Distal Interphalangeal Joint Involvement May Be Associated with Disease Activity and Affected Joint Distribution in Rheumatoid Arthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Composite Disease Activity Index for RA
2.3. DIP Involvement
2.4. Joint Index
2.5. Statistical Analysis
3. Results
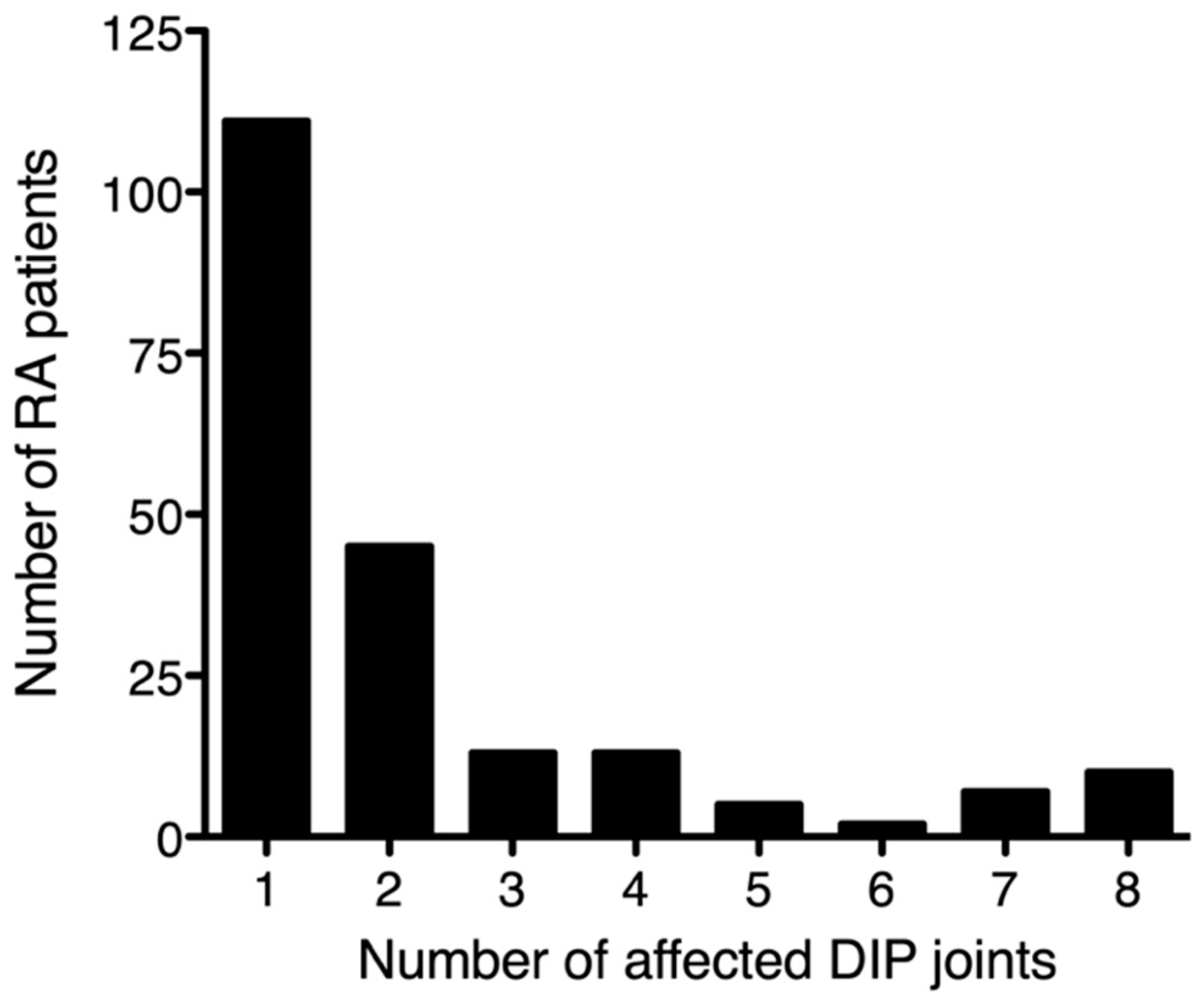
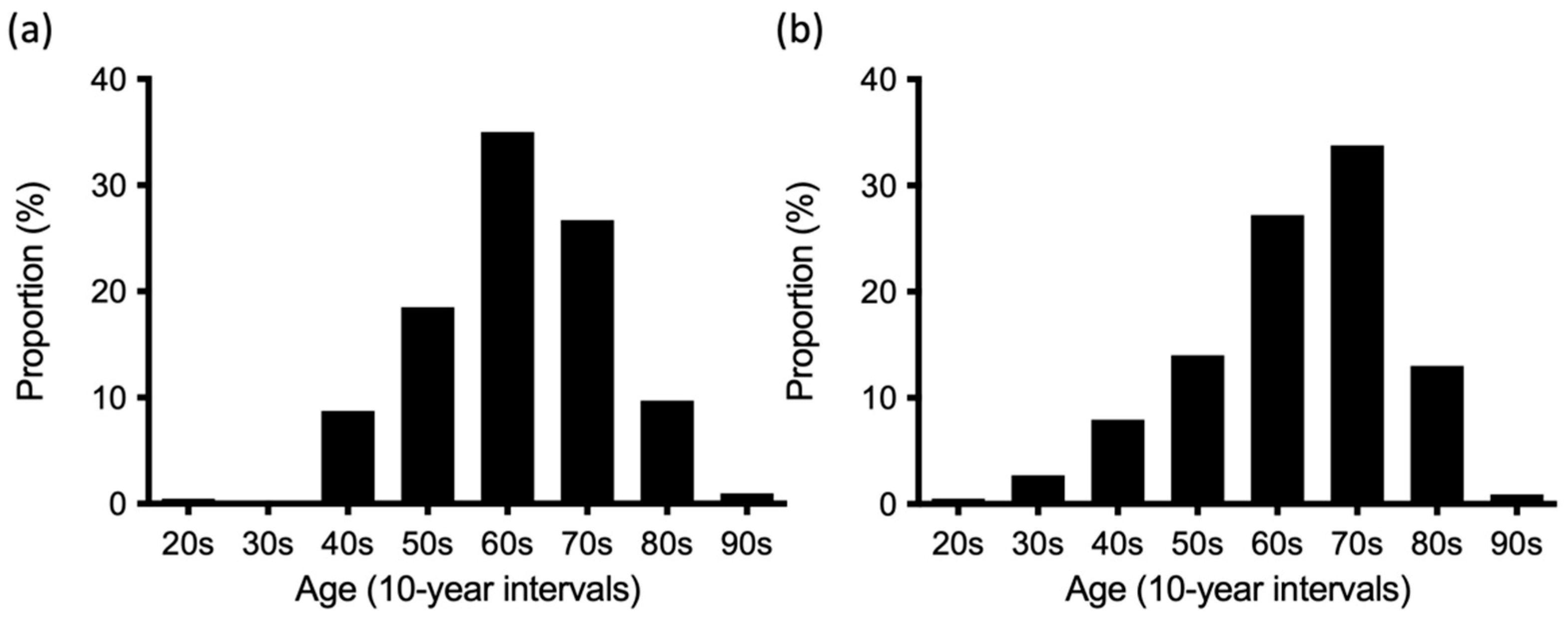
3.1. DIP Involvement in RA
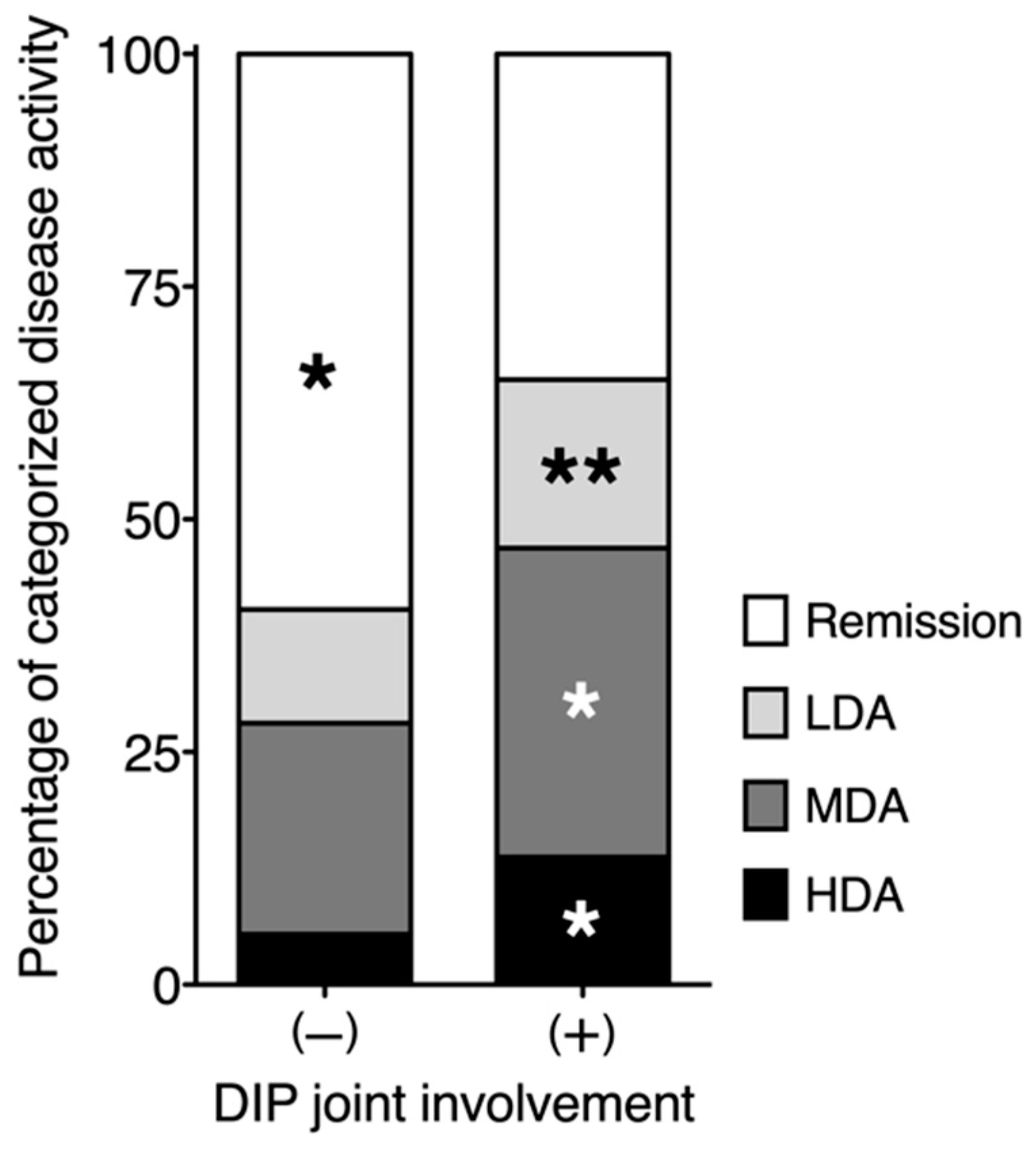
3.2. Relationship between DIP Joint Involvement and RA Disease Activity
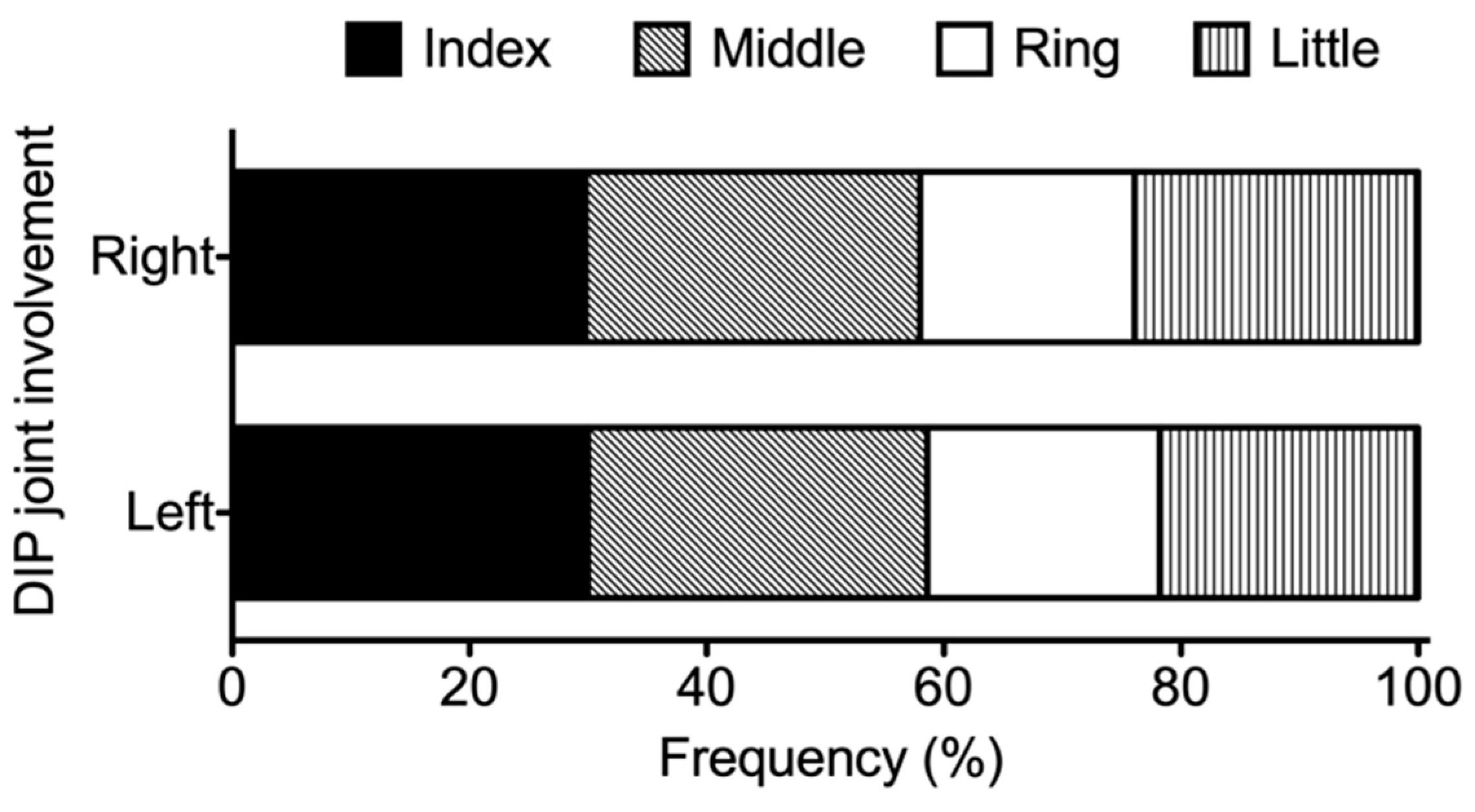
3.3. Distribution of Affected Joints in Patients with RA Exhibiting DIP Joint Involvement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- England, B.; Mikuls, T. Clinical features of rheumatoid arthritis. In Firestein & Kelly’s Textbook of Rheumatology, 11th ed.; Firestein, G., Budd, R., Gabriel, S., Koretzky, G., McInnes, I., O’Dell, J., Eds.; Elsevier: Philadelphia, PA, USA, 2020; pp. 1236–1257. [Google Scholar]
- Fleming, A.; Benn, R.T.; Corbett, M.; Wood, P.H. Early rheumatoid disease. II. Patterns of joint involvement. Ann. Rheum. Dis. 1976, 35, 361–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparks, J.A. Rheumatoid Arthritis. Ann. Intern. Med. 2019, 170, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, E. Arthritis associated with psoriasis and other skin diseases. In Rheumatology Secrets, 4th ed.; Sterling, G.W., Ed.; Elsevier: Philadelphia, PA, USA, 2020; pp. 308–314. [Google Scholar]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Peter, J.B.; Pearson, C.M.; Marmor, L. Erosive osteoarthritis of the hands. Arthritis Rheum. 1966, 9, 365–388. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Alarcón, G.; Appelrouth, D.; Bloch, D.; Borenstein, D.; Brandt, K.; Brown, C.; Cooke, T.D.; Daniel, W.; Gray, R.; et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum. 1990, 33, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Kloppenburg, M.; Kwok, W.Y. Hand osteoarthritis—A heterogeneous disorder. Nat. Rev. Rheumatol. 2011, 8, 22–31. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Driban, J.B.; Roberts, M.B.; Duryea, J.; Haugen, I.K.; Schaefer, L.F.; Smith, S.E.; Mathiessen, A.; Eaton, C. Erosive Hand Osteoarthritis: Incidence and Predictive Characteristics Among Participants in the Osteoarthritis Initiative. Arthritis Rheumatol. 2021, 73, 2015–2024. [Google Scholar] [CrossRef]
- Van der Heijde, D.; Sharp, J.; Wassenberg, S.; Gladman, D.D. Psoriatic arthritis imaging: A review of scoring methods. Ann. Rheum. Dis. 2005, 64 (Suppl. S2), ii61–ii64. [Google Scholar] [CrossRef] [Green Version]
- Poggenborg, R.P.; Bird, P.; Boonen, A.; Wiell, C.; Pedersen, S.J.; Sørensen, I.J.; Madsen, O.R.; Slot, O.; Møller, J.M.; Bøyesen, P.; et al. Pattern of bone erosion and bone proliferation in psoriatic arthritis hands: A high-resolution computed tomography and radiography follow-up study during adalimumab therapy. Scand. J. Rheumatol. 2014, 43, 202–208. [Google Scholar] [CrossRef]
- Jacob, J.; Sartoris, D.; Kursunoglu, S.; Pate, D.; Pineda, C.J.; Braun, R.M.; Resnick, D.; Weisman, M.H. Distal interphalangeal joint involvement in rheumatoid arthritis. Arthritis Rheum. 1986, 29, 10–15. [Google Scholar] [CrossRef]
- Harris, E.J. Rheumatoid arthritis: The clinical spectrum. In Textbook of Rheumatology; Kelley, W., Harris, E., Jr., Ruddy, S., Sledge, C., Eds.; WB Saunders: Philadelphia, PA, USA, 1981; pp. 928–963. [Google Scholar]
- Williams, R.; McCarty, D. Clinical picture of rheumatoid arthritis. In Arthritis and Allied Condition, 10th ed.; McCarty, D., Ed.; Lea & Febiger: Philadelphia, PA, USA, 1985; pp. 605–619. [Google Scholar]
- McCarty, D.; Gatter, R. A study of distal interphalangeal joint tenderness in rheumatoid arthritis. Arthritis Rheum. 1966, 9, 325–336. [Google Scholar] [CrossRef]
- Nishiyama, S.; Aita, T.; Yoshinaga, Y.; Kishimoto, H.; Toda, M.; Yoshihara, Y.; Miyoshi, S.; Manki, A.; Miyawaki, S. Proposing a method of regional assessment and a novel outcome measure in rheumatoid arthritis. Rheumatol. Int. 2012, 32, 2569–2571. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, S.; Sawada, T.; Nishino, J.; Tohma, S. Joint index vector: A novel assessment measure for stratified medicine in patients with rheumatoid arthritis. J. Big Data 2018, 5, 37. [Google Scholar] [CrossRef]
- Yamanaka, H.; Tohma, S. Potential impact of observational cohort studies in Japan on rheumatoid arthritis research and practice. Mod. Rheumatol. 2006, 16, 75–76. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; McShane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef]
- Steinbrocker, O.; Traeger, C.H.; Batterman, R.C. Therapeutic criteria in rheumatoid arthritis. J. Am. Med. Assoc. 1949, 140, 659–662. [Google Scholar] [CrossRef]
- Felson, D.T.; Anderson, J.J.; Boers, M.; Bombardier, C.; Chernoff, M.; Fried, B.; Furst, D.; Goldsmith, C.; Kieszak, S.; Lightfoot, R.; et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum. 1993, 36, 729–740. [Google Scholar] [CrossRef]
- Anderson, J.K.; Zimmerman, L.; Caplan, L.; Michaud, K. Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and Disease Activity Score with 28-Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score-II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), Chronic Arthritis Systemic Index (CASI), Patient-Based Disease Activity Score with ESR (PDAS1) and Patient-Based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI-RA). Arthritis Care Res. 2011, 63 (Suppl. S11), S14–S36. [Google Scholar]
- Inoue, E.; Yamanaka, H.; Hara, M.; Tomatsu, T.; Kamatani, N. Comparison of Disease Activity Score (DAS)28-erythrocyte sedimentation rate and DAS28-C-reactive protein threshold values. Ann. Rheum. Dis. 2007, 66, 407–409. [Google Scholar] [CrossRef]
- Oka, S.; Furukawa, H.; Shimada, K.; Hashimoto, A.; Komiya, A.; Tsunoda, S.; Saisho, K.; Tsuchiya, N.; Katayama, M.; Shinohara, S.; et al. Association of HLA-DRB1 genotype with younger age onset and elder age onset rheumatoid arthritis in Japanese populations. Medicine 2019, 98, e18218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terao, C.; Brynedal, B.; Chen, Z.; Jiang, X.; Westerlind, H.; Hansson, M.; Jakobsson, P.J.; Lundberg, K.; Skriner, K.; Serre, G.; et al. Distinct HLA Associations with Rheumatoid Arthritis Subsets Defined by Serological Subphenotype. Am. J. Hum. Genet. 2019, 105, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.P.; Guo, Y.; Lahiri, M. Predominance of large joint active synovitis in Asian patients with established rheumatoid arthritis. Clin. Exp. Rheumatol. 2016, 34, 157. [Google Scholar] [PubMed]
- Halla, J.T.; Fallahi, S.; Hardin, J.G. Small joint involvement: A systematic roentgenographic study in rheumatoid arthritis. Ann. Rheum. Dis. 1986, 45, 327–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanna, D.; Ranganath, V.K.; Fitzgerald, J.; Park, G.S.; Altman, R.D.; Elashoff, D.; Gold, R.H.; Sharp, J.T.; Furst, D.E.; Paulus, H.E. Increased radiographic damage scores at the onset of seropositive rheumatoid arthritis in older patients are associated with osteoarthritis of the hands, but not with more rapid progression of damage. Arthritis Rheum. 2005, 52, 2284–2292. [Google Scholar] [CrossRef]
- Lechtenboehmer, C.A.; Jaeger, V.K.; Kyburz, D.; Walker, U.A.; Hügle, T. Brief Report: Influence of disease activity in rheumatoid arthritis on radiographic progression of concomitant interphalangeal joint osteoarthritis. Arthritis Rheumatol. 2019, 71, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Rees, F.; Doherty, S.; Hui, M.; Maciewicz, R.; Muir, K.; Zhang, W.; Doherty, M. Distribution of finger nodes and their association with underlying radiographic features of osteoarthritis. Arthritis Care Res. 2012, 64, 533–538. [Google Scholar] [CrossRef] [Green Version]
- Mody, E.; Husni, M.E.; Schur, P.; Qureshi, A.A. Multidisciplinary evaluation of patients with psoriasis presenting with musculoskeletal pain: A dermatology: Rheumatology clinic experience. Br. J. Dermatol. 2007, 157, 1050–1051. [Google Scholar] [CrossRef]
- Chen, K.L.; Chiu, H.Y.; Lin, J.H.; Ye, J.D.; Cho, Y.H.; Li, K.J.; Tsai, T.F. Prevalence, clinical features and treatment pattern of patients with concurrent diagnoses of rheumatoid arthritis and psoriatic disease: Results of a 14-year retrospective study in a tertiary referral center. Ther. Adv. Chronic Dis. 2019, 10, 2040622319847900. [Google Scholar] [CrossRef]
- Kato, E.; Sawada, T.; Tahara, K.; Hayashi, H.; Tago, M.; Mori, H.; Nishino, J.; Matsui, T.; Tohma, S. The age at onset of rheumatoid arthritis is increasing in Japan: A nationwide database study. Int. J. Rheum. Dis. 2017, 20, 839–845. [Google Scholar] [CrossRef]
| DIP Involvement | |||
|---|---|---|---|
| Presence (n = 206) | Absence (n = 9832) | p-Value | |
| Age, mean ± SD years | 65.5 ± 11.3 | 66.8 ± 12.6 | <0.05 |
| Older adult population (≥65 years of age) | 115 (55.8%) | 6301 (64.1%) | <0.05 |
| Female | 182 (88.4%) | 7842 (79.8%) | <0.01 |
| Age at RA onset, mean ± SD years | 52.9 ± 14.7 | 52.2 ± 14.8 | NS |
| Disease duration, mean ± SD years | 13.3 ± 10.4 | 13.9 ± 11.1 | NS |
| Stage III-IV | 79 (38.4%) | 4310 (43.8%) | NS |
| Class 3–4 | 23 (11.2%) | 1793 (18.3%) | <0.01 |
| TJC, mean ± SD cm * | 5.8 ± 8.3 | 1.8 ± 3.9 | <0.001 |
| SJC, mean ± SD cm * | 4.7 ± 5.2 | 1.3 ± 2.7 | <0.001 |
| Pain VAS, mean ± SD cm * | 3.0 ± 2.4 | 2.4 ± 2.3 | <0.001 |
| PGA, mean ± SD | 3.1 ± 2.4 | 2.4 ± 2.2 | <0.001 |
| PhGA, mean ± SD | 2.3 ± 1.5 | 1.5 ± 1.5 | <0.001 |
| mHAQ, mean ± SD | 0.41 ± 0.60 | 0.38 ± 0.60 | NS |
| DAS28-CRP, mean ± SD | 2.9 ± 1.1 | 2.3 ± 1.0 | <0.001 |
| CRP mean ± SD mg/dL | 0.48 ± 1.1 | 0.54 ± 1.2 | NS |
| Positive rheumatoid factor | 143 (69.4%) | 7206 (73.3%) | NS |
| Positive anti-CCP Ab (n = 4835 **) | 69/97 (71.1%) | 3428/4738 (72.4%) | NS |
| Joint index x, mean ± SD | 0.36 ± 0.39 | 0.17 ± 0.28 | <0.001 |
| y, mean ± SD | 0.30 ± 0.41 | 0.12 ± 0.27 | <0.001 |
| z, mean ± SD | −0.04 ± 0.44 | 0.08 ± 0.30 | <0.001 |
| DIP Count | TJC | SJC | Pain VAS | PGA | PhGA | mHAQ | DAS28-CRP | JI x | JI y | JI z | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DIP count | 1 | ||||||||||
| TJC | 0.43 ** | 1 | |||||||||
| SJC | 0.30 ** | −0.03 | 1 | ||||||||
| Pain VAS | 0.17 * | 0.50 ** | −0.02 | 1 | |||||||
| PGA | 0.18 * | 0.51 ** | −0.02 | 0.93 ** | 1 | ||||||
| PhGA | 0.19 ** | 0.58 ** | 0.21 ** | 0.54 ** | 0.53 ** | 1 | |||||
| mHAQ | 0.09 | 0.40 ** | −0.03 | 0.41 ** | 0.46 ** | 0.21 ** | 1 | ||||
| DAS28-CRP | 0.26 ** | 0.73 ** | 0.21 ** | 0.71 ** | 0.74 ** | 0.63 ** | 0.43 ** | 1 | |||
| JI x | 0.32 ** | 0.76 ** | 0.45 ** | 0.45 ** | 0.47 ** | 0.56 ** | 0.35 ** | 0.81 ** | 1 | ||
| JI y | 0.23 ** | 0.67 ** | 0.38 ** | 0.25 ** | 0.26 ** | 0.54 ** | 0.31 ** | 0.48 ** | 0.60 ** | 1 | |
| JI z | −0.16 * | −0.14 | −0.39 ** | 0.06 | 0.10 | −0.15 * | 0.07 | −0.03 | −0.13 | −0.23 ** | 1 |
| DIP Involvement | ||||
|---|---|---|---|---|
| Presence (n = 206) | Absence (n = 9832) | Odds Ratio (95% CI) | p-Value | |
| PIP joint | 129 (62.6%) | 1855 (18.9%) | 7.2 (5.4–9.6) | <0.01 |
| MCP joint | 106 (51.5%) | 2399 (24.4%) | 3.3 (2.5–4.3) | <0.01 |
| Wrist | 90 (43.7%) | 2598 (26.4%) | 2.2 (1.6–2.9) | <0.01 |
| Elbow | 39 (18.9%) | 1177 (12.0%) | 1.7 (1.2–2.5) | <0.01 |
| Shoulder | 43 (20.9%) | 1109 (11.3%) | 2.1 (1.5–2.9) | <0.01 |
| Hip | 6 (2.9%) | 165 (1.7%) | 1.8 (0.8–4.0) | NS |
| Knee | 45 (21.8%) | 1454 (14.8%) | 1.6 (1.2–2.3) | <0.01 |
| Ankle | 51 (24.8%) | 1327 (13.5%) | 2.1 (1.5–2.9) | <0.01 |
| MTP joint | 73 (35.4%) | 898 (9.1%) | 5.5 (4.1–7.3) | <0.01 |
| DIP | PIP | MCP | Wrist | Elbow | Shoulder | Hip | Knee | Ankle | MTP | |
|---|---|---|---|---|---|---|---|---|---|---|
| DIP | 1.00 | |||||||||
| PIP | 0.43 ** | 1.00 | ||||||||
| MCP | 0.33 ** | 0.55 ** | 1.00 | |||||||
| Wrist | 0.11 | 0.18 * | 0.32 ** | 1.00 | ||||||
| Elbow | 0.18 ** | 0.29 ** | 0.45 ** | 0.36 ** | 1.00 | |||||
| Shoulder | 0.11 | 0.26 ** | 0.34 ** | 0.36 ** | 0.36 ** | 1.00 | ||||
| Hip | 0.17 * | 0.11 | 0.07 | 0.06 | 0.04 | 0.12 #3 | 1.00 | |||
| Knee | 0.13 #1 | 0.20 ** | 0.26 ** | 0.32 ** | 0.18 ** | 0.28 ** | 0.22 ** | 1.00 | ||
| Ankle | 0.19 ** | 0.32 ** | 0.34 ** | 0.35 ** | 0.39 ** | 0.30 ** | 0.01 | 0.25 ** | 1.00 | |
| MTP | 0.13 #2 | 0.39 ** | 0.36 ** | 0.25 ** | 0.26 ** | 0.23 ** | −0.08 | 0.09 | 0.24 ** | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizuuchi, T.; Sawada, T.; Nishiyama, S.; Tahara, K.; Hayashi, H.; Mori, H.; Kato, E.; Tago, M.; Matsui, T.; Tohma, S. Distal Interphalangeal Joint Involvement May Be Associated with Disease Activity and Affected Joint Distribution in Rheumatoid Arthritis. J. Clin. Med. 2022, 11, 1405. https://doi.org/10.3390/jcm11051405
Mizuuchi T, Sawada T, Nishiyama S, Tahara K, Hayashi H, Mori H, Kato E, Tago M, Matsui T, Tohma S. Distal Interphalangeal Joint Involvement May Be Associated with Disease Activity and Affected Joint Distribution in Rheumatoid Arthritis. Journal of Clinical Medicine. 2022; 11(5):1405. https://doi.org/10.3390/jcm11051405
Chicago/Turabian StyleMizuuchi, Takahiro, Tetsuji Sawada, Susumu Nishiyama, Koichiro Tahara, Haeru Hayashi, Hiroaki Mori, Eri Kato, Mayu Tago, Toshihiro Matsui, and Shigeto Tohma. 2022. "Distal Interphalangeal Joint Involvement May Be Associated with Disease Activity and Affected Joint Distribution in Rheumatoid Arthritis" Journal of Clinical Medicine 11, no. 5: 1405. https://doi.org/10.3390/jcm11051405
APA StyleMizuuchi, T., Sawada, T., Nishiyama, S., Tahara, K., Hayashi, H., Mori, H., Kato, E., Tago, M., Matsui, T., & Tohma, S. (2022). Distal Interphalangeal Joint Involvement May Be Associated with Disease Activity and Affected Joint Distribution in Rheumatoid Arthritis. Journal of Clinical Medicine, 11(5), 1405. https://doi.org/10.3390/jcm11051405





