI | Spinal cord injury |
| Sg | Glucose effectiveness |
| Si | Insulin sensitivity |
| T2DM | Type 2 diabetes mellitus |
References
- Gordon, P.S.; Farkas, G.J.; Gater, D.R. Neurogenic Obesity-Induced Insulin Resistance and Type 2 Diabetes Mellitus in Chronic Spinal Cord Injury. Top. Spinal Cord Inj. Rehabil. 2021, 27, 36–56. [Google Scholar] [CrossRef]
- Muniyappa, R.; Lee, S.; Chen, H.; Quon, M.J. Current approaches for assessing insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. Am. J. Physiol. Metab. 2008, 294, E15–E26. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of Medical Care in Diabetes—2018 Abridged for Primary Care Providers. Clin. Diabetes 2017, 36, 14–37. [Google Scholar] [CrossRef]
- Nash, M.S.; Groah, S.L.; Gater, D.R.; Dyson-Hudson, T.A.; Lieberman, J.A.; Myers, J.; Sabharwal, S.; Taylor, A.J. Identification and Management of Cardiometabolic Risk after Spinal Cord Injury. J. Spinal Cord Med. 2019, 42, 643–677. [Google Scholar] [CrossRef]
- Duckworth, W.C.; Jallepalli, P.; Solomon, S.S. Glucose Intolerance in Spinal Cord Injury. Arch. Phys. Med. Rehabil. 1983, 64, 107–110. [Google Scholar] [PubMed]
- Gater, D.R.; Farkas, G.J.; Berg, A.S.; Castillo, C. Prevalence of metabolic syndrome in veterans with spinal cord injury. J. Spinal Cord Med. 2019, 42, 86–93. [Google Scholar] [CrossRef]
- LaVela, S.L.; Weaver, F.M.; Goldstein, B.; Chen, K.; Miskevics, S.; Rajan, S.; Gater, D.R. Diabetes Mellitus in Individuals with Spinal Cord Injury or Disorder. J. Spinal Cord Med. 2006, 29, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Gater, D.R., Jr.; Farkas, G.J.; Dolbow, D.R.; Berg, A.S.; Gorgey, A.S. Body Composition and Metabolic Assessment After Motort Complete Spinal Cord Injury: Development of A Clinically Relevant Equation to Estimate Body Fat. Top. Spinal. Cord. Inj. Rehabil. 2021, 27, 11–22. [Google Scholar] [CrossRef]
- Cragg, J.J.; Noonan, V.K.; Dvorak, M.; Krassioukov, A.; Mancini, G.J.; Borisoff, J.F. Spinal cord injury and type 2 diabetes: Results from a population health survey. Neurology 2013, 81, 1864–1868. [Google Scholar] [CrossRef]
- Lai, Y.-J.; Lin, C.-L.; Chang, Y.-J.; Lin, M.-C.; Lee, S.-T.; Sung, F.-C.; Lee, W.-Y.; Kao, C.-H. Spinal cord injury increases the risk of Type 2 diabetes: A population-based cohort study. Spine J. 2014, 14, 1957–1964. [Google Scholar] [CrossRef]
- Peterson, M.D.; Berri, M.; Lin, P.; Kamdar, N.; Rodriguez, G.; Mahmoudi, E.; Tate, D. Cardiovascular and metabolic morbidity following spinal cord injury. Spine J. 2021, 21, 1520–1527. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tobin, J.D.; Andres, R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am. J. Physiol. Metab. 1979, 237, E214-23. [Google Scholar] [CrossRef]
- Bergman, R.N.; Ider, Y.Z.; Bowden, C.R.; Cobelli, C. Quantitative estimation of insulin sensitivity. Am. J. Physiol. Metab. 1979, 236, E667-77. [Google Scholar] [CrossRef] [PubMed]
- Bergman, R.N.; Prager, R.; Volund, A.; Olefsky, J.M. Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J. Clin. Investig. 1987, 79, 790–800. [Google Scholar] [CrossRef]
- Bergman, R.N.; Phillips, L.S.; Cobelli, C. Physiologic evaluation of factors controlling glucose tolerance in man: Measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J. Clin. Investig. 1981, 68, 1456–1467. [Google Scholar] [CrossRef]
- Pacini, G.; Bergman, R.N. MINMOD: A computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput. Methods Programs Biomed. 1986, 23, 113–122. [Google Scholar] [CrossRef]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative Insulin Sensitivity Check Index: A Simple, Accurate Method for Assessing Insulin Sensitivity in Humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.C.; Matthews, D.R.; Hermans, M.P. Correct Homeostasis Model Assessment (HOMA) Evaluation Uses the Computer Program. Diabetes Care 1998, 21, 2191–2192. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Matsuda, M.D.R. Web Calculator for Matsuda Index. Available online: Http://Mmatsuda.Diabetes-Smc.Jp/Mindex.Html (accessed on 13 November 2020).
- Mojiminiyi, O.A.; Abdella, N.A. Effect of homeostasis model assessment computational method on the definition and associations of insulin resistance. Clin. Chem. Lab. Med. 2010, 48, 1629–1634. [Google Scholar] [CrossRef]
- Demir, A.K.; Şahin, Ş.; Kaya, S.U.; Bütün, I.; Çıtıl, R.; Önder, Y.; Taşlıyurt, T.; Demir, O.; Deveci, K.; Kutlutürk, F. Prevalence of insulin resistance and identifying HOMA1-IR and HOMA2-IR indexes in the Middle Black Sea region of Turkey. Afr. Health Sci. 2020, 20, 277–286. [Google Scholar] [CrossRef]
- Ghasemi, A.; Tohidi, M.; Derakhshan, A.; Hasheminia, M.; Azizi, F.; Hadaegh, F. Cut-off points of homeostasis model assessment of insulin resistance, beta-cell function, and fasting serum insulin to identify future type 2 diabetes: Tehran Lipid and Glucose Study. Acta Diabetol. 2015, 52, 905–915. [Google Scholar] [CrossRef]
- Geloneze, B.; Vasques, A.C.J.; Stabe, C.F.C.; Pareja, J.C.; de Lima Rosado, L.E.F.P.; De Queiroz, E.C.; Tambascia, M.A. HOMA1-IR and HOMA2-IR indexes in identifying insulin resistance and metabolic syndrome: Brazilian Metabolic Syndrome Study (BRAMS). Arq. Bras. Endocrinol. Metabol. 2009, 53, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-I.; Taylor, J.A.; Tan, C.O.; Park, H.; Kim, J.Y.; Park, S.-Y.; Chung, K.-M.; Lee, Y.-H.; Lee, B.-S.; Jeon, J.Y. A pilot randomized controlled trial of 6-week combined exercise program on fasting insulin and fitness levels in individuals with spinal cord injury. Eur. Spine J. 2019, 28, 1082–1091. [Google Scholar] [CrossRef]
- Yoon, E.S.; Heffernan, K.S.; Jae, S.Y.; Kim, H.J.; Bunsawat, K.; Fernhall, B. Metabolically healthy obesity and subclinical atherosclerosis in persons with spinal cord injury. J. Rehabil. Med. 2018, 50, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Bresnahan, J.J.; Farkas, G.J.; Clasey, J.L.; Yates, J.W.; Gater, D.R. Arm crank ergometry improves cardiovascular disease risk factors and community mobility independent of body composition in high motor complete spinal cord injury. J. Spinal Cord Med. 2017, 42, 272–280. [Google Scholar] [CrossRef] [PubMed]
- La Fountaine, M.F.; Cirnigliaro, C.M.; Kirshblum, S.C.; McKenna, C.; Bauman, W.A. Effect of functional sympathetic nervous system impairment of the liver and abdominal visceral adipose tissue on circulating triglyceride-rich lipoproteins. PLoS ONE 2017, 12, e0173934. [Google Scholar] [CrossRef]
- Kim, D.-I.; Lee, H.; Lee, B.-S.; Kim, J.; Jeon, J.Y. Effects of a 6-Week Indoor Hand-Bike Exercise Program on Health and Fitness Levels in People with Spinal Cord Injury: A Randomized Controlled Trial Study. Arch. Phys. Med. Rehabil. 2015, 96, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Barbonetti, A.; Vassallo, M.R.C.; Pacca, F.; Cavallo, F.; Costanzo, M.; Felzani, G.; Francavilla, S. Correlates of low testosterone in men with chronic spinal cord injury. Andrology 2014, 2, 721–728. [Google Scholar] [CrossRef] [PubMed]
- D’Oliveira, G.L.C.; Figueiredo, F.A.; Passos, M.C.F.; Chain, A.; Bezerra, F.F.; Koury, J.C. Physical exercise is associated with better fat mass distribution and lower insulin resistance in spinal cord injured individuals. J. Spinal Cord Med. 2013, 37, 79–84. [Google Scholar] [CrossRef]
- Ryan, T.E.; Brizendine, J.T.; Backus, D.; McCully, K.K. Electrically Induced Resistance Training in Individuals with Motor Complete Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2013, 94, 2166–2173. [Google Scholar] [CrossRef] [PubMed]
- Koury, J.C.; Passos, M.C.F.; Figueiredo, F.A.; Chain, A.; Franco, J.G. Time of physical exercise practice after injury in cervical spinal cord-injured men is related to the increase in insulin sensitivity. Spinal Cord 2012, 51, 116–119. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Farkas, G.J.; Gorgey, A.S.; Dolbow, D.R.; Berg, A.S.; Gater, D.R. Energy Expenditure, Cardiorespiratory Fitness, and Body Composition Following Arm Cycling or Functional Electrical Stimulation Exercises in Spinal Cord Injury: A 16-Week Randomized Controlled Trial. Top. Spinal Cord Inj. Rehabil. 2021, 27, 121–134. [Google Scholar] [CrossRef]
- Solinsky, R.; Betancourt, L.; Schmidt-Read, M.; Kupfer, M.; Owens, M.; Schwab, J.M.; Dusseau, N.B.; Szlachcic, Y.; Sutherland, L.; Taylor, J.A.; et al. Acute Spinal Cord Injury Is Associated with Prevalent Cardiometabolic Risk Factors. Arch. Phys. Med. Rehabil. 2021. [Google Scholar] [CrossRef] [PubMed]
- Cirnigliaro, C.M.; La Fountaine, M.F.; Hobson, J.C.; Kirshblum, S.C.; Dengel, D.R.; Spungen, A.M.; Bauman, W.A. Predicting Cardiometabolic Risk from Visceral Abdominal Adiposity in Persons with Chronic Spinal Cord Injury. J. Clin. Densitom. 2021, 24, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Nash, M.S.; Tractenberg, R.E.; Mendez, A.J.; David, M.; Ljungberg, I.H.; Tinsley, E.A.; Burns-Drecq, P.A.; Betancourt, L.F.; Groah, S.L. Cardiometabolic Syndrome in People with Spinal Cord Injury/Disease: Guideline-Derived and Nonguideline Risk Components in a Pooled Sample. Arch. Phys. Med. Rehabil. 2016, 97, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, T.E.; Walhin, J.-P.; Thompson, D.; Bilzon, J.L.J. Impact of Exercise on Cardiometabolic Component Risks in Spinal Cord–injured Humans. Med. Sci. Sports Exerc. 2017, 49, 2469–2477. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Demirel, A.; Azuero, A.; Womack, E.D.; Kroeger, E.N.; McLain, A.; Yarar-Fisher, C. Limited Association between the Total Healthy Eating Index-2015 Score and Cardiovascular Risk Factors in Individuals with Long-Standing Spinal Cord Injury: An Exploratory Study: An Exploratory Study. J. Acad. Nutr. Diet. 2021, 121, 2260–2266. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hunter, G.R.; Chen, Y.; McLain, A.; Smith, D.L.; Yarar-Fisher, C. Differences in Glucose Metabolism Among Women With Spinal Cord Injury May Not Be Fully Explained by Variations in Body Composition. Arch. Phys. Med. Rehabil. 2018, 100, 1061–1067. [Google Scholar] [CrossRef]
- Li, J.; Polston, K.F.L.; Eraslan, M.; Bickel, C.S.; Windham, S.T.; McLain, A.B.; Oster, R.A.; Bamman, M.M.; Yarar-Fisher, C. A high-protein diet or combination exercise training to improve metabolic health in individuals with long-standing spinal cord injury: A pilot randomized study. Physiol. Rep. 2018, 6, e13813. [Google Scholar] [CrossRef]
- Alajam, R.A.; Alqahtani, A.S.; Moon, S.; Sarmento, C.V.M.; Frederick, J.; Smirnova, I.V.; Liu, W. Effects of walking training on risk markers of cardiovascular disease in individuals with chronic spinal cord injury. J. Spinal Cord Med. 2021, 1–9. [Google Scholar] [CrossRef]
- Rankin, K.C.; O’Brien, L.C.; Segal, L.; Khan, M.R.; Gorgey, A.S. Liver Adiposity and Metabolic Profile in Individuals with Chronic Spinal Cord Injury. BioMed Res. Int. 2017, 2017, 1364818. [Google Scholar] [CrossRef] [PubMed]
- Abilmona, S.M.; Sumrell, R.M.; Gill, R.S.; Adler, R.A.; Gorgey, A.S. Serum testosterone levels may influence body composition and cardiometabolic health in men with spinal cord injury. Spinal Cord 2018, 57, 229–239. [Google Scholar] [CrossRef]
- Farkas, G.J.; Gorgey, A.S.; Dolbow, D.R.; Berg, A.S.; Gater, D.R. The influence of level of spinal cord injury on adipose tissue and its relationship to inflammatory adipokines and cardiometabolic profiles. J. Spinal Cord Med. 2017, 41, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Farkas, G.J.; Gorgey, A.S.; Dolbow, D.R.; Berg, A.S.; Gater, D.R. Sex dimorphism in the distribution of adipose tissue and its influence on proinflammatory adipokines and cardiometabolic profiles in motor complete spinal cord injury. J. Spinal Cord Med. 2018, 42, 430–436. [Google Scholar] [CrossRef]
- Kirshblum, S.C.; Burns, S.P.; Biering-Sørensen, F.; Donovan, W.; Graves, D.E.; Jha, A.; Johansen, M.; Jones, L.; Krassioukov, A.; Mulcahey, M.J.; et al. International standards for neurological classification of spinal cord injury (Revised 2011). J. Spinal Cord Med. 2011, 34, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Farkas, G.J.; Sneij, A.; McMillan, D.W.; Tiozzo, E.; Nash, M.S.; Gater, D.R. Energy Expenditure and Nutrient Intake after Spinal Cord Injury: A Comprehensive Review and Practical Recommendations. Br. J. Nutr. 2021, 1–77. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Mather, K.J.; Gater, D.R. Central adiposity associations to carbohydrate and lipid metabolism in individuals with complete motor spinal cord injury. Metabolism 2011, 60, 843–851. [Google Scholar] [CrossRef]
- Doherty, J.G.; Burns, A.S.; O’Ferrall, D.M.; Ditunno, J.F. Prevalence of Upper Motor Neuron vs Lower Motor Neuron Lesions in Complete Lower Thoracic and Lumbar Spinal Cord Injuries. J. Spinal Cord Med. 2002, 25, 289–292. [Google Scholar] [CrossRef]
- Farkas, G.J.; Gorgey, A.S.; Dolbow, D.R.; Berg, A.S.; Gater, D.R. Caloric Intake Relative to Total Daily Energy Expenditure Using a Spinal Cord Injury-Specific Correction Factor: An Analysis by Level of Injury. Am. J. Phys. Med. Rehabil. 2019, 98, 947–952. [Google Scholar] [CrossRef]
- Farkas, G.J.; Swartz, A.M.; Gorgey, A.S.; Berg, A.S.; Gater, D.R. Acute exercise improves glucose effectiveness but not insulin sensitivity in paraplegia. Disabil. Rehabil. 2021, 27, 1–7. [Google Scholar] [CrossRef]
- Cheng, C.; Campbell, K.L.; Kushner, H.; Falkner, B.E. Correlation of oral glucose tolerance test-derived estimates of insulin sensitivity with insulin clamp measurements in an African-American cohort. Metabolism 2004, 53, 1107–1112. [Google Scholar] [CrossRef]
- Chen, H.; Sullivan, G.; Quon, M.J. Assessing the Predictive Accuracy of QUICKI as a Surrogate Index for Insulin Sensitivity Using a Calibration Model. Diabetes 2005, 54, 1914–1925. [Google Scholar] [CrossRef]
- Radziuk, J. Insulin Sensitivity and Its Measurement: Structural Commonalities among the Methods 1. J. Clin. Endocrinol. Metab. 2000, 85, 4426–4433. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer International Publishing: New York, NY, USA, 2016. [Google Scholar]
- Gutch, M.; Kumar, S.; Razi, S.M.; Gupta, K.K.; Gupta, A. Assessment of insulin sensitivity/resistance. Indian J. Endocrinol. Metab. 2015, 19, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Otten, J.; Ahrén, B.; Olsson, T. Surrogate measures of insulin sensitivity vs the hyperinsulinaemic–euglycaemic clamp: A meta-analysis. Diabetologia 2014, 57, 1781–1788. [Google Scholar] [CrossRef]
- Mather, K.J.; Hunt, A.E.; Steinberg, H.O.; Paradisi, G.; Hook, G.; Katz, A.; Quon, M.J.; Baron, A.D. Repeatability Characteristics of Simple Indices of Insulin Resistance: Implications for Research Applications. J. Clin. Endocrinol. Metab. 2001, 86, 5457–5464. [Google Scholar] [CrossRef] [PubMed]
- Sarafidis, P.A.; Lasaridis, A.N.; Nilsson, P.M.; Pikilidou, M.I.; Stafilas, P.C.; Kanaki, A.; Kazakos, K.; Yovos, J.; Bakris, G.L. Validity and reproducibility of HOMA-IR, 1/HOMA-IR, QUICKI and McAuley’s indices in patients with hypertension and type II diabetes. J. Hum. Hypertens. 2007, 21, 709–716. [Google Scholar] [CrossRef]
- Antuna-Puente, B.; Faraj, M.; Karelis, A.D.; Garrel, D.; Prud’Homme, D.; Rabasa-Lhoret, R.; Bastard, J.-P. HOMA or QUICKI: Is it useful to test the reproducibility of formulas? Diabetes Metab. 2008, 34, 294–296. [Google Scholar] [CrossRef]
- Henríquez, S.; Jara, N.; Bunout, D.; Hirsch, S.; De La Maza, M.P.; Leiva, L.; Barrera, G. Variability of Formulas to Assess Insulin Sensitivity and Their Association with The Matsuda Index. Nutr. Hosp. 2013, 28, 1594–1598. [Google Scholar] [CrossRef]
- Cowie, C.C.; Rust, K.F.; Byrd-Holt, D.D.; Gregg, E.W.; Ford, E.S.; Geiss, L.S.; Bainbridge, K.E.; Fradkin, J.E. Prevalence of Diabetes and High Risk for Diabetes Using A1C Criteria in the U.S. Population in 1988–2006. Diabetes Care 2010, 33, 562–568. [Google Scholar] [CrossRef]
- Stillman, M.D.; Williams, S. Guideline for the identification and management of cardiometabolic risk after spinal cord injury: A case of unsubstantiated recommendations. Spinal Cord Ser. Cases 2019, 5, 97. [Google Scholar] [CrossRef] [PubMed]
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997, 20, 1183–1197. [Google Scholar] [CrossRef] [PubMed]
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2003, 26, S5–S20. [Google Scholar] [CrossRef] [PubMed]
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2000, 23 (Suppl. S1), S4–S19. [Google Scholar]
- Gillett, M.J. International Expert Committee Report on the Role of the A1C Assay in the Diagnosis of Diabetes. Clin. Biochem. Rev. 2009, 32, 1327–1334. [Google Scholar]
- Duckworth, W.C.; Solomon, S.S.; Jallepalli, P.; Heckemeyer, C.; Finnern, J.; Powers, A. Glucose Intolerance Due to Insulin Resistance in Patients with Spinal Cord Injuries. Diabetes 1980, 29, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Bauman, W.A.; Spungen, A.M. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: A model of premature aging. Metabolism 1994, 43, 749–756. [Google Scholar] [CrossRef]
- Stillman, M.; Graves, D.; Lenneman, C.; Williams, S. Neurogenic Bowel, Disordered Glycemic Control And Chronic Spinal Cord Injury: A Preliminary Investigation. Phys. Med. Rehabil. Int. 2017, 4, 1–3. [Google Scholar] [CrossRef]
- Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43 (Suppl. S1), S14–S31. [CrossRef]
- Bonora, E.; Tuomilehto, J. The Pros and Cons of Diagnosing Diabetes with A1C. Diabetes Care 2011, 34, S184–S190. [Google Scholar] [CrossRef]
- Lorenzo, C.; Wagenknecht, L.E.; Hanley, A.J.; Rewers, M.J.; Karter, A.J.; Haffner, S.M. A1C Between 5.7 and 6.4% as a Marker for Identifying Pre-Diabetes, Insulin Sensitivity and Secretion, and Cardiovascular Risk Factors: The Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care 2010, 33, 2104–2109. [Google Scholar] [CrossRef] [PubMed]
- Camacho, J.E.; Shah, V.O.; Schrader, R.; Wong, C.S.; Burge, M.R. Performance of A1C Versus Ogtt for the Diagnosis of Prediabetes in a Community-Based Screening. Endocr. Pract. 2016, 22, 1288–1295. [Google Scholar] [CrossRef]
- Yan, S.-T.; Xiao, H.-Y.; Tian, H.; Li, C.-L.; Fang, F.-S.; Li, X.-Y.; Cheng, X.-L.; Li, N.; Miao, X.-Y.; Yang, Y.; et al. The cutoffs and performance of glycated hemoglobin for diagnosing diabetes and prediabetes in a young and middle-aged population and in an elderly population. Diabetes Res. Clin. Pract. 2015, 109, 238–245. [Google Scholar] [CrossRef]
- Nadon, G.W.; Little, J.A.; Hall, W.E.; O’Sullivan, M.O. A Comparison of the Oral and Intravenous Glucose Tolerance Tests in Non-Diabetic, Possible Diabetic and Diabetic Subjects. Can. Med Assoc. J. 1964, 91, 1350–1353. [Google Scholar] [PubMed]
- Hanley, A.J.G.; Williams, K.; Stern, M.P.; Haffner, S.M. Homeostasis Model Assessment of Insulin Resistance in Relation to the Incidence of Cardiovascular Disease: The San Antonio Heart Study. Diabetes Care 2002, 25, 1177–1184. [Google Scholar] [CrossRef]
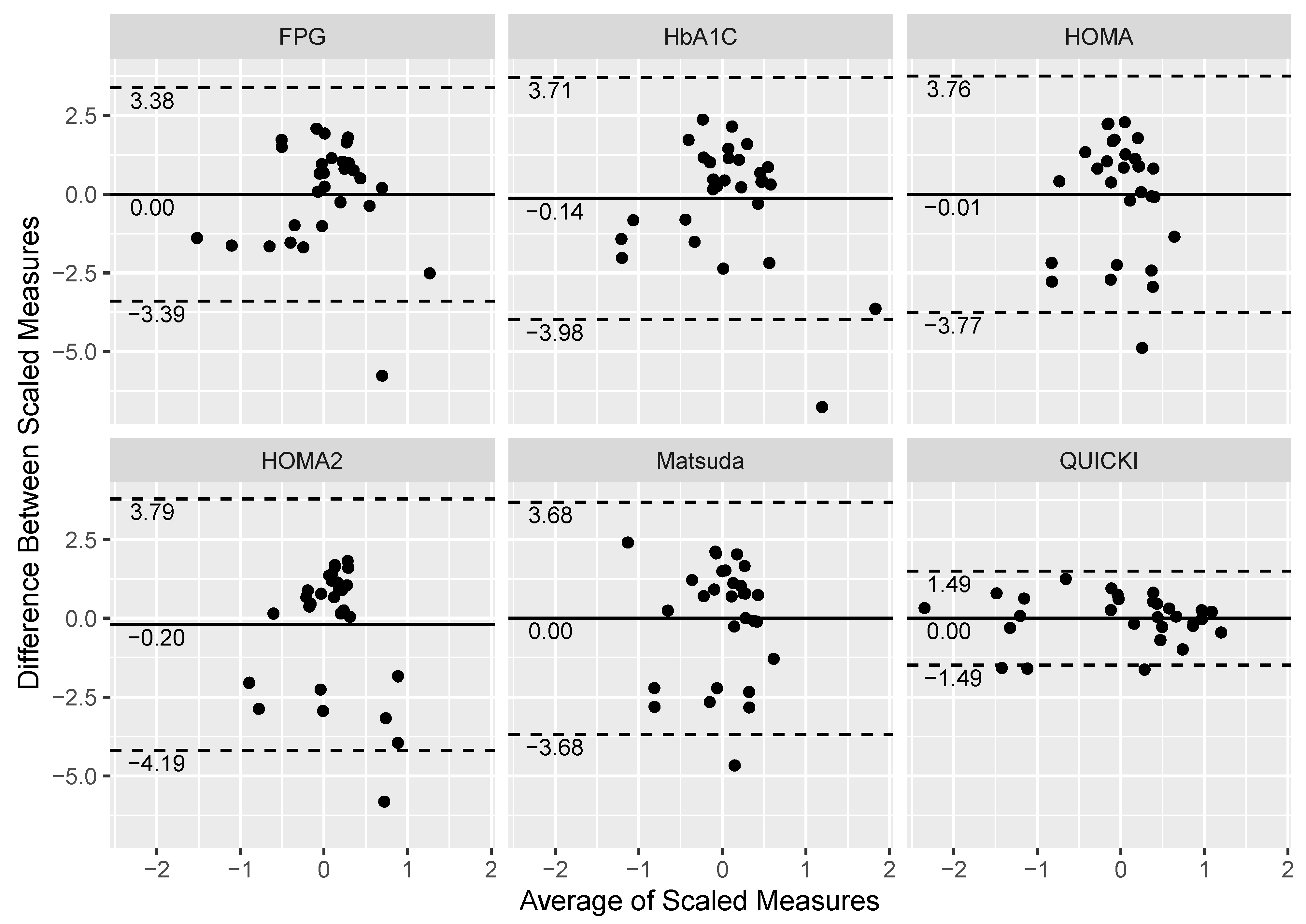
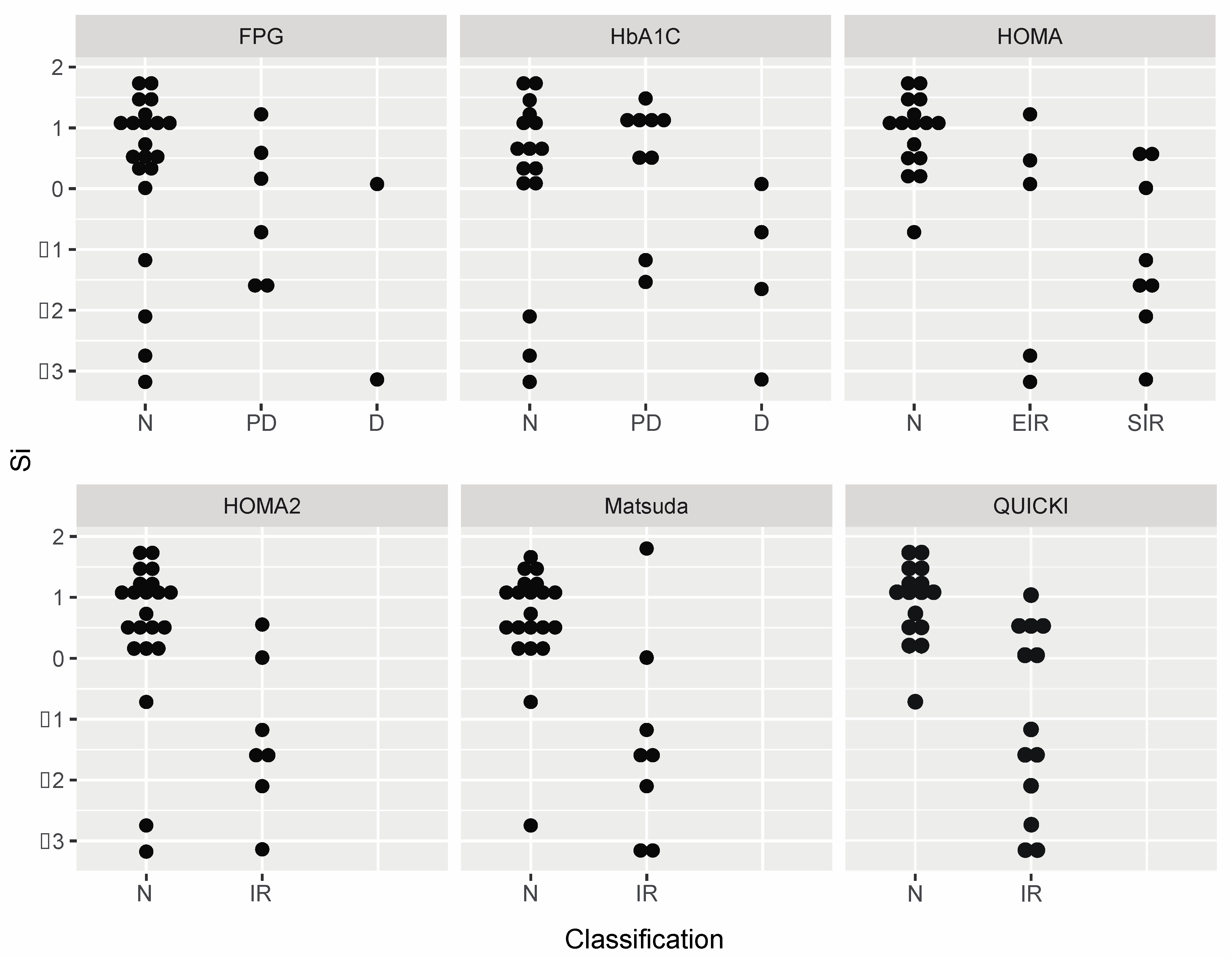
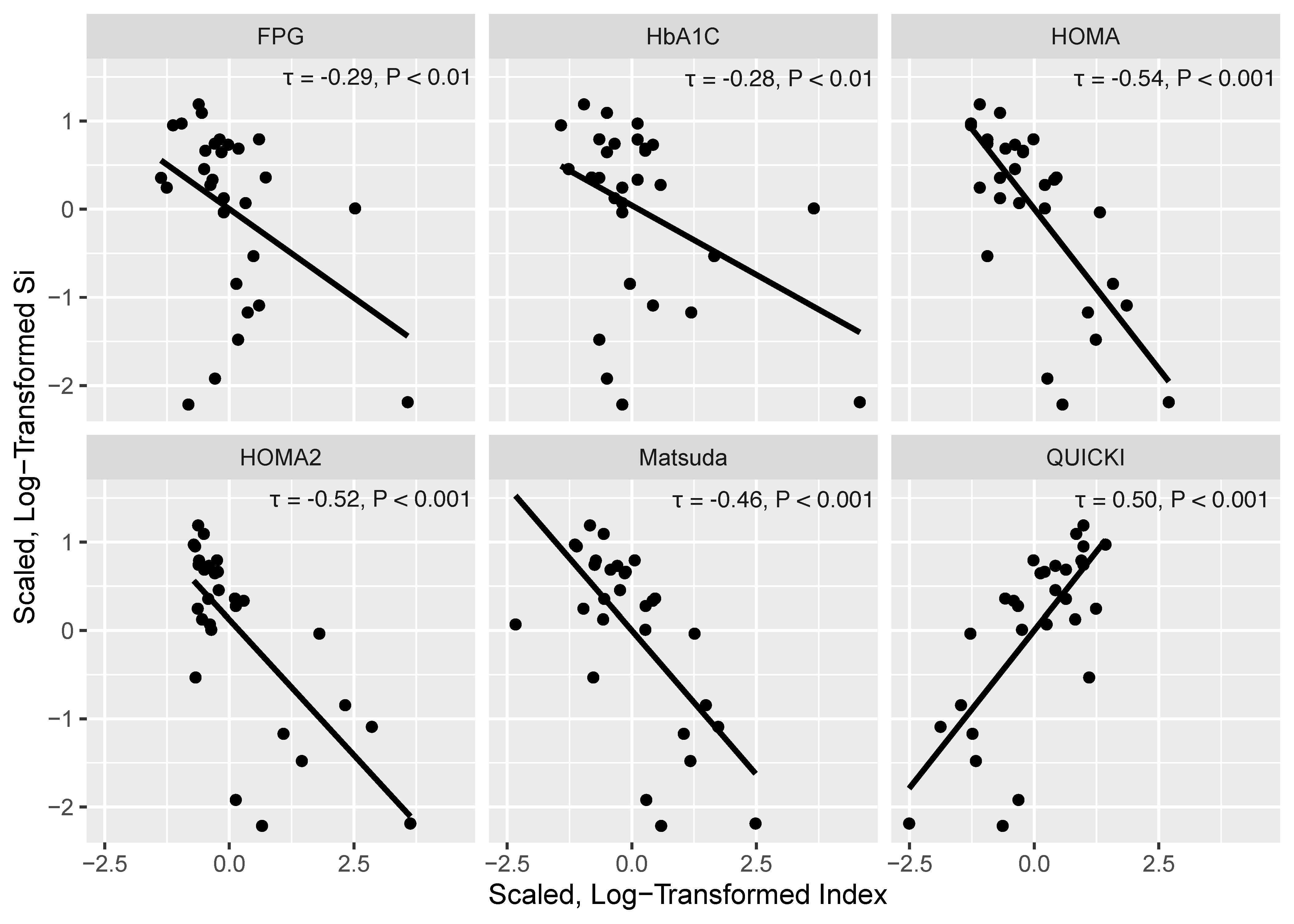
| Index | Reference Ranges |
|---|---|
| Fasting plasma glucose [1,3] | Normal: <100 mg/dL |
| Prediabetes: 100–125 mg/dL | |
| T2DM: ≥126 mg/dL | |
| Hemoglobin A1C [1,3] | Normal: <5.7% |
| Prediabetes: 5.7–6.4% | |
| T2DM: ≥6.5% | |
| Homeostatic Model Assessment of IR [1,18] | Normal: ≤1.6 |
| Early IR: 1.7–2.4 | |
| Significant IR: ≥2.5 | |
| Homeostatic Model Assessment 2 of IR [1] * | Normal: < 1.4 |
| IR: ≥1.4 | |
| Matsuda Index [1,20,21] | Normal: >2.5 |
| IR: ≤2.5 | |
| Quantitative Insulin-sensitivity Check Index [1,17] | Normal: >0.339 |
| IR: ≤0.339 |
| Demographic and Injury Characteristics | |
|---|---|
| Age (years) | 42.2 (11.4) |
| Sex (% male) | 79.3% |
| Body mass index (kg/m2) | 28.6 (6.4) |
| Body weight (kg) | 87.8 (22.9) |
| Height (m) | 1.8 (0.09) |
| Time since injury (years) | 14.5 (11.6) |
| Level of injury | C4-T10 |
| Injury severity (ASIA Impairment Scale %A/%B) | (86.2/13.8%) |
| Body Composition | |
| Fat free mass (kg) | 52.8 (11.6) |
| Lean body mass (kg) | 48.7 (10.7) |
| Total body fat (%) | 40.4 (8.9) |
| Glucose Metabolism | |
| Insulin sensitivity (min−1/(µU/mL−1) × 10−4) | 2.3 (1.8) |
| Glucose effectiveness (min−1) | 0.02 (0.01) |
| Fasting plasma insulin (uU/L) | 9.7 (9.0) |
| Fasting plasma glucose (mg/dL) | 95.4 (28.4) |
| Hemoglobin A1C (%) | 5.7 (0.7) |
| Homeostatic Model Assessment for Insulin Resistance | 2.7 (3.8) |
| Homeostatic Model Assessment 2 for Insulin Resistance | 1.3 (1.2) |
| Matsuda Index | 6.9 (4.5) |
| Quantitative Insulin-sensitivity Check Index | 0.36 (0.04) |
| Data presented as mean (SD). | |
| n (%) | |
|---|---|
| Fasting Plasma Glucose (mg/dL) | |
| Normal | 22 (75.9%) |
| Prediabetes | 6 (20.7%) |
| Diabetes | 1 (3.4%) |
| Hemoglobin A1C (%) | |
| Normal | 18 (62.1%) |
| Prediabetes | 8 (27.6%) |
| Diabetes | 3 (10.3%) |
| Homeostatic Model Assessment for Insulin Resistance | |
| Normal | 18 (62.1%) |
| Early Insulin Resistance | 7 (24.1%) |
| Significant Insulin Resistance | 4 (13.8%) |
| Homeostatic Model Assessment 2 for Insulin Resistance | |
| Normal | 22 (75.9%) |
| Insulin Resistance | 7 (24.1%) |
| Matsuda Index | |
| Normal | 22 (75.9%) |
| Insulin Resistance | 7 (24.1%) |
| Quantitative Insulin-sensitivity Check Index | |
| Normal | 18 (62.1%) |
| Insulin Resistance | 11 (37.9%) |
| Trinary Scale | ||||
|---|---|---|---|---|
| R2 | Adjusted R2 | Akaike Information Criterion | p-Value | |
| Fasting plasma glucose | 0.124 | 0.056 | 107.5 | 0.1799 |
| Hemoglobin A1C | 0.164 | 0.100 | 106.1 | 0.0975 |
| HOMA | 0.422 | 0.378 | 95.4 | 0.0008 |
| HOMA2 | 0.282 | 0.256 | 99.7 | 0.0030 |
| Matsuda Index | 0.379 | 0.356 | 95.5 | 0.0004 |
| QUICKI | 0.501 | 0.463 | 91.1 | 0.0001 |
| Binary Scale * | ||||
| R2 | Adjusted R2 | Akaike Information Criterion | p-Value | |
| Fasting plasma glucose | 0.087 | 0.053 | 106.7 | 0.1206 |
| Hemoglobin A1C | 0.009 | −0.027 | 109.1 | 0.6175 |
| HOMA | 0.420 | 0.398 | 93.5 | 0.0001 |
| HOMA2 | 0.282 | 0.256 | 99.7 | 0.0030 |
| Matsuda Index | 0.379 | 0.356 | 95.5 | 0.0004 |
| QUICKI | 0.501 | 0.463 | 91.1 | 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farkas, G.J.; Gordon, P.S.; Trewick, N.; Gorgey, A.S.; Dolbow, D.R.; Tiozzo, E.; Berg, A.S.; Gater, D.R., Jr. Comparison of Various Indices in Identifying Insulin Resistance and Diabetes in Chronic Spinal Cord Injury. J. Clin. Med. 2021, 10, 5591. https://doi.org/10.3390/jcm10235591
Farkas GJ, Gordon PS, Trewick N, Gorgey AS, Dolbow DR, Tiozzo E, Berg AS, Gater DR Jr. Comparison of Various Indices in Identifying Insulin Resistance and Diabetes in Chronic Spinal Cord Injury. Journal of Clinical Medicine. 2021; 10(23):5591. https://doi.org/10.3390/jcm10235591
Chicago/Turabian StyleFarkas, Gary J., Phillip S. Gordon, Nareka Trewick, Ashraf S. Gorgey, David R. Dolbow, Eduard Tiozzo, Arthur S. Berg, and David R. Gater, Jr. 2021. "Comparison of Various Indices in Identifying Insulin Resistance and Diabetes in Chronic Spinal Cord Injury" Journal of Clinical Medicine 10, no. 23: 5591. https://doi.org/10.3390/jcm10235591
APA StyleFarkas, G. J., Gordon, P. S., Trewick, N., Gorgey, A. S., Dolbow, D. R., Tiozzo, E., Berg, A. S., & Gater, D. R., Jr. (2021). Comparison of Various Indices in Identifying Insulin Resistance and Diabetes in Chronic Spinal Cord Injury. Journal of Clinical Medicine, 10(23), 5591. https://doi.org/10.3390/jcm10235591











