Combined Coronary CT-Angiography and TAVI Planning: Utility of CT-FFR in Patients with Morphologically Ruled-Out Obstructive Coronary Artery Disease
Abstract
:1. Introduction
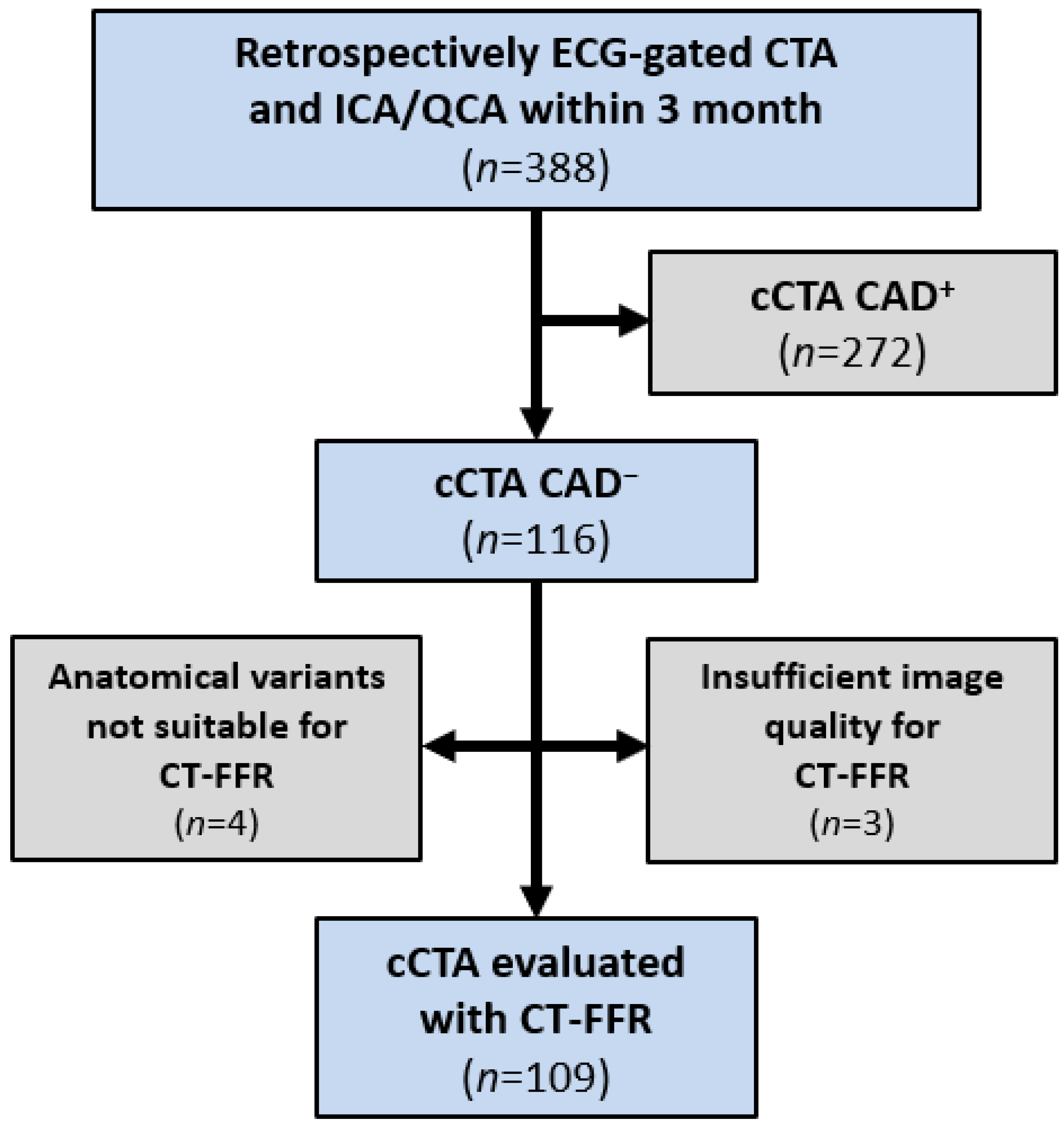
2. Materials and Methods
2.1. Study Design
2.2. CT Acquisition
2.3. cCTA, ICA and QCA
2.4. Image Quality of cCTA and CAC
- 0 = nondiagnostic (excluded from this analysis, as CAD could not be excluded)
- 1 = diagnostic
- 2 = good
- 3 = excellent
2.5. CT-FFR
2.6. Statistical Analysis
3. Results
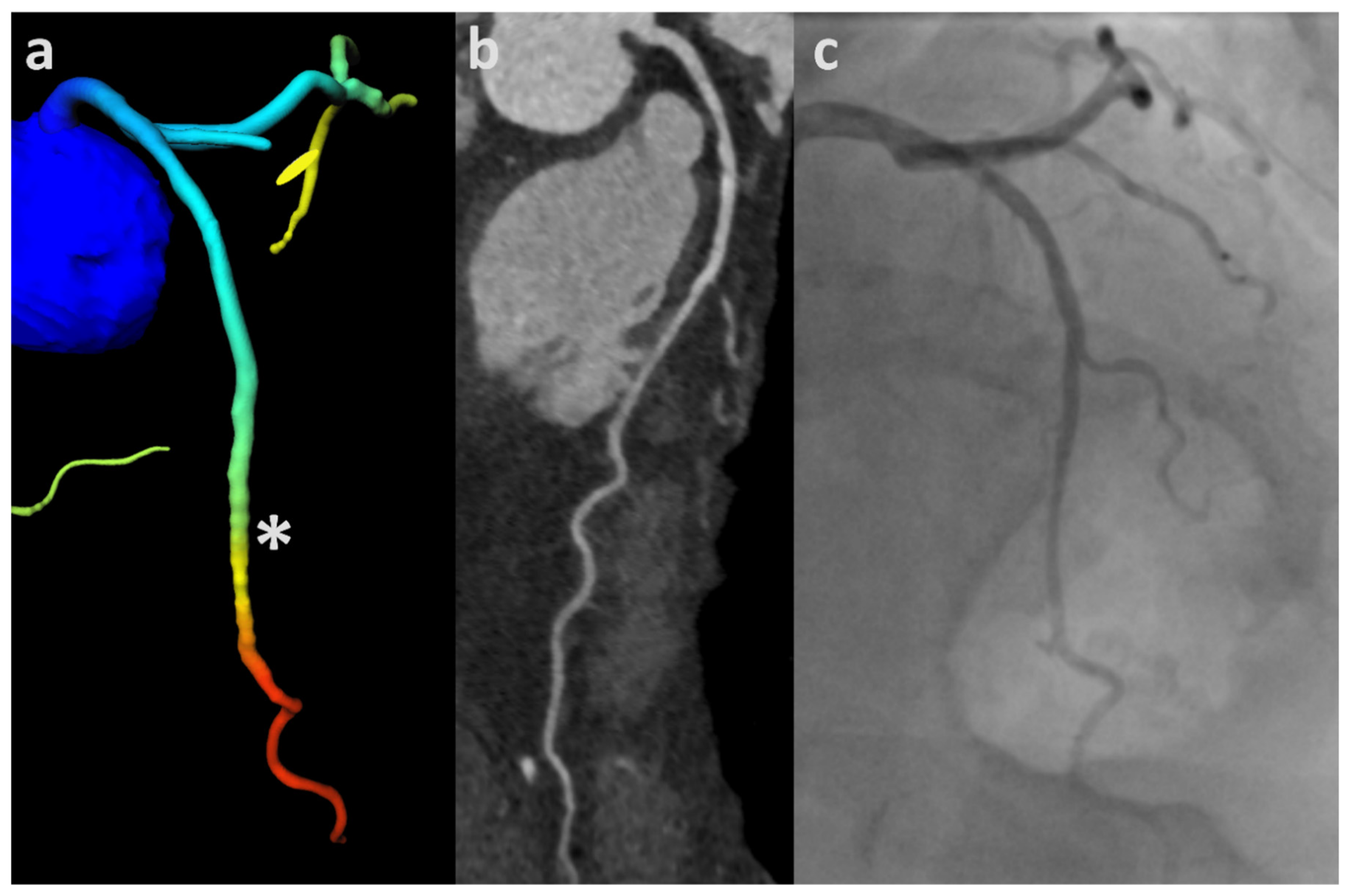
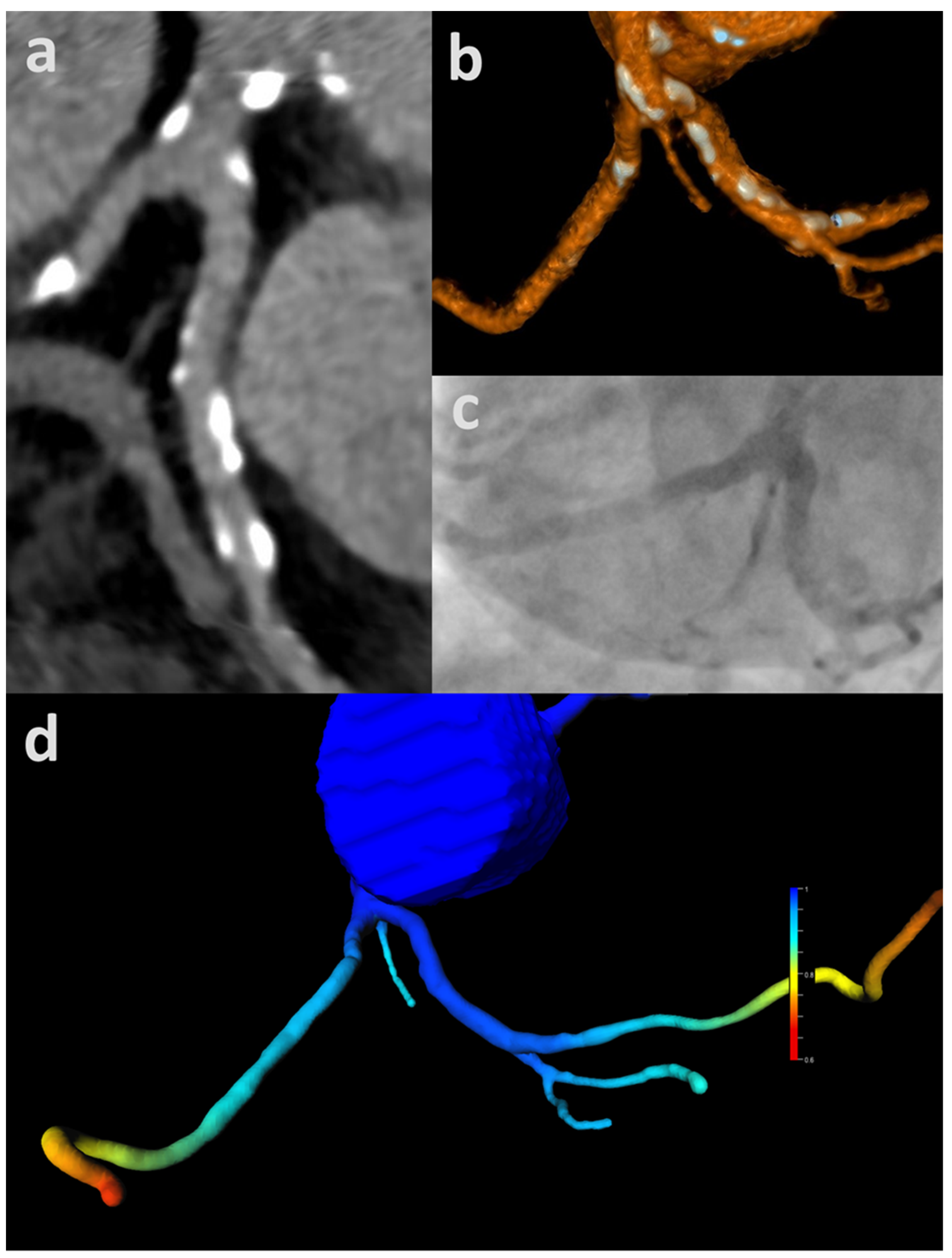
3.1. ML-Based CT-FFR
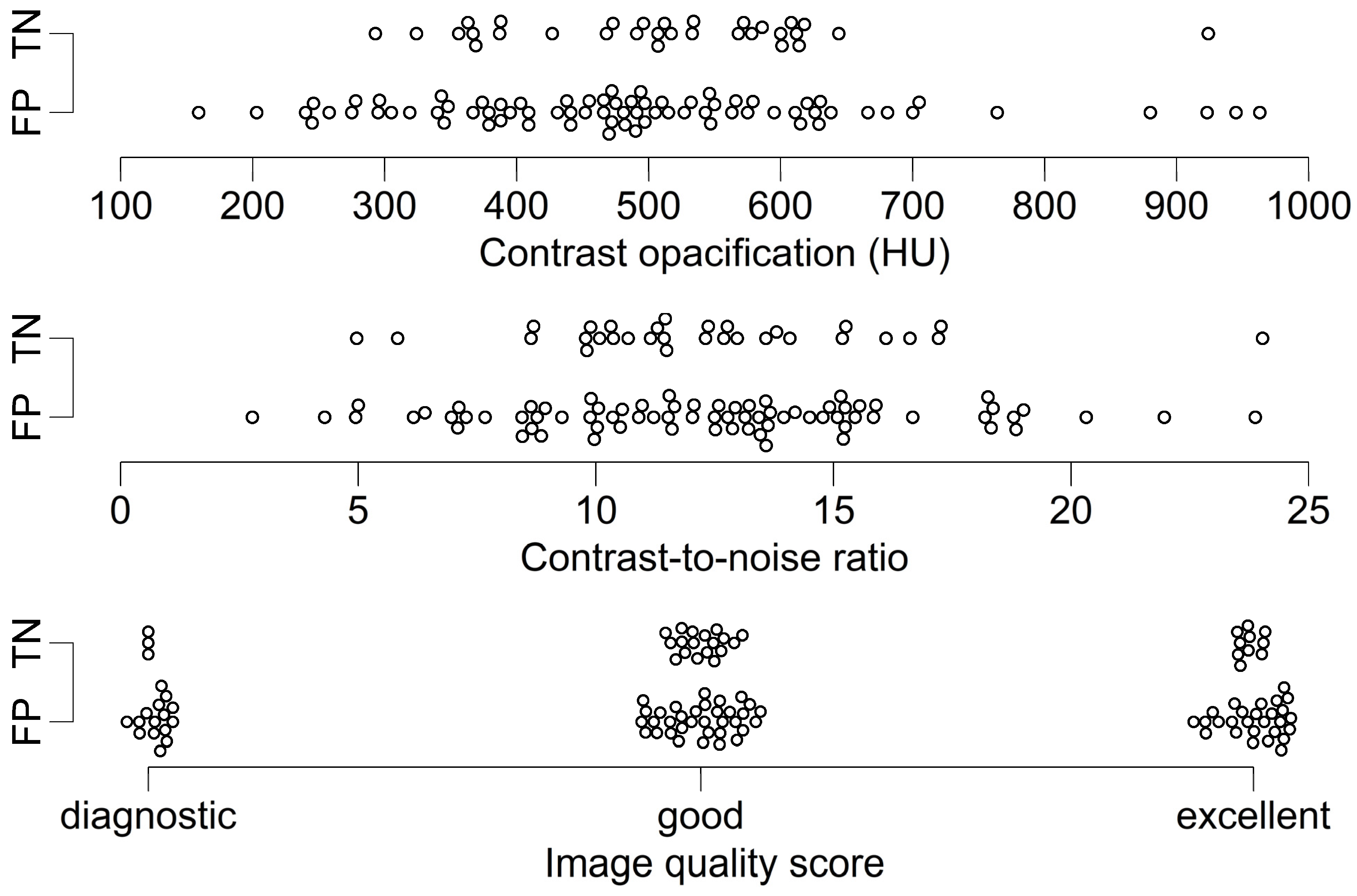
3.2. Analysis According to Image Quality and CAC
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baumgartner, H.; Falk, V.; Bax, J.J.; de Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Muñoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease. J. Am. Coll. Cardiol. 2017, 70, 252–289. [Google Scholar] [CrossRef]
- Blanke, P.; Weir-McCall, J.R.; Achenbach, S.; Delgado, V.; Hausleiter, J.; Jilaihawi, H.; Marwan, M.; Nørgaard, B.L.; Piazza, N.; Schoenhagen, P.; et al. Computed Tomography Imaging in the Context of Transcatheter Aortic Valve Implantation (TAVI)/Transcatheter Aortic Valve Replacement (TAVR): An Expert Consensus Document of the Society of Cardiovascular Computed Tomography. JACC Cardiovasc. Imaging 2019, 12, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Francone, M.; Budde, R.P.J.; Bremerich, J.; Dacher, J.N.; Loewe, C.; Wolf, F.; Natale, L.; Pontone, G.; Redheuil, A.; Vliegenthart, R.; et al. CT and MR imaging prior to transcatheter aortic valve implantation: Standardisation of scanning protocols, measurements and reporting—A consensus document by the European Society of Cardiovascular Radiology (ESCR). Eur. Radiol. 2020, 30, 2627–2650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2438–2488. [Google Scholar] [CrossRef] [Green Version]
- Andreini, D.; Pontone, G.; Mushtaq, S.; Bartorelli, A.L.; Ballerini, G.; Bertella, E.; Segurini, C.; Conte, E.; Annoni, A.; Baggiano, A.; et al. Diagnostic accuracy of multidetector computed tomography coronary angiography in 325 consecutive patients referred for transcatheter aortic valve replacement. Am. Heart J. 2014, 168, 332–339. [Google Scholar] [CrossRef]
- Annoni, A.D.; Andreini, D.; Pontone, G.; Mancini, M.E.; Formenti, A.; Mushtaq, S.; Baggiano, A.; Conte, E.; Guglielmo, M.; Muscogiuri, G.; et al. CT angiography prior to TAVI procedure using third-generation scanner with wide volume coverage: Feasibility, renal safety and diagnostic accuracy for coronary tree. Br. J. Radiol. 2018, 91, 20180196. [Google Scholar] [CrossRef]
- Gohmann, R.F.; Lauten, P.; Seitz, P.; Krieghoff, C.; Lücke, C.; Gottschling, S.; Mende, M.; Weiß, S.; Wilde, J.; Kiefer, P.; et al. Combined Coronary CT-Angiography and TAVI-Planning: A Contrast-Neutral Routine Approach for Ruling-out Significant Coronary Artery Disease. J. Clin. Med. 2020, 9, 1623. [Google Scholar] [CrossRef]
- Hamdan, A.; Wellnhofer, E.; Konen, E.; Kelle, S.; Goitein, O.; Andrada, B.; Raanani, E.; Segev, A.; Barbash, I.; Klempfner, R.; et al. Coronary CT angiography for the detection of coronary artery stenosis in patients referred for transcatheter aortic valve replacement. J. Cardiovasc. Comput. Tomogr. 2015, 9, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.S.; de Cecco, C.N.; Schoepf, U.J.; Steinberg, D.H.; Bayer, R.R.; Krazinski, A.W.; Dyer, K.T.; Sandhu, M.K.; Zile, M.R.; Meinel, F.G. Dual-source CT imaging to plan transcatheter aortic valve replacement: Accuracy for diagnosis of obstructive coronary artery disease. Radiology 2015, 275, 80–88. [Google Scholar] [CrossRef]
- Matsumoto, S.; Yamada, Y.; Hashimoto, M.; Okamura, T.; Yamada, M.; Yashima, F.; Hayashida, K.; Fukuda, K.; Jinzaki, M. CT imaging before transcatheter aortic valve implantation (TAVI) using variable helical pitch scanning and its diagnostic performance for coronary artery disease. Eur. Radiol. 2017, 27, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Opolski, M.P.; Kim, W.-K.; Liebetrau, C.; Walther, C.; Blumenstein, J.; Gaede, L.; Kempfert, J.; van Linden, A.; Walther, T.; Hamm, C.W.; et al. Diagnostic accuracy of computed tomography angiography for the detection of coronary artery disease in patients referred for transcatheter aortic valve implantation. Clin. Res. Cardiol. 2015, 104, 471–480. [Google Scholar] [CrossRef]
- Pontone, G.; Andreini, D.; Bartorelli, A.L.; Annoni, A.; Mushtaq, S.; Bertella, E.; Formenti, A.; Cortinovis, S.; Alamanni, F.; Fusari, M.; et al. Feasibility and accuracy of a comprehensive multidetector computed tomography acquisition for patients referred for balloon-expandable transcatheter aortic valve implantation. Am. Heart J. 2011, 161, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; de Cecco, C.N.; Kennon, S.R.O.; Zou, L.; Meinel, F.G.; Toscano, W.; Segreto, S.; Achenbach, S.; Hausleiter, J.; Schoepf, U.J.; et al. CT angiography to evaluate coronary artery disease and revascularization requirement before trans-catheter aortic valve replacement. J. Cardiovasc. Comput. Tomogr. 2017, 11, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Strong, C.; Ferreira, A.; Teles, R.C.; Mendes, G.; Abecasis, J.; Cardoso, G.; Guerreiro, S.; Freitas, P.; Santos, A.C.; Saraiva, C.; et al. Diagnostic accuracy of computed tomography angiography for the exclusion of coronary artery disease in candidates for transcatheter aortic valve implantation. Sci. Rep. 2019, 9, 19942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grover, F.L.; Vemulapalli, S.; Carroll, J.D.; Edwards, F.H.; Mack, M.J.; Thourani, V.H.; Brindis, R.G.; Shahian, D.M.; Ruiz, C.E.; Jacobs, J.P.; et al. 2016 Annual Report of The Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. J. Am. Coll. Cardiol. 2017, 69, 1215–1230. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, J.; Pedersen, K.S.; Budoff, M.; Kofoed, K.F. Influence of coronary calcification on the diagnostic accuracy of 64-slice computed tomography coronary angiography: A systematic review and meta-analysis. Int. J. Cardiovasc. Imaging 2012, 28, 943–953. [Google Scholar] [CrossRef]
- Gohmann, R.F.; Pawelka, K.; Seitz, P.; Majunke, N.; Heiser, L.; Renatus, K.; Desch, S.; Lauten, P.; Holzhey, D.; Noack, T.; et al. Combined Coronary CT-Angiography and TAVR Planning for Ruling Out Significant Coronary Artery Disease: Added Value of Machine-Learning-Based CT-FFR. JACC Cardiovasc. Imaging 2021. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Michail, M.; Ihdayhid, A.-R.; Comella, A.; Thakur, U.; Cameron, J.D.; McCormick, L.M.; Gooley, R.P.; Nicholls, S.J.; Mathur, A.; Hughes, A.D.; et al. Feasibility and Validity of Computed Tomography-Derived Fractional Flow Reserve in Patients With Severe Aortic Stenosis: The CAST-FFR Study. Circ. Cardiovasc. Interv. 2021, 14, e009586. [Google Scholar] [CrossRef] [PubMed]
- Artzner, C.; Daubert, M.; Ehieli, W.; Kong, D.; Mammarappallil, J.; Nikolaou, K.; Boll, D.T.; Koweek, L. Impact of computed tomography (CT)-derived fractional flow reserve on reader confidence for interpretation of coronary CT angiography. Eur. J. Radiol. 2018, 108, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Karády, J.; Mayrhofer, T.; Ivanov, A.; Foldyna, B.; Lu, M.T.; Ferencik, M.; Pursnani, A.; Salerno, M.; Udelson, J.E.; Mark, D.B.; et al. Cost-effectiveness Analysis of Anatomic vs Functional Index Testing in Patients With Low-Risk Stable Chest Pain. JAMA Netw. Open 2020, 3, e2028312. [Google Scholar] [CrossRef]
- O’Riordan, M. No Cost Savings with Routine FFRCT for New-Onset Chest Pain: FORECAST: FFRCT Did Reduce the Use of Invasive Angiography, but the Savings Weren’t Enough to Offset the Up-Front Costs of Testing. Available online: https://www.tctmd.com/news/no-cost-savings-routine-ffrct-new-onset-chest-pain-forecast (accessed on 8 January 2022).
- Graby, J.; Metters, R.; Kandan, S.R.; McKenzie, D.; Lowe, R.; Carson, K.; Hudson, B.J.; Rodrigues, J.C.L. Real-world clinical and cost analysis of CT coronary angiography and CT coronary angiography-derived fractional flow reserve (FFRCT)-guided care in the National Health Service. Clin. Radiol. 2021, 76, 862.e19. [Google Scholar] [CrossRef]
- Itu, L.; Rapaka, S.; Passerini, T.; Georgescu, B.; Schwemmer, C.; Schoebinger, M.; Flohr, T.; Sharma, P.; Comaniciu, D. A machine-learning approach for computation of fractional flow reserve from coronary computed tomography. J. Appl. Physiol. 2016, 121, 42–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coenen, A.; Kim, Y.-H.; Kruk, M.; Tesche, C.; de Geer, J.; Kurata, A.; Lubbers, M.L.; Daemen, J.; Itu, L.; Rapaka, S.; et al. Diagnostic Accuracy of a Machine-Learning Approach to Coronary Computed Tomographic Angiography-Based Fractional Flow Reserve: Result From the MACHINE Consortium. Circ. Cardiovasc. Imaging 2018, 11, e007217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leipsic, J.; Abbara, S.; Achenbach, S.; Cury, R.; Earls, J.P.; Mancini, G.J.; Nieman, K.; Pontone, G.; Raff, G.L. SCCT guidelines for the interpretation and reporting of coronary CT angiography: A report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J. Cardiovasc. Comput. Tomogr. 2014, 8, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef] [Green Version]
- Nørgaard, B.L.; Fairbairn, T.A.; Safian, R.D.; Rabbat, M.G.; Ko, B.; Jensen, J.M.; Nieman, K.; Chinnaiyan, K.M.; Sand, N.P.; Matsuo, H.; et al. Coronary CT Angiography-derived Fractional Flow Reserve Testing in Patients with Stable Coronary Artery Disease: Recommendations on Interpretation and Reporting. Radiol. Cardiothorac. Imaging 2019, 1, e190050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Kueh, S.H.; Mooney, J.; Ohana, M.; Kim, U.; Blanke, P.; Grover, R.; Sellers, S.; Ellis, J.; Murphy, D.; Hague, C.; et al. Fractional flow reserve derived from coronary computed tomography angiography reclassification rate using value distal to lesion compared to lowest value. J. Cardiovasc. Comput. Tomogr. 2017, 11, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, Y.O.; Fujimoto, S.; Aoshima, C.; Kamo, Y.; Kawaguchi, Y.O.; Takamura, K.; Kudo, A.; Takahashi, D.; Hiki, M.; Kato, Y.; et al. Comparison of diagnostic performance in on-site based CT-derived fractional flow reserve measurements. Int. J. Cardiol. Heart Vasc. 2021, 35, 100815. [Google Scholar] [CrossRef] [PubMed]
- de Bruyne, B.; Hersbach, F.; Pijls, N.H.; Bartunek, J.; Bech, J.W.; Heyndrickx, G.R.; Gould, K.L.; Wijns, W. Abnormal epicardial coronary resistance in patients with diffuse atherosclerosis but “Normal” coronary angiography. Circulation 2001, 104, 2401–2406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coenen, A.; Lubbers, M.M.; Kurata, A.; Kono, A.; Dedic, A.; Chelu, R.G.; Dijkshoorn, M.L.; Gijsen, F.J.; Ouhlous, M.; van Geuns, R.-J.M.; et al. Fractional flow reserve computed from noninvasive CT angiography data: Diagnostic performance of an on-site clinician-operated computational fluid dynamics algorithm. Radiology 2015, 274, 674–683. [Google Scholar] [CrossRef]
- Choy, J.S.; Kassab, G.S. Scaling of myocardial mass to flow and morphometry of coronary arteries. J. Appl. Physiol. 2008, 104, 1281–1286. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.P.; Li, J.H.; Zhou, F.; Jiang, M.D.; Zhou, C.S.; Lu, M.J.; Tang, C.X.; Zhang, X.L.; Yang, L.; Zhang, Y.X.; et al. The influence of image quality on diagnostic performance of a machine learning-based fractional flow reserve derived from coronary CT angiography. Eur. Radiol. 2020, 30, 2525–2534. [Google Scholar] [CrossRef] [PubMed]
- Leipsic, J.; Yang, T.-H.; Thompson, A.; Koo, B.-K.; Mancini, G.B.J.; Taylor, C.; Budoff, M.J.; Park, H.-B.; Berman, D.S.; Min, J.K. CT angiography (CTA) and diagnostic performance of noninvasive fractional flow reserve: Results from the Determination of Fractional Flow Reserve by Anatomic CTA (DeFACTO) study. AJR Am. J. Roentgenol. 2014, 202, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.O.; Fujimoto, S.; Kumamaru, K.K.; Kato, E.; Dohi, T.; Takamura, K.; Aoshima, C.; Kamo, Y.; Kato, Y.; Hiki, M.; et al. The predictive factors affecting false positive in on-site operated CT-fractional flow reserve based on fluid and structural interaction. Int. J. Cardiol. Heart Vasc. 2019, 23, 100372. [Google Scholar] [CrossRef] [PubMed]
- Tesche, C.; Otani, K.; de Cecco, C.N.; Coenen, A.; de Geer, J.; Kruk, M.; Kim, Y.-H.; Albrecht, M.H.; Baumann, S.; Renker, M.; et al. Influence of Coronary Calcium on Diagnostic Performance of Machine Learning CT-FFR: Results From MACHINE Registry. JACC Cardiovasc. Imaging 2020, 13, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Cami, E.; Tagami, T.; Raff, G.; Fonte, T.A.; Renard, B.; Gallagher, M.J.; Chinnaiyan, K.; Bilolikar, A.; Fan, A.; Hafeez, A.; et al. Assessment of lesion-specific ischemia using fractional flow reserve (FFR) profiles derived from coronary computed tomography angiography (FFRCT) and invasive pressure measurements (FFRINV): Importance of the site of measurement and implications for patient referral for invasive coronary angiography and percutaneous coronary intervention. J. Cardiovasc. Comput. Tomogr. 2018, 12, 480–492. [Google Scholar] [CrossRef]
- Takagi, H.; Ishikawa, Y.; Orii, M.; Ota, H.; Niiyama, M.; Tanaka, R.; Morino, Y.; Yoshioka, K. Optimized interpretation of fractional flow reserve derived from computed tomography: Comparison of three interpretation methods. J. Cardiovasc. Comput. Tomogr. 2019, 13, 134–141. [Google Scholar] [CrossRef]
- Ahmad, Y.; Götberg, M.; Cook, C.; Howard, J.P.; Malik, I.; Mikhail, G.; Frame, A.; Petraco, R.; Rajkumar, C.; Demir, O.; et al. Coronary Hemodynamics in Patients With Severe Aortic Stenosis and Coronary Artery Disease Undergoing Transcatheter Aortic Valve Replacement: Implications for Clinical Indices of Coronary Stenosis Severity. JACC Cardiovasc. Interv. 2018, 11, 2019–2031. [Google Scholar] [CrossRef]
- Scarsini, R.; Pesarini, G.; Zivelonghi, C.; Piccoli, A.; Ferrero, V.; Lunardi, M.; Gottin, L.; Zanetti, C.; Faggian, G.; Ribichini, F. Physiologic evaluation of coronary lesions using instantaneous wave-free ratio (iFR) in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. EuroIntervention 2018, 13, 1512–1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| n | TP | TN | FP | FN | Sen. | Spe. | PPV | NPV | Acc. | |
|---|---|---|---|---|---|---|---|---|---|---|
| Patients cCTA | 109 | 0 | 107 | 0 | 2 | 0.0% | 100.0% | 98.2% | 98.2% | |
| Patients CT-FFR | 2 | 31 | 76 | 0 | 100.0% | 29.0% | 2.6% | 100.0% | 30.3% | |
| Difference Δ: patient level | 2 | −76 | 76 | −2 | +100.0% | −71.0% | +1.8% | −67.9% | ||
| Vessels cCTA | 436 | 0 | 434 | 0 | 2 | 0.0% | 100.0% | 99.5% | 99.5% | |
| Vessels CT-FFR | 0 | 308 | 126 | 2 | 0.0% | 71.0% | 0.0% | 99.4% | 70.6% | |
| Difference Δ: vessel level | 0 | −126 | 126 | 0 | 0.0% | −29.0% | −0.2% | −28.9% | ||
| Segments cCTA | 1456 | 0 | 1454 | 0 | 2 | 0.0% | 100.0% | 99.9% | 99.9% | |
| Segments CT-FFR | 0 | 1268 | 186 | 2 | 0.0% | 87.2% | 0.0% | 99.8% | 87.1% | |
| Difference Δ: segment level | 0 | −186 | 186 | 0 | 0.0% | −12.8% | 0.0% | −12.8% |
| n | FP (%) | |
|---|---|---|
| Pat. | 109 | 76 (70) |
| RCA | 109 | 46 (42) |
| Seg. 1 | 109 | 0 (0) |
| Seg. 2 | 108 | 2 (2) |
| Seg. 3 | 101 | 13 (13) |
| Seg. 4 | 76 | 30 (39) |
| Seg. 16 | 80 | 26 (33) |
| LM/Seg. 5 | 109 | 0 (0) |
| LAD | 109 | 53 (49) |
| Seg. 6 | 109 | 0 (0) |
| Seg. 7 | 109 | 9 (8) |
| Seg. 8 | 108 | 50 (46) |
| Seg. 9 | 88 | 11 (13) |
| Seg. 10 | 56 | 11 (20) |
| Seg. 17 | 34 | 3 (9) |
| CX | 109 | 27 (25) |
| Seg. 11 | 109 | 1 (1) |
| Seg. 12 | 88 | 7 (8) |
| Seg. 13 | 90 | 6 (7) |
| Seg. 14 | 58 | 7 (12) |
| Seg. 15 | 11 | 4 (36) |
| Seg. 18 | 13 | 6 (46) |
| Variables | TN (n = 31) | FP (n = 76) | p | Correlation Coefficient | CI | p |
|---|---|---|---|---|---|---|
| Contrast opacification (HU) | 510.9 ± 125.8 | 487.3 ± 165.2 | 0.43 | 0.07 | −0.12, 0.26 | 0.48 |
| CNR | 12.33 ± 3.67 | 12.38 ± 4.19 | 0.94 | −0.007 | −0.20, 0.18 | 0.95 |
| Image quality score | 2 (1) | 2 (1) | 0.74 | 0.03 | −0.15, 0.21 | 0.73 |
| CACPatient | 343.4 (584.1) | 189.6 (538.1) | 0.10 | 0.16 | −0.03, 0.34 | 0.10 |
| CACRCA | 47.2 (225.5) | 22.3 (80.1) | 0.39 | 0.08 | −0.11, 0.27 | 0.39 |
| CACLAD | 42.6 (183.8) | 118.0 (315.1) | 0.04 | −0.20 | −0.38, −0.01 | 0.03 |
| CACCX | 9.0 (80.4) | 9.6 (85.9) | 0.91 | −0.01 | −0.21, 0.19 | 0.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gohmann, R.F.; Seitz, P.; Pawelka, K.; Majunke, N.; Schug, A.; Heiser, L.; Renatus, K.; Desch, S.; Lauten, P.; Holzhey, D.; et al. Combined Coronary CT-Angiography and TAVI Planning: Utility of CT-FFR in Patients with Morphologically Ruled-Out Obstructive Coronary Artery Disease. J. Clin. Med. 2022, 11, 1331. https://doi.org/10.3390/jcm11051331
Gohmann RF, Seitz P, Pawelka K, Majunke N, Schug A, Heiser L, Renatus K, Desch S, Lauten P, Holzhey D, et al. Combined Coronary CT-Angiography and TAVI Planning: Utility of CT-FFR in Patients with Morphologically Ruled-Out Obstructive Coronary Artery Disease. Journal of Clinical Medicine. 2022; 11(5):1331. https://doi.org/10.3390/jcm11051331
Chicago/Turabian StyleGohmann, Robin Fabian, Patrick Seitz, Konrad Pawelka, Nicolas Majunke, Adrian Schug, Linda Heiser, Katharina Renatus, Steffen Desch, Philipp Lauten, David Holzhey, and et al. 2022. "Combined Coronary CT-Angiography and TAVI Planning: Utility of CT-FFR in Patients with Morphologically Ruled-Out Obstructive Coronary Artery Disease" Journal of Clinical Medicine 11, no. 5: 1331. https://doi.org/10.3390/jcm11051331
APA StyleGohmann, R. F., Seitz, P., Pawelka, K., Majunke, N., Schug, A., Heiser, L., Renatus, K., Desch, S., Lauten, P., Holzhey, D., Noack, T., Wilde, J., Kiefer, P., Krieghoff, C., Lücke, C., Ebel, S., Gottschling, S., Borger, M. A., Thiele, H., ... Gutberlet, M. (2022). Combined Coronary CT-Angiography and TAVI Planning: Utility of CT-FFR in Patients with Morphologically Ruled-Out Obstructive Coronary Artery Disease. Journal of Clinical Medicine, 11(5), 1331. https://doi.org/10.3390/jcm11051331






