Glycaemic Variability and Hyperglycaemia as Prognostic Markers of Major Cardiovascular Events in Diabetic Patients Hospitalised in Cardiology Intensive Care Unit for Acute Heart Failure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Capillary Blood Glucose Values
2.3. Measurement of Glycaemic Variability
2.4. Diabetes Care
2.5. Outcomes
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Incidence of MACE
3.3. Causes of Cardiovascular and Non-Cardiovascular Death
3.4. Univariate Regression Analysis
3.5. Multivariate Regression Analysis
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. SYNTAX Score and Global Registry of Acute Coronary Events (GRACE) Risk Score
| Baseline Characteristics | GV ≤ 50 mg/dL | GV > 50 mg/dL | p Value |
|---|---|---|---|
| Age (years) | 72 ± 11.1 | 74 ± 10.0 | 0.22 |
| Age > 75 (years) | 84 (47.7) | 107 (49.5) | 0.79 |
| Female sex (%) | 54 (30.7) | 67 (31.0) | 0.92 |
| Prior HF hospitalisation | 60 (34.1) | 65 (30.1) | 0.68 |
| History of chronic heart failure | 108 (61.4) | 106 (49.1) | 0.06 |
| Systolic blood pressure at admission (mmHg) | 130 ± 31.7 | 132 ± 30.9 | 0.96 |
| Heart rate at admission (beats/min) | 85 ± 22.8 | 88 ± 28.0 | 0.26 |
| Smoking status (%) | |||
| Non-smoker | 108 (61.4) | 116 (53.7) | 0.15 |
| Former smoker | 60 (34.1) | 66 (30.6) | 0.41 |
| Current smoker | 23 (13.1) | 19 (8.8) | 0.23 |
| Hypertension * | 147 (83.5) | 182 (84.3) | 0.84 |
| Type of diabetes | |||
| Type 1 | 5 (2.8) | 7 (3.2) | 0.53 |
| Type 2 | 171 (97.1) | 210 (97.2) | 0.92 |
| Secondary (chronic pancreatitis) | 1 (0.5) | 1 (0.4) | 0.70 |
| HbA1c ≥ 6.5% | 93 (52.8) | 107 (49.5) | 0.58 |
| Total cholesterol (mg/dL) | 164 ± 51 | 154 ± 46 | 0.11 |
| LDL cholesterol (mg/dL) | 93 ± 38 | 92 ± 39 | 0.86 |
| HDL cholesterol (mg/dL) | 43 ± 15 | 41 ± 12 | 0.43 |
| TG (mmol/L) (mg/dL) | 272 ± 189 | 256 ± 215 | 0.72 |
| BMI (kg/m2) | 29.2 ± 6.2 | 29.1 ± 6.0 | 0.83 |
| Family history of CAD (%) | 15 (8.5) | 18 (8.3) | 0.92 |
| Personal history of CAD (%) | 87 (49.4) | 89 (41.2) | 0.13 |
| Chronic respiratory failure (%) | 40 (22.7) | 56 (25.9) | 0.38 |
| eGFR (mL/min/1.73 m2) | 57.5 ± 25.7 | 53.1 ± 26.5 | 0.09 |
| CKD with RRT (%) | 3 (1.7) | 5 (2.3) | 0.74 |
| Left ventricular ejection fraction (LVEF) (%) | 44.6 ± 12.6 | 42.3 ± 13.1 | 0.06 |
| Pre-existent aetiologies of cardiopathies predisposing to HF | |||
| Ischaemic heart disease | 67 (38.1) | 77 (35.6) | 0.64 |
| Toxic damage | 8 (4.5) | 11 (5.1) | 0.81 |
| Immune-mediated and inflammatory damage | 1 (0.5) | 1 (0.4) | 0.70 |
| Infiltration | 1 (0.5) | 1 (0.4) | 0.70 |
| Metabolic derangements | 2 (1.1) | 3 (1.4) | 0.60 |
| Genetic abnormalities | 12 (6.8) | 8 (3.7) | 0.17 |
| Valve and myocardium structural defects | 45 (25.6) | 57 (26.4) | 0.90 |
| Pericardial and endomyocardial pathologies | 3 (1.7) | 2 (0.9) | 0.66 |
| Tachycardia-induced cardiomyopathy | 3 (1.7) | 4 (1.9) | 0.62 |
| Heart failure with preserved ejection fraction | 19 (10.8) | 17 (7.9) | 0.34 |
| No pre-existent cardiopathy | 26 (14.8) | 26 (12.0) | 0.43 |
| Factors triggering acute heart failure | |||
| Acute coronary syndrome (ACS) | 79 (44.9) | 80 (37.0) | 0.14 |
| Myocardial rupture complicating ACS † | 2 (1.1) | 1 (0.4) | 0.59 |
| Hypertensive emergency | 16 (9.1) | 24 (11.1) | 0.63 |
| Tachyarrhythmia | 20 (11.4) | 30 (13.9) | 0.55 |
| Bradyarrhythmia | 5 (2.8) | 7 (3.2) | 0.53 |
| Acute native or prosthetic valve incompetence ‡ | 14 (7.9) | 13 (6.0) | 0.58 |
| Vigorous fluid administration | 5 (2.8) | 5 (2.3) | 0.76 |
| Non-adherence with salt/fluid intake or medications | 17 (9.6) | 15 (6.9) | 0.43 |
| Worsening renal failure | 9 (5.1) | 11 (5.1) | 0.82 |
| Severe anaemia § | 8 (4.5) | 12 (5.6) | 0.82 |
| Infection (e.g., pneumonia, sepsis) | 37 (21.0) | 39 (18.1) | 0.54 |
| Stress-related cardiomyopathy | 1 (0.5) | 2 (0.9) | 0.58 |
| Metabolic/hormonal derangements ¶ | 3 (1.7) | 4 (1.9) | 0.62 |
| Toxic substances, cardiodepressant and other drugs # | 2 (1.1) | 3 (1.4) | 0.60 |
| BNP value at admission (pg/mL) | 1003 ± 1163 | 1005 ± 1005 | 0.98 |
| Arterial pH at admission (n = 200) | 7.37 ± 0.10 | 7.34 ± 0.12 | 0.21 |
| Arterial blood lactate at admission (mmol/L) (n = 200) | 2.39 ± 3.33 | 2.32 ± 1.78 | 0.86 |
| Acute kidney failure ** (%) | 65 (36.9) | 80 (37.0) | 0.92 |
| Cardiogenic shock †† | 19 (10.8) | 33 (15.3) | 0.23 |
| Peak troponin I (ng/mL) (normal < 0.04) | 0.42 (0.06–9) | 0.26 (0.13–7) | 0.39 |
| Presence and extent of CAD (%) | |||
| No invasive angiography | 73 (41.5) | 101 (46.3) | 0.34 |
| No CAD | 9 (5.1) | 13 (6.0) | 0.82 |
| No significant stenosis | 20 (11.4) | 24 (11.1) | 0.92 |
| One-vessel disease | 20 (11.4) | 20 (9.3) | 0.60 |
| Two-vessel disease | 22 (12.5) | 22 (10.2) | 0.57 |
| Left main and/or three-vessel disease | 30 (17.0) | 38 (17.6) | 0.89 |
| Syntax score | 18.0 ± 13.3 | 21.6 ± 12.9 | 0.06 |
| GRACE Score (n = 159) | 164 ± 38 | 171 ± 37 | 0.25 |
| GRACE Score > 140 | 58 (80.6) | 60 (70.0) | 0.14 |
Appendix A.2. Association of Hypoglycaemia, Admission Glycaemia and Mean Glycaemia with MACE
References
- Adams, K.F.; Fonarow, G.C.; Emerman, C.L.; LeJemtel, T.H.; Costanzo, M.R.; Abraham, W.T.; Berkowitz, R.L.; Galvao, M.; Horton, D.P.; ADHERE Scientific Advisory Committee and Investigators. Characteristics and outcomes of patients hospitalized for heart failure in the United States: Rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am. Heart J. 2005, 149, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Rawshani, A.; Rawshani, A.; Franzén, S.; Sattar, N.; Eliasson, B.; Svensson, A.M.; Zethelius, B.; Miftaraj, M.; McGuire, D.K.; Rosengren, A.; et al. Risk Factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N. Eng. J. Med. 2018, 379, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Ofstad, A.P.; Atar, D.; Gullestad, L.; Langslet, G.; Johansen, O.E. The heart failure burden of type 2 diabetes mellitus-a review of pathophysiology and interventions. Heart Fail. Rev. 2018, 23, 303–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeVries, J.H. Glucose variability: Where it is important and how to measure it. Diabetes 2013, 62, 1405–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frontoni, S.; Di Bartolo, P.; Avogaro, A.; Bosi, E.; Paolisso, G.; Ceriello, A. Glucose variability: An emerging target for the treatment of diabetes mellitus. Diabetes Res. Clin. Pract. 2013, 102, 86–95. [Google Scholar] [CrossRef]
- Smith-Palmer, J.; Brändle, M.; Trevisan, R.; Orsini Federici, M.; Liabat, S.; Valentine, W. Assessment of the association between glycemic variability and diabetes-related complications in type 1 and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 273–284. [Google Scholar] [CrossRef]
- Benalia, M.; Zeller, M.; Mouhat, B.; Guenancia, C.; Yameogo, V.; Greco, C.; Yao, H.; Maza, M.; Vergès, B.; Cottin, Y. Glycaemic variability is associated with severity of coronary artery disease in patients with poorly controlled type 2 diabetes and acute myocardial infarction. Diabetes Metab. 2019, 45, 446–452. [Google Scholar] [CrossRef]
- Suh, S.; Kim, J.H. Glycemic variability: How do we measure it and why is it important? Diabetes Metab. J. 2015, 39, 273–282. [Google Scholar] [CrossRef]
- Costantino, S.; Paneni, F.; Battista, R.; Castello, L.; Capretti, G.; Chiandotto, S.; Tanese, L.; Russo, G.; Pitocco, D.; Lanza, G.A.; et al. Impact of glycemic variability on chromatin remodeling, oxidative stress, and endothelial dysfunction in patients with type 2 diabetes and with target HbA1c levels. Diabetes 2017, 66, 2472–2482. [Google Scholar] [CrossRef] [Green Version]
- Monnier, L.; Mas, E.; Ginet, C.; Michel, F.; Villon, L.; Cristol, J.P.; Colette, C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006, 295, 1681–1687. [Google Scholar] [CrossRef] [Green Version]
- Brownlee, M.; Hirsch, I.B. Glycemic variability: A hemoglobin A1c-independent risk factor for diabetic complications. JAMA 2006, 295, 1707–1708. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Santamarina, J.; Pavesi, A.; Musso, C.; Umpierrez, G.E. Glycaemic variability and cardiovascular disease in patients with type 2 diabetes. BMJ Open Diab. Res. Care 2021, 9, e002032. [Google Scholar] [CrossRef] [PubMed]
- Gerbaud, E.; Darier, R.; Montaudon, M.; Beauvieux, M.C.; Coffin-Boutreux, C.; Coste, P.; Douard, H.; Ouattara, A.; Catargi, B. Glycemic variability is a powerful independent predictive factor of midterm major adverse cardiac events in patients with diabetes with acute coronary syndrome. Diabetes Care 2019, 42, 674–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baudu, J.; Gerbaud, E.; Catargi, B.; Montaudon, M.; Beauvieux, M.C.; Sagnier, S.; Debruxelles, S.; Renou, P.; Poli, M.; Olindo, S.; et al. High glycemic variability: An underestimated determinant of stroke functional outcome following large vessel occlusion. Rev. Neurol. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Dungan, K.M.; Binkley, P.; Nagaraja, H.N.; Schuster, D.; Osei, K. The effect of glycaemic control and glycaemic variability on mortality in patients hospitalized with congestive heart failure. Diabetes Metab. Res. Rev. 2011, 27, 85–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazzeri, C.; Valente, S.; Chiostri, M.; D’Alfonso, M.G.; Gensini, G.F. Prognostic impact of early glucose variability in acute heart failure patients: A pilot study. Int. J. Cardiol. 2014, 177, 693–695. [Google Scholar] [CrossRef]
- Cunha, F.M.; Cidade-Rodrigues, C.; Elias, C.; Oliveira, D.; Bettencourt, P.; Lourenço, P. Glucose variability predicts 6-month mortality in patients hospitalized with acute heart failure. Intern. Emerg. Med. 2021, 16, 2121–2128. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [Green Version]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghissi, E.S.; Korytkowski, M.T.; DiNardo, M.; Einhorn, D.; Hellman, R.; Hirsch, I.B.; Inzucchi, S.E.; Ismail-Beigi, F.; Kirkman, M.S.; Kirkman, G.E. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009, 32, 1119–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rydén, L.; Grant, P.J.; Anker, S.D.; Berne, C.; Cosentino, F.; Danchin, N.; Deaton, C.; Escaned, J.; Hammes, H.-P.; Huikuri, H.; et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur. Heart J. 2013, 34, 3035–3087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peduzzi, P.; Concato, J.; Feinstein, A.R.; Holford, T.R. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J. Clin. Epidemiol. 1995, 48, 1503–1510. [Google Scholar] [CrossRef]
- Hicks, K.A.; Tcheng, J.E.; Bozkurt, B.; Chaitman, B.R.; Cutlip, D.E.; Farb, A.; Fonarow, G.C.; Jacobs, J.P.; Jaff, M.R.; Lichtman, J.H.; et al. 2014 ACC/AHA Key data elements and definitions for cardiovascular endpoint events in clinical trials. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards. Circulation 2015, 132, 302–361. [Google Scholar] [CrossRef] [Green Version]
- Logeart, D.; Isnard, R.; Resche-Rigon, M.; Seronde, M.F.; de Groote, P.; Jondeau, G.; Galinier, M.; Mulak, G.; Donal, E.; Delahaye, F.; et al. Current aspects of the spectrum of acute heart failure syndromes in a real-life setting: The OFICA study. Eur. J. Heart Fail. 2013, 15, 465–476. [Google Scholar] [CrossRef] [Green Version]
- Crespo-Leiro, M.G.; Anker, S.D.; Maggioni, A.P.; Coats, A.J.; Filippatos, G.; Ruschitzka, F.; Ferrari, R.; Piepoli, M.F.; Delgado Jimenez, J.F.; Metra, M.; et al. European Society of Cardiology heart failure long-term registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur. J. Heart Fail. 2016, 18, 613–625. [Google Scholar] [CrossRef] [Green Version]
- Moliner, P.; Lupon, J.; de Antonio, M.; Domingo, M.; Santiago-Vacas, E.; Zamora, E.; Santesmases, J.; Díez-Quevedo, C.; Troya, M.I.; Boldó, M. Trends in modes of death in heart failure over the last two decades: Less sudden death but cancer deaths on the rise. Eur. J. Heart Fail. 2019, 21, 1259–1266. [Google Scholar] [CrossRef]
- Metra, M.; Mentz, R.J.; Chiswell, K.; Bloomfield, D.M.; Cleland, J.G.; Cotter, G.; Davison, B.A.; Dittrich, H.C.; Fiuzat, M.; Givertz, M.M.; et al. Acute heart failure in elderly patients: Worse outcomes and differential utility of standard prognostic variables. Insights from the PROTECT trial. Eur. J. Heart Fail. 2015, 17, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Curtis, J.P.; Sokol, S.I.; Wang, Y.; Rathore, S.S.; Ko, D.T.; Jadbabaie, F.; Portnay, E.L.; Portnay, E.L.; Marshalko, S.J.; Radford, M.J.; et al. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J. Am. Coll. Cardiol. 2003, 42, 736–742. [Google Scholar] [CrossRef] [Green Version]
- Yandrapalli, S.; Malik, A.; Pemmasani, G.; Aronow, W.; Shah, F.; Lanier, G.; Cooper, H.; Jain, D.; Naidu, S.; Frishman, W.; et al. Sex differences in heart failure hospitalization risk following acute myocardial infarction. Heart 2021, 107, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; Valente, M.A.; Voors, A.A.; O’Connor, C.M.; van Veldhuisen, D.J.; Hillege, H.L. Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta-analysis. Eur. Heart J. 2014, 35, 455–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Januzzi, J.L., Jr.; Maisel, A.S.; Silver, M.; Xue, Y.; DeFilippi, C. Natriuretic peptide testing for predicting adverse events following heart failure hospitalization. Congest. Heart Fail. 2012, 18, S9–S13. [Google Scholar] [CrossRef] [PubMed]
- Gohbara, M.; Iwahashi, N.; Kataoka, S.; Hayakawa, Y.; Sakamaki, K.; Akiyama, E.; Maejima, N.; Tsukahara, K.; Hibi, K.; Kosuge, M.; et al. Glycemic variability determined by continuous glucose monitoring system predicts left ventricular remodeling in patients with a first ST-Segment elevation myocardial infarction. Circ. J. 2015, 79, 1092–1099. [Google Scholar] [CrossRef] [Green Version]
- Danne, T.; Nimri, R.; Battelino, T.; Bergenstal, R.M.; Close, K.L.; DeVries, J.H.; Garg, S.; Heinemann, L.; Hirsch, I.; Amiel, S.A.; et al. International consensus on use of continuous glucose monitoring. Diabetes Care 2017, 40, 1631–1640. [Google Scholar] [CrossRef] [Green Version]
- Lanspa, M.J.; Dickerson, J.; Morris, A.H.; Orme, J.F.; Holmen, J.; Hirshberg, E.L. Coefficient of glucose variation is independently associated with mortality in critically ill patients receiving intravenous insulin. Crit. Care 2014, 18, R86. [Google Scholar] [CrossRef] [Green Version]
- Rodbard, H.W.; Peters, A.L.; Slee, A.; Cao, A.; Traina, S.B.; Alba, M. The effect of canaglifozin, a sodium glucose cotransporter 2 inhibitor, on glycemic end points assessed by continuous glucose monitoring and patient-reported outcomes among people with type 1 diabetes. Diabetes Care 2017, 40, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Faerch, K.; Blond, M.B.; Bruhn, L.; Amahid, H.; Vistisen, D.; Clemmensen, K.K.B.; Vainø, C.T.; Pedersen, C.; Tvermosegaard, M.; Dejgaard, T.F.; et al. The effects of dapaglifozin, metformin or exercise on glycaemic variability in overweight or obese individuals with prediabetes (the PRE-D Trial): A multi-arm, randomized, controlled trial. Diabetologia 2021, 64, 42–55. [Google Scholar] [CrossRef]
- Santos-Gallego, C.G.; Vargas-Delgado, A.P.; Requena-Ibanez, J.A.; Garcia-Ropero, A.; Mancini, D.; Pinney, S.; Macaluso, F.; Sartori, S.; Roque, M.; Sabatel-Perez, F.; et al. Randomized trial of empaglifozin in nondiabetic patients with heart failure and reduced ejection fraction. J. Am. Coll. Cardiol. 2021, 77, 243–255. [Google Scholar] [CrossRef]
- Kim, M.K.; Han, K.; Park, Y.M.; Kwon, H.S.; Kang, G.; Yoon, K.H.; Lee, S.H. Associations of variability in blood pressure, glucose and cholesterol concentrations, and body mass index with mortality and cardiovascular outcomes in the general population. Circulation 2018, 138, 2627–2637. [Google Scholar] [CrossRef]
- Rodbard, D. The challenges of measuring glycemic variability. J. Diabetes Sci. Technol. 2012, 6, 712–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baghurst, P.A.; Rodbard, D.; Cameron, F.J. The minimum frequency of glucose measurements from which glycemic variation can be consistently assessed. J. Diabetes Sci. Technol. 2010, 4, 1382–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serruys, P.W.; Morice, M.C.; Kappetein, A.P.; Colombo, A.; Holmes, D.R.; Mack, M.J.; Ståhle, E.; Feldman, T.E.; van den Brand, M.; SYNTAX Investigators; et al. Percutaneous coronary intervention versus coronary-artery by-pass grafting for severe coronary artery disease. N. Engl. J. Med. 2009, 360, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Eagle, K.A.; Lim, M.J.; Dabbous, O.H.; Pieper, K.S.; Goldberg, R.J.; Van de Werf, F.; Goodman, S.G.; Granger, C.B.; Steg, P.G.; Gore, J.M.; et al. A validated prediction model for all forms of acute coronary syndrome: Estimating the risk of 6-month postdischarge death in an international registry. JAMA 2004, 291, 2727–2733. [Google Scholar] [CrossRef] [PubMed]


| Baseline Characteristics | N = 392 |
|---|---|
| Age (years) | 73 ± 10.2 |
| Age > 75 (years) | 191 (48.7) |
| Female sex (%) | 121 (30.9) |
| Prior HF hospitalisation | 125 (31.9) |
| History of chronic heart failure | 214 (54.6) |
| Systolic blood pressure at admission (mmHg) | 131 ± 41.2 |
| Heart rate at admission (beats/min) | 87 ± 25.9 |
| Smoking status (%) | |
| Non-smoker | 224 (57.1) |
| Former smoker | 126 (32.2) |
| Current smoker | 42 (10.7) |
| Hypertension * | 329 (83.9) |
| Type of diabetes | |
| Type 1 | 12 (3.1) |
| Type 2 | 378 (96.4) |
| Secondary (chronic pancreatitis) | 2 (0.5) |
| HbA1c (%) | 7.38 ± 1.48 |
| Total cholesterol (mg/dL) | 159 ± 57 |
| LDL cholesterol (mg/dL) | 96 ± 48 |
| HDL cholesterol (mg/dL) | 42 ± 16 |
| TG (mg/dL) | 262 ± 209 |
| BMI (kg/m2) | 29.1 ± 6.5 |
| Family history of CAD (%) | 33 (8.4) |
| Personal history of CAD (%) | 163 (41.6) |
| Atrial fibrillation | 76 (19.4) |
| Chronic respiratory failure (%) | 96 (24.5) |
| eGFR (mL/min/1.73 m2) † | 55.1 ± 29.2 |
| CKD with RRT (%) | 8 (2.0) |
| Left ventricular ejection fraction (LVEF) (%) | 43.3 ± 13.0 |
| LVEF < 30% | 81 (20.7) |
| LVEF 30–40% | 72 (18.4) |
| LVEF 40–49% | 95 (24.2) |
| LVEF ≥ 50% | 144 (36.7) |
| Pre-existent aetiologies of cardiopathies predisposing to AHF | |
| Ischaemic heart disease | 142 (36.2) |
| Toxic damage | 19 (4.8) |
| Immune-mediated and inflammatory damage | 2 (0.5) |
| Infiltration | 2 (0.5) |
| Metabolic derangements | 5 (1.3) |
| Genetic abnormalities | 20 (5.1) |
| Valve and myocardium structural defects | 102 (26.0) |
| Pericardial and endomyocardial pathologies | 5 (1.3) |
| Tachycardia-induced cardiomyopathy | 7 (1.8) |
| Prior HF hospitalisation in patient with preserved LVEF | 36 (9.2) |
| No pre-existent cardiopathy | 52 (13.3) |
| Factors triggering AHF | |
| Acute coronary syndrome (ACS) | 159 (40.6) |
| Myocardial rupture complicating ACS ‡ | 3 (0.7) |
| Hypertensive emergency | 40 (10.2) |
| Tachyarrhythmia | 50 (12.8) |
| Bradyarrhythmia | 12 (3.1) |
| Acute native or prosthetic valve incompetence § | 27 (6.9) |
| Vigorous fluid administration | 10 (7.7) |
| Non-adherence with salt/fluid intake or medications | 32 (8.2) |
| Worsening renal failure | 20 (5.1) |
| Severe anaemia ¶ | 20 (5.1) |
| Infection (e.g., pneumonia, sepsis) | 76 (19.4) |
| Stress-related cardiomyopathy | 3 (0.7) |
| Metabolic/hormonal derangements # | 7 (1.8) |
| Toxic substances, cardiodepressant and other drugs ** | 5 (1.3) |
| BNP value at admission (pg/mL) | 597 (348–1300) |
| Arterial pH at admission (n = 200) | 7.35 ± 0.11 |
| Arterial blood lactate at admission (mmol/L) (n = 200) | 2.35 ± 2.57 |
| Acute kidney failure †† (%) | 145 (37.0) |
| Cardiogenic shock ‡‡ | 52 (13.3) |
| Peak troponin I (ng/mL) (normal < 0.04) §§ | 0.34 (0.09–6) |
| Presence and extent of CAD (%) | |
| No invasive angiography | 174 (44.4) |
| No CAD | 22 (5.6) |
| No significant stenosis | 44 (11.2) |
| One-vessel disease | 40 (10.2) |
| Two-vessel disease | 44 (11.2) |
| Left main and/or three-vessel disease | 68 (17.4) |
| SYNTAX score | 20.0 ± 13.2 |
| GRACE Score (n = 159) | 168 ± 37 |
| GRACE Score > 140 | 118 (74.2) |
| Management | |
| Oxygen therapy | 385 (98.2) |
| Diuretics | 375 (95.7) |
| Intravenous vasodilators | 104 (26.5) |
| Inotropic agents ¶¶ | 65 (16.6) |
| Vasopressors ## | 43 (11.0) |
| Non-invasive positive pressure ventilation | 114 (29.1) |
| Mechanical ventilation | 47 (12.0) |
| Duration of mechanical ventilation (days) | 3.3 ± 5.6 |
| Renal replacement therapy | 34 (8.7) |
| Intra-aortic balloon pump | 8 (2.0) |
| Mechanical circulatory support *** | 10 (2.6) |
| Heart transplantation | 2 (0.5) |
| Myocardial revascularisation | |
| PCI | 126 (32.1) |
| CABG | 23 (5.9) |
| Hybrid strategy | 2 (0.5) |
| Medical treatment only ††† | 8 (2.0) |
| Electrical cardioversion | 20 (5.1) |
| Ablation for arrhythmias | 5 (1.2) |
| Pacemaker | 14 (3.6) |
| Non-surgical device treatment of heart failure | |
| Cardiac resynchronisation therapy | 9 (2.3) |
| Implantable cardioverter-defibrillator | 5 (1.2) |
| Valvular heart disease treatment | |
| Trans-aortic valve replacement | 14 (3.6) |
| Valve surgery | 22 (5.6) |
| Treatment at hospital discharge | |
| Diuretics | 301 (76.8) |
| RAASI | 241 (61.5) |
| ARNi | 5 (1.2) |
| ß-Blocker | 260 (66.3) |
| Mineralocorticoid antagonists | 124 (31.6) |
| Antithrombotic treatment (%) | |
| Single APT (SAPT) | 77 (19.6) |
| DAPT | 96 (24.5) |
| OAC monotherapy | 75 (19.1) |
| Dual therapy (OAC + SAPT) | 62 (15.8) |
| Triple therapy (OAC + DAPT) | 27 (6.9) |
| Statin | 269 (68.6) |
| Oral glucose-lowering therapies | 197 (50.3) |
| Biguanides | 116 (29.6) |
| Sulfonylureas and meglitinides | 123 (31.4) |
| α-glucosidase inhibitors | 5 (1.2) |
| DDP-4 inhibitors | 49 (12.5) |
| GLP-1 receptor agonists | 5 (1.2) |
| Insulin therapy | 193 (49.2) |
| Glycaemic status | |
| Glycaemia assays per patient | 45 (23–78) |
| Glycaemia assays per patient per day | 5 (3–8) |
| Admission glycaemia (mg/dL) | 193 (87) |
| Mean glycaemia (mg/dL) | 164 (32) |
| Percentage of hypoglycaemia ‡‡‡ (%) | 0.9 |
| Number of patients with hypoglycaemia (%) | 84 (21.4) |
| Hypoglycaemia events per patient, n | 2 (1–2) |
| Percentage of hyperglycaemia §§§ (%) | 32.6 |
| Number of patients with hyperglycaemia (%) | 357 (91.1) |
| Glycaemic variability (SD, mg/dL) | 53 (22) |
| All-Cause Death, n (%) | 131 (33.4) |
|---|---|
| Cause-specific CV and non-CV death | |
| Cardiovascular cause of death, n (%) | 92 (23.5) |
| Sudden cardiac death, n (%) | 15 (3.8) |
| Heart failure/Cardiogenic shock, n (%) | 50 (12.8) |
| Acute myocardial infarction, n (%) | 16 (4.1) |
| Stroke, n (%) | 1 (0.3) |
| Cardiovascular haemorrhage, n (%) | 1 (0.3) |
| Cardiovascular procedure, n (%) | 6 (1.5) |
| Other cardiovascular causes, n (%) | 3 (0.8) |
| Non-cardiovascular cause of death, n (%) | 39 (9.9) |
| Malignancy, n (%) | 15 (3.8) |
| Infection (including sepsis), n (%) | 13 (3.3) |
| Other, n (%) | 10 (2.6) |
| Undetermined death, n (%) | 1 (0.3) |
| Variables | Risk Ratio | 95% CI | p Value |
|---|---|---|---|
| Age, years | 1.02 | 1.01–1.04 | <0.001 |
| Age > 75 years | 1.27 | 1.11–1.45 | <0.001 |
| Female sex | 1.17 | 1.01–1.35 | 0.03 |
| Prior HF hospitalisation | 1.04 | 1.01–1.08 | 0.07 |
| History of chronic heart failure | 0.95 | 0.87–1.05 | 0.31 |
| Current smoker status | 0.92 | 0.71–1.20 | 0.10 |
| Hypertension | 0.88 | 0.74–1.05 | 0.15 |
| Diabetes type | 0.61 | 0.25–1.47 | 0.27 |
| HbA1c ≥ 6.5% | 1.03 | 0.64–1.50 | 0.19 |
| TC (mg/dL) | 0.80 | 0.56–1.15 | 0.23 |
| LDL-C (mg/dL) | 0.89 | 0.57–1.38 | 0.60 |
| HDL-C (mg/dL) | 0.78 | 0.23–2.67 | 0.70 |
| TG (mg/dL) | 0.92 | 0.71–1.19 | 0.53 |
| BMI (kg/m2) | 0.99 | 0.97–1.02 | 0.46 |
| eGFR < 50 mL/min/1.73 m2 | 1.78 | 1.36–2.34 | <0.001 |
| CKD with RRT | 2.22 | 0.98–5.03 | 0.06 |
| Family history of CAD | 1.00 | 0.85–1.14 | 0.96 |
| Personal history of CAD | 1.09 | 0.95–1.25 | 0.21 |
| Atrial fibrillation | 1.07 | 0.86–1.34 | 0.53 |
| Chronic respiratory failure | 1.05 | 0.89–1.22 | 0.58 |
| Systolic blood pressure at admission (mmHg) | 1.00 | 0.99–1.00 | 0.14 |
| Heart rate at admission (beats/min) | 1.00 | 0.99–1.00 | 0.57 |
| LVEF (%) | 0.99 | 0.98–1.00 | 0.10 |
| LVEF of <30% (compared with LVEF of ≥30%) | 1.51 | 1.11–2.05 | 0.009 |
| LVEF of <40% (compared with LVEF of ≥40%) | 1.08 | 0.94–1.24 | 0.28 |
| Factors triggering acute heart failure | |||
| Acute coronary syndrome (ACS) | 0.91 | 0.80–1.05 | 0.20 |
| Myocardial rupture complicating ACS | 0.66 | 0.40–1.10 | 0.11 |
| Hypertensive emergency | 1.00 | 0.94–1.07 | 0.94 |
| Tachyarrhythmia | 1.01 | 0.93–1.08 | 0.88 |
| Bradyarrhythmia | 1.01 | 0.96–1.05 | 0.52 |
| Acute native or prosthetic valve incompetence | 1.05 | 1.01–1.10 | 0.06 |
| Vigorous fluid administration | 1.00 | 0.98–1.02 | 0.75 |
| Non-adherence with salt/fluid intake or medications | 1.02 | 0.97–1.07 | 0.45 |
| Worsening renal failure | 1.02 | 0.98–1.05 | 0.63 |
| Severe anaemia | 1.01 | 0.94–1.07 | 0.50 |
| Infection (e.g., pneumonia, sepsis) | 1.03 | 0.88–1.21 | 0.58 |
| Stress-related cardiomyopathy | 1.01 | 0.94–1.09 | 0.76 |
| Metabolic/hormonal derangements | 1.01 | 0.98–1.03 | 0.46 |
| Toxic substances, cardiodepressant and other drugs | 1.03 | 1.00–1.05 | 0.07 |
| BNP value at admission > 615 pg/mL | 1.84 | 1.40–2.42 | <0.001 |
| Arterial pH at admission | 0.96 | 0.20–4.72 | 0.96 |
| Arterial blood lactate at admission (mmol/L) | 0.99 | 0.91–1.08 | 0.89 |
| Acute kidney failure during hospitalisation | 1.65 | 1.25–2.17 | <0.001 |
| Cardiogenic shock | 1.87 | 1.30–2.70 | <0.001 |
| Peak troponin (ng/mL) | 1.00 | 0.99–1.01 | 0.38 |
| Extent of CAD | |||
| One-vessel disease | 0.67 | 0.45–1.10 | 0.94 |
| Two-vessel disease | 0.85 | 0.39–1.47 | 0.58 |
| Left main and/or three-vessel disease | 1.37 | 1.10–1.70 | 0.004 |
| SYNTAX Score ≤ 22 | 1.12 | 0.84–1.49 | 0.45 |
| 22 < SYNTAX Score ≤ 33 | 1.15 | 0.96–1.35 | 0.13 |
| SYNTAX Score > 33 | 1.73 | 0.99–3.01 | 0.04 |
| GRACE score | 0.99 | 0.99–1.00 | 0.71 |
| GRACE score > 140 | 1.98 | 1.16–3.40 | 0.01 |
| Length of stay (number of days) | 1.00 | 1.00–1.01 | 0.04 |
| Management | |||
| Oxygen therapy | 0.92 | 0.64–1.33 | 0.66 |
| Diuretics | 0.78 | 0.45–1.34 | 0.36 |
| Intravenous vasodilators | 0.70 | 0.44–1.10 | 0.11 |
| Vasopressors/inotropic agents | 1.85 | 1.31–2.62 | <0.001 |
| Non-invasive positive pressure ventilation | 0.93 | 0.70–1.24 | 0.61 |
| Mechanical ventilation | 1.56 | 1.03–2.38 | 0.04 |
| Duration of mechanical ventilation (days) | 1.05 | 1.02–1.09 | 0.006 |
| Renal replacement therapy | 1.84 | 1.12–3.03 | 0.02 |
| Intra-aortic balloon pump | 0.83 | 0.27–2.62 | 0.76 |
| Mechanical circulatory support | 4.30 | 0.60–30.7 | 0.15 |
| Myocardial revascularisation | |||
| PCI | 1.07 | 0.86–1.34 | 0.53 |
| CABG | 1.11 | 0.40–3.07 | 0.85 |
| Hybrid strategy | 1.19 | 0.59–2.39 | 0.97 |
| Medical treatment only | 1.03 | 0.49–2.22 | 0.63 |
| Glycaemia assays per patient | 1.00 | 1.00–1.01 | 0.02 |
| Admission glucose level (mg/dL) | 1.00 | 1.00–1.01 | 0.008 |
| Mean glycaemia (mg/dL) | 1.01 | 1.00–1.01 | 0.03 |
| Hypoglycaemia (%) | 1.84 | 1.36–2.47 | <0.001 |
| Hyperglycaemia (%) | 3.10 | 1.72–5.58 | <0.001 |
| GV (SD, mg/dL) | 1.02 | 1.01–1.04 | <0.001 |
| GV > 2.70 (SD, mg/dL) | 5.01 | 1.78–3.01 | <0.001 |
| GV tertiles | |||
| First | 1.23 | 0.39–3.88 | 0.72 |
| Second | 1.70 | 0.45–6.35 | 0.43 |
| Third | 6.22 | 2.08–18.6 | <0.001 |
| Variables | Risk Ratio | 95% CI | p Value |
|---|---|---|---|
| Age > 75 years | 1.54 | 1.14–2.08 | 0.005 |
| Female sex | 1.43 | 1.05–1.94 | 0.03 |
| LVEF of <30% (compared with LVEF of ≥30%) | 1.47 | 1.06–2.07 | 0.02 |
| BNP value at admission > 615 pg/mL | 1.30 | 0.95–1.77 | 0.09 |
| eGFR < 50 mL/min/1.73 m2 | 1.10 | 0.80–1.52 | 0.54 |
| Acute kidney failure during hospitalisation | 1.27 | 0.92–1.76 | 0.15 |
| Mechanical ventilation | 1.23 | 0.70–2.16 | 0.48 |
| Vasopressor/inotropic agents | 1.60 | 0.97–2.64 | 0.06 |
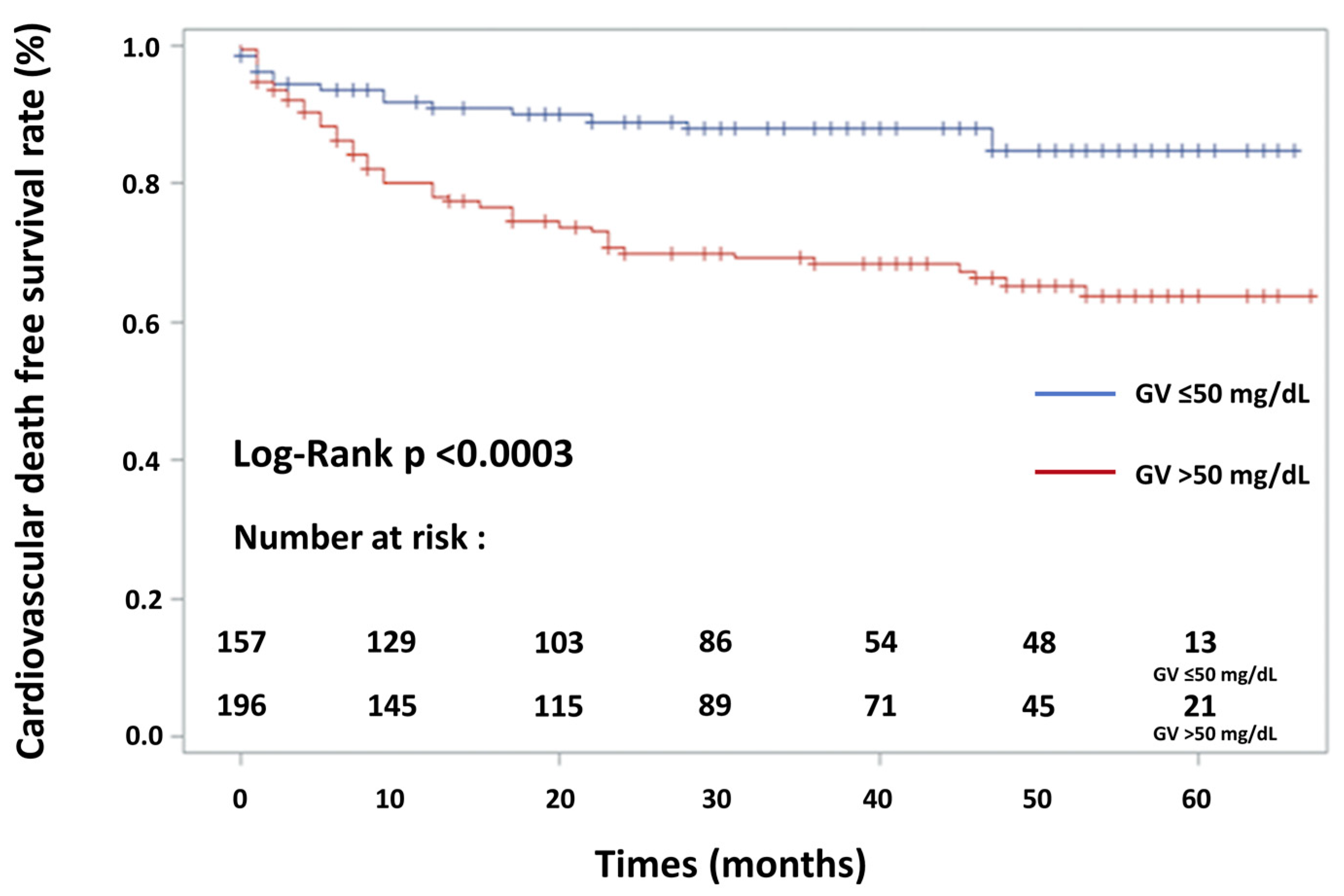
| GV > 50 mg/dL (or >2.70 mmol/L) | 3.16 | 2.25–4.43 | <0.001 |
| Length of stay (days) | 1.00 | 0.99–1.01 | 0.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerbaud, E.; Bouchard de La Poterie, A.; Baudinet, T.; Montaudon, M.; Beauvieux, M.-C.; Lemaître, A.-I.; Cetran, L.; Seguy, B.; Picard, F.; Vélayoudom, F.-L.; et al. Glycaemic Variability and Hyperglycaemia as Prognostic Markers of Major Cardiovascular Events in Diabetic Patients Hospitalised in Cardiology Intensive Care Unit for Acute Heart Failure. J. Clin. Med. 2022, 11, 1549. https://doi.org/10.3390/jcm11061549
Gerbaud E, Bouchard de La Poterie A, Baudinet T, Montaudon M, Beauvieux M-C, Lemaître A-I, Cetran L, Seguy B, Picard F, Vélayoudom F-L, et al. Glycaemic Variability and Hyperglycaemia as Prognostic Markers of Major Cardiovascular Events in Diabetic Patients Hospitalised in Cardiology Intensive Care Unit for Acute Heart Failure. Journal of Clinical Medicine. 2022; 11(6):1549. https://doi.org/10.3390/jcm11061549
Chicago/Turabian StyleGerbaud, Edouard, Ambroise Bouchard de La Poterie, Thomas Baudinet, Michel Montaudon, Marie-Christine Beauvieux, Anne-Iris Lemaître, Laura Cetran, Benjamin Seguy, François Picard, Fritz-Line Vélayoudom, and et al. 2022. "Glycaemic Variability and Hyperglycaemia as Prognostic Markers of Major Cardiovascular Events in Diabetic Patients Hospitalised in Cardiology Intensive Care Unit for Acute Heart Failure" Journal of Clinical Medicine 11, no. 6: 1549. https://doi.org/10.3390/jcm11061549
APA StyleGerbaud, E., Bouchard de La Poterie, A., Baudinet, T., Montaudon, M., Beauvieux, M.-C., Lemaître, A.-I., Cetran, L., Seguy, B., Picard, F., Vélayoudom, F.-L., Ouattara, A., Kabore, R., Coste, P., Domingues-Dos-Santos, P., & Catargi, B. (2022). Glycaemic Variability and Hyperglycaemia as Prognostic Markers of Major Cardiovascular Events in Diabetic Patients Hospitalised in Cardiology Intensive Care Unit for Acute Heart Failure. Journal of Clinical Medicine, 11(6), 1549. https://doi.org/10.3390/jcm11061549






