Selecting the Target Population for Screening of Hepatic Fibrosis in Primary Care Centers in Korea
(This article belongs to the Section Gastroenterology & Hepatopancreatobiliary Medicine)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Rationale for Abdominal Sonography and MRE as Health Check-Up
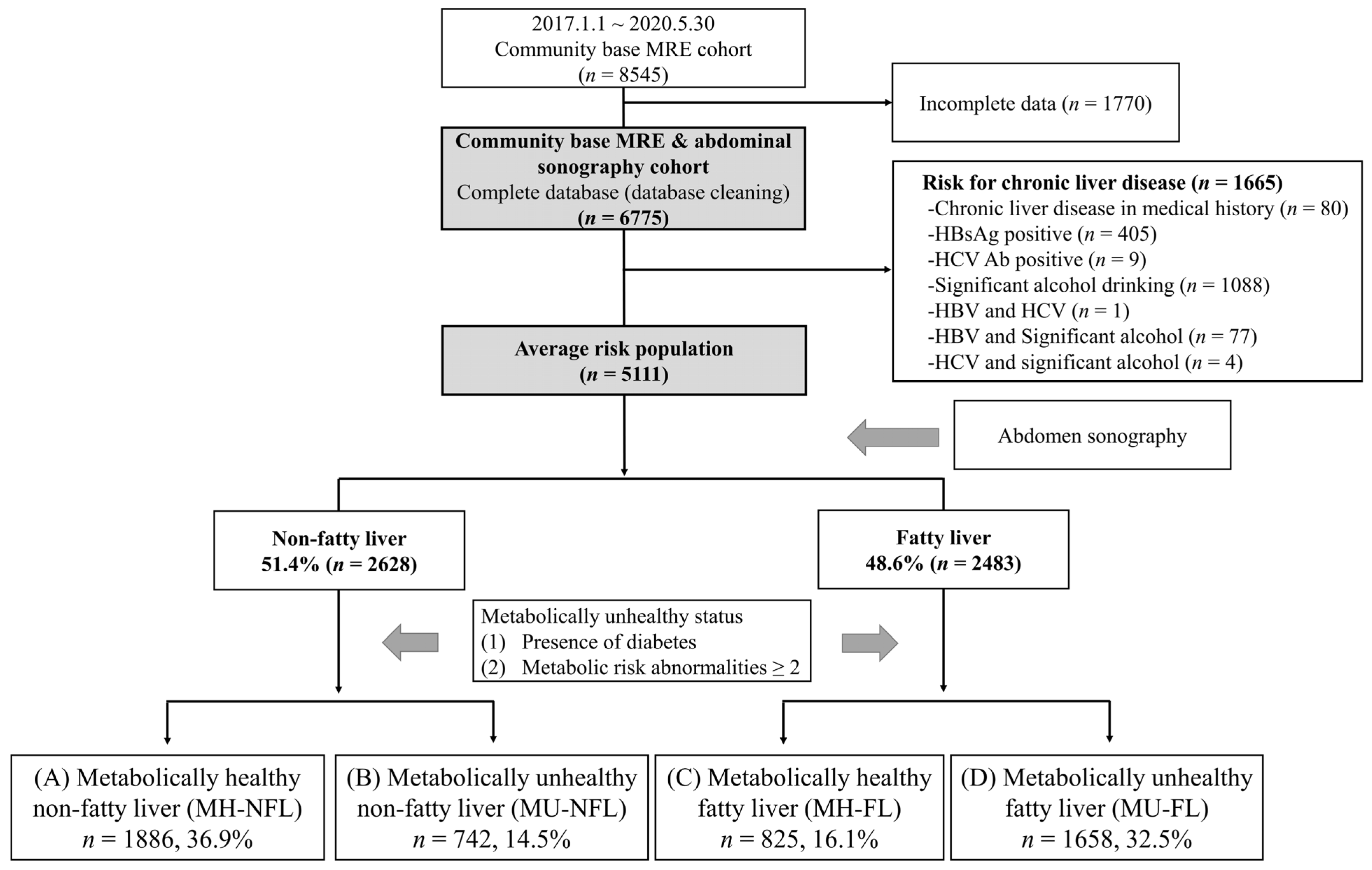
2.3. Inclusion and Exclusion Criteria
2.4. Clinical Parameters of Participants
2.5. Assessment of Fatty Liver and Hepatic Fibrosis Severity
2.6. Definition of Abnormality
2.7. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Prevalence of Hepatic Fibrosis According to Metabolic Risk Factor Type
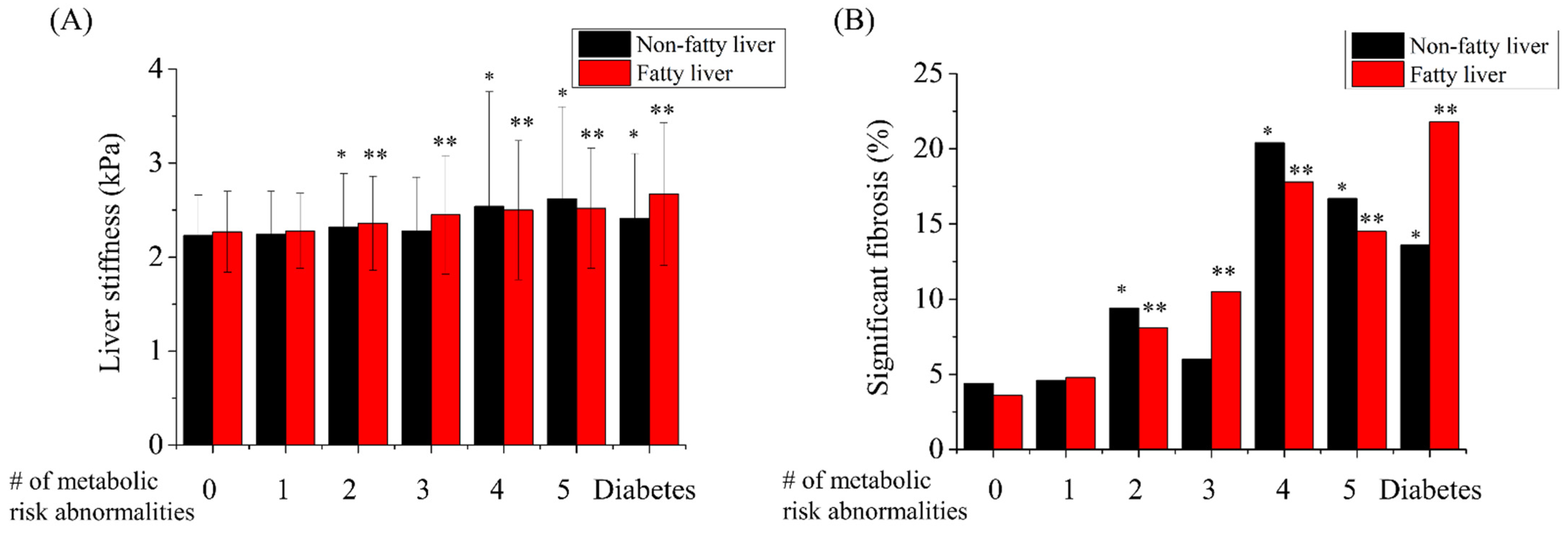
3.3. Hepatic Fibrosis Burden Increased When Subjects Had Two or More Metabolic Risk Abnormalities or Diabetes
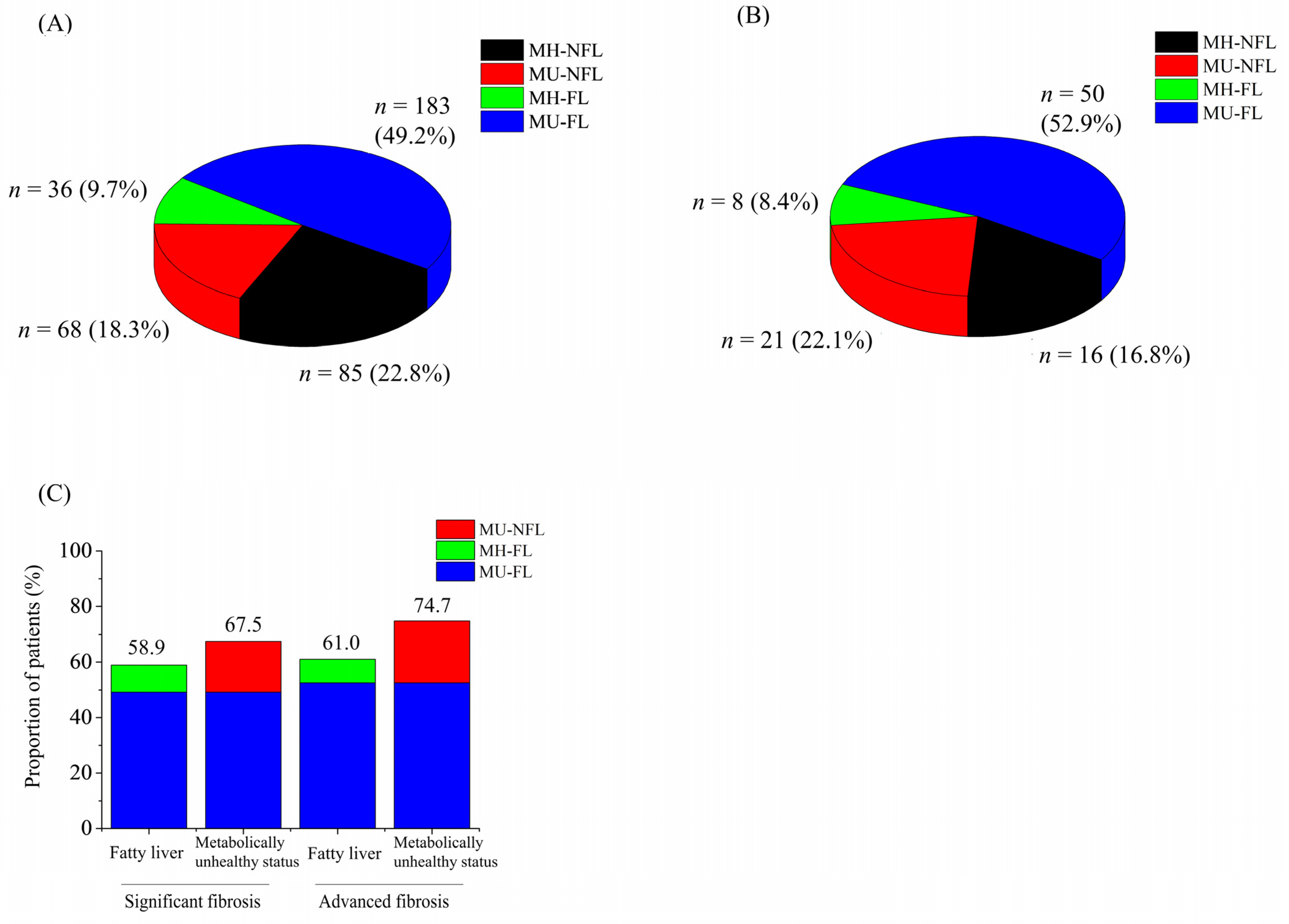
3.4. Metabolically Unhealthy Status Shared Considerable Hepatic Fibrosis Regardless of Fatty Liver
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, K.A.; Jun, D.W.; Kim, M.S.; Kwon, H.J.; Nguyen, M.H. Prevalence of significant hepatic fibrosis using magnetic resonance elastography in a health check-up clinic population. Aliment. Pharmacol. Ther. 2020, 51, 388–396. [Google Scholar] [CrossRef]
- Nah, E.-H.; Cho, S.; Kim, S.; Chu, J.; Kwon, E.; Cho, H.-I. Prevalence of liver fibrosis and associated risk factors in the Korean general population: A retrospective cross-sectional study. BMJ Open 2021, 11, e046529. [Google Scholar] [CrossRef] [PubMed]
- Lackner, C.; Tiniakos, D. Fibrosis and alcohol-related liver disease. J. Hepatol. 2019, 70, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Germani, G.; Hytiroglou, P.; Fotiadu, A.; Burroughs, A.K.; Dhillon, A.P. Assessment of fibrosis and cirrhosis in liver biopsies: An update. Semin. Liver Dis. 2011, 31, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Milovanović, T.; Pantić, I.; Dragašević, S.; Lugonja, S.; Dumić, I.; Rajilić-Stojanović, M. The interrelationship among non-alcoholic fatty liver disease, colonic diverticulosis and metabolic syndrome. J. Gastrointestin. Liver Dis. 2021, 30, 1–9. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Calzadilla-Bertot, L.; Wong, V.W.-S.; Castellanos, M.; Aller-de la Fuente, R.; Metwally, M.; Eslam, M.; Gonzalez-Fabian, L.; Sanz, M.A.-Q.; Conde-Martin, A.F. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: A multi-national cohort study. Gastroenterology 2018, 155, 443–457.e17. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Marchesini, G. Referral pathways for NAFLD fibrosis in primary care—No longer a ‘needle in a haystack’. J. Hepatol. 2019, 71, 246–248. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, A.; Gailer, R.; Tanwar, S.; Trembling, P.; Parkes, J.; Rodger, A.; Suri, D.; Thorburn, D.; Sennett, K.; Morgan, S. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J. Hepatol. 2019, 71, 371–378. [Google Scholar] [CrossRef] [Green Version]
- Vieira Barbosa, J.; Lai, M. Nonalcoholic Fatty Liver Disease Screening in Type 2 Diabetes Mellitus Patients in the Primary Care Setting. Hepatol. Commun. 2021, 5, 158–167. [Google Scholar] [CrossRef]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Petta, S.; Eslam, M.; Valenti, L.; Bugianesi, E.; Barbara, M.; Cammà, C.; Porzio, M.; Rosso, C.; Fargion, S.; George, J. Metabolic syndrome and severity of fibrosis in nonalcoholic fatty liver disease: An age-dependent risk profiling study. Liver Int. 2017, 37, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Barb, D.; Repetto, E.M.; Stokes, M.E.; Shankar, S.S.; Cusi, K. Type 2 diabetes mellitus increases the risk of hepatic fibrosis in individuals with obesity and nonalcoholic fatty liver disease. Obesity 2021, 29, 1950–1960. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, H.; Craig, D.; Barker, R.; Spiers, G.; Stow, D.; Anstee, Q.M.; Hanratty, B. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of population-based observational studies. PLoS Med. 2020, 17, e1003100. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.R.; Li, L.; Dai, J.; Natarajan, Y.; Yu, X.; Asch, S.M.; El-Serag, H.B. Effect of metabolic traits on the risk of cirrhosis and hepatocellular cancer in nonalcoholic fatty liver disease. Hepatology 2020, 71, 808–819. [Google Scholar] [CrossRef]
- Golabi, P.; Otgonsuren, M.; de Avila, L.; Sayiner, M.; Rafiq, N.; Younossi, Z.M. Components of metabolic syndrome increase the risk of mortality in nonalcoholic fatty liver disease (NAFLD). Medicine 2018, 97, e0214. [Google Scholar] [CrossRef] [PubMed]
- Sohn, W.; Lee, Y.-S.; Lee, J.G.; An, J.; Jang, E.S.; Lee, D.H.; Sinn, D.H. A Survey of Liver Cancer Specialists’ Views on the National Liver Cancer Screening Program in Korea. J. Liver Cancer 2020, 20, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Mittelstaedt, C. Retroperitoneum. In General Ultrasound; Churchill Livingstone: New York, NY, USA, 1992; p. 749. [Google Scholar]
- Yin, M.; Talwalkar, J.A.; Glaser, K.J.; Manduca, A.; Grimm, R.C.; Rossman, P.J.; Fidler, J.L.; Ehman, R.L. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin. Gastroenterol. Hepatol. 2007, 5, 1207–1213.e2. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.; Caussy, C.; Imajo, K.; Chen, J.; Singh, S.; Kaulback, K.; Le, M.-D.; Hooker, J.; Tu, X.; Bettencourt, R. Magnetic resonance vs. transient elastography analysis of patients with nonalcoholic fatty liver disease: A systematic review and pooled analysis of individual participants. Clin. Gastroenterol. Hepatol. 2019, 17, 630–637.e8. [Google Scholar] [CrossRef] [Green Version]
- Alberti, K.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Trépo, E.; Romeo, S.; Zucman-Rossi, J.; Nahon, P. PNPLA3 gene in liver diseases. J. Hepatol. 2016, 65, 399–412. [Google Scholar] [CrossRef] [Green Version]
- Koo, B.K.; Kim, D.; Joo, S.K.; Kim, J.H.; Chang, M.S.; Kim, B.G.; Lee, K.L.; Kim, W. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J. Hepatol. 2017, 66, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Wiest, R.; Albillos, A.; Trauner, M.; Bajaj, J.S.; Jalan, R. Targeting the gut-liver axis in liver disease. J. Hepatol. 2017, 67, 1084–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippatos, T.; Alexakis, K.; Mavrikaki, V.; Mikhailidis, D. Nonalcoholic fatty pancreas disease: Role in metabolic syndrome, “prediabetes,” diabetes and atherosclerosis. Dig. Dis. Sci. 2022, 67, 26–41. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total n = 5111 | Non-Fatty Liver | Fatty Liver | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (A) MH-NFL n = 1886 (36.9%) | (B) MU-NFL n = 742 (14.5%) | (C) MH-FL n = 825 (16.1%) | (D) MU-FL n = 1658 (32.5%) | A vs. B | A vs. C | A vs. D | B vs. C | B vs. D | C vs. D | ||
| Age (years) † | 46.9 ± 10.4 | 44.7 ± 10.5 | 50.6 ± 10.7 | 45.9 ± 9.3 | 48.2 ± 10 | <0.001 | 0.004 | <0.001 | <0.001 | <0.001 | <0.001 |
| Male/Female | 4170/942 (81.6/18.4) | 1349/537 (71.5/28.5) | 592/150 (79.8/20.2) | 730/95 (88.5/11.5) | 1499/159 (90.4/9.6) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.136 |
| Hypertension | 1475 (28.9) | 184 (9.8) | 375 (50.5) | 63 (7.6) | 853 (51.4) | <0.001 | 0.078 | <0.001 | <0.001 | 0.681 | <0.001 |
| Diabetes | 424 (8.3) | 0 (0) | 103 (13.9) | 0 (0) | 321 (19.4) | <0.001 | - | <0.001 | <0.001 | 0.001 | <0.001 |
| Number of metabolic risk abnormalities † | 1.5 ± 1.3 | 0.4 ± 0.4 | 2.3 ± 0.7 | 0.6 ± 0.4 | 2.8 ± 0.8 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Metabolic syndrome | 1189 (23.3) | 0 (0) | 243 (32.7) | 0 (0) | 946 (57.1) | <0.001 | - | <0.001 | <0.001 | <0.001 | <0.001 |
| BMI (kg/m2) † | 24.8 ± 3.2 | 22.8 ± 2.3 | 24.9 ± 2.8 | 24.8 ± 2.4 | 27.1 ± 3 | <0.001 | <0.001 | <0.001 | 0.79 | <0.001 | <0.001 |
| Waist circumference (cm) † | 85.5 ± 9 | 79.2 ± 7.2 | 86.3 ± 7.8 | 85.4 ± 6.3 | 92.3 ± 7.2 | <0.001 | <0.001 | <0.001 | 0.013 | <0.001 | <0.001 |
| Total fat mass (kg) † | 18.5 ± 5.8 | 15.2 ± 4.1 | 18.7 ± 5.3 | 18.3 ± 4.2 | 22.4 ± 5.8 | <0.001 | <0.001 | <0.001 | 0.085 | <0.001 | <0.001 |
| Lean mass (kg) † | 49.3 ± 8.8 | 46.1 ± 8.5 | 48.9 ± 8.6 | 49.7 ± 7.4 | 52.8 ± 8.5 | <0.001 | <0.001 | <0.001 | 0.051 | <0.001 | <0.001 |
| Lean mass/BW (%) † | 68.7 ± 6.9 | 70.8 ± 6.8 | 68.4 ± 6.9 | 68.8 ± 6.1 | 66.3 ± 6.5 | <0.001 | <0.001 | <0.001 | 0.227 | <0.001 | <0.001 |
| SBP (mmHg) † | 116 ± 13 | 111 ± 11 | 121 ± 14 | 113 ± 9 | 122 ± 13 | <0.001 | <0.001 | <0.001 | <0.001 | 0.192 | <0.001 |
| DBP (mmHg) † | 75 ± 9 | 71 ± 7 | 78 ± 10 | 72 ± 7 | 78 ± 9 | <0.001 | 0.001 | <0.001 | <0.001 | 0.09 | <0.001 |
| AST (IU/L) † | 30 ± 18 | 25 ± 11 | 28 ± 15 | 29 ± 21 | 36 ± 22 | <0.001 | <0.001 | <0.001 | 0.132 | <0.001 | <0.001 |
| ALT (IU/L) † | 32 ± 30 | 22 ± 21 | 27 ± 23 | 34 ± 36 | 45 ± 34 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| GGT (U/L) † | 57 ± 88 | 37 ± 46 | 59 ± 70 | 55 ± 114 | 80 ± 110 | <0.001 | <0.001 | <0.001 | 0.422 | <0.001 | <0.001 |
| Triglyceride (mg/dL) † | 145 ± 111 | 92 ± 51 | 162 ± 100 | 121 ± 69 | 211 ± 141 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| HDL (mg/dL) † | 52 ± 12 | 59 ± 12 | 51 ± 12 | 52 ± 10 | 46 ± 10 | <0.001 | <0.001 | <0.001 | 0.018 | <0.001 | <0.001 |
| Glucose (mg/dL) † | 99 ± 21 | 90 ± 8 | 105 ± 23 | 92 ± 8 | 109 ± 27 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 |
| HbA1c (%) † | 5.7 ± 0.7 | 5.4 ± 0.3 | 5.9 ± 0.9 | 5.6 ± 0.3 | 6.1 ± 0.9 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Significant Fibrosis * | Advanced Fibrosis * | |
|---|---|---|
| Total subjects n = 5111 | 372/5111 (7.3) | 95/5111 (1.9) |
| Subjects with metabolic risk abnormality | ||
| Central obesity n = 1812 (35.5) | 189/1812 (10.4) | 48/1812 (2.6) |
| High blood pressure or hypertension medication n = 1156 (22.6) | 108/1156 (9.3) | 29/1156 (2.5) |
| High triglyceride n = 1811 (35.6) | 170/1811 (9.4) | 44/1811 (2.4) |
| Low HDL or dyslipidemia medication n = 967 (18.9) | 87/967 (9.0) | 26/967 (2.7) |
| Prediabetes or diabetes n = 1705 (33.4) | 207/1705 (12.1) | 67/1705 (3.9) |
| Variables | OR | CI (95%) | p-Value |
|---|---|---|---|
| (a) Univariate analysis | |||
| Age | 1.04 | 1.03–1.05 | <0.001 |
| Male sex | 2.13 | 1.50–3.02 | <0.001 |
| BMI (kg/m2) | 1.12 | 1.09–1.16 | <0.001 |
| Fatty liver (≥mild) | 1.56 | 1.26–1.93 | <0.001 |
| Hypertension | 1.69 | 1.36–2.11 | <0.001 |
| Metabolic risk abnormalities (≥2) | 2.54 | 2.03–3.17 | <0.001 |
| Metabolic risk abnormalities (≥3) | 2.37 | 1.91–2.95 | <0.001 |
| Diabetes | 3.77 | 2.89–4.92 | <0.001 |
| Metabolically unhealthy status | 2.50 | 1.99–3.12 | <0.001 |
| Abnormal aminotransferase | 2.96 | 2.39–3.68 | <0.001 |
| Component of metabolic risk abnormality | |||
| Central obesity | 1.98 | 1.60–2.45 | <0.001 |
| High blood pressure or hypertension medication | 1.44 | 1.14–1.82 | 0.002 |
| High triglyceride | 1.58 | 1.27–1.95 | <0.001 |
| Low HDL or dyslipidemia medication | 1.33 | 1.04–1.72 | 0.023 |
| Prediabetes or diabetes | 2.71 | 2.19–3.36 | <0.001 |
| (b) Multivariate analysis | |||
| Age | 1.04 | 1.03–1.05 | <0.001 |
| Male sex | 1.85 | 1.29–2.65 | 0.001 |
| Fatty liver (≥mild) | 0.86 | 0.67–1.10 | 0.256 |
| Abnormal aminotransferase | 2.80 | 2.21–3.55 | <0.001 |
| Metabolically unhealthy status | 1.76 | 1.37–2.26 | <0.001 |
| (A) MH-NFL | (B) MU-NFL | (C) MH-FL | (D) MU-FL | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A vs. B | A vs. C | A vs. D | B vs. C | B vs. D | C vs. D | |||||
| Liver stiffness (kPa) † | 2.24 ± 0.44 | 2.33 ± 0.64 | 2.27 ± 0.42 | 2.43 ± 0.58 | <0.001 | 0.044 | <0.001 | 0.05 | <0.001 | <0.001 |
| Significant fibrosis | 85 (4.5) | 68 (9.2) | 36 (4.4) | 183 (11) | <0.001 | 0.868 | <0.001 | <0.001 | 0.166 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Yoon, E.L.; Kim, M.; Cho, S.; Kim, J.-H.; Jun, D.W.; Nah, E.-H. Selecting the Target Population for Screening of Hepatic Fibrosis in Primary Care Centers in Korea. J. Clin. Med. 2022, 11, 1474. https://doi.org/10.3390/jcm11061474
Park H, Yoon EL, Kim M, Cho S, Kim J-H, Jun DW, Nah E-H. Selecting the Target Population for Screening of Hepatic Fibrosis in Primary Care Centers in Korea. Journal of Clinical Medicine. 2022; 11(6):1474. https://doi.org/10.3390/jcm11061474
Chicago/Turabian StylePark, Huiyul, Eileen L. Yoon, Mimi Kim, Seon Cho, Jung-Hwan Kim, Dae Won Jun, and Eun-Hee Nah. 2022. "Selecting the Target Population for Screening of Hepatic Fibrosis in Primary Care Centers in Korea" Journal of Clinical Medicine 11, no. 6: 1474. https://doi.org/10.3390/jcm11061474
APA StylePark, H., Yoon, E. L., Kim, M., Cho, S., Kim, J.-H., Jun, D. W., & Nah, E.-H. (2022). Selecting the Target Population for Screening of Hepatic Fibrosis in Primary Care Centers in Korea. Journal of Clinical Medicine, 11(6), 1474. https://doi.org/10.3390/jcm11061474






