Access Site Bleeding Complications with NOACs versus VKAs in Patients with Atrial Fibrillation Undergoing Cardiac Implantable Device Intervention
Abstract
:1. Introduction
2. Methods
- Need for post-procedural surgical evacuation of pocket hematoma (e.g., in the case of tense, painful, extended hematoma causing compression of superficial perfusion, with subsequent high risk of erosion).
- Post-procedural pocket infection, defined as clinical presentation with inflammatory skin changes, including pain, swelling and redness, often associated with skin and soft tissue ulceration and drainage.
- Major bleeding at 30 days, defined as fatal or overt bleeding with a drop in hemoglobin level ≥23 g/dL, or requiring transfusion of at least 2 units packed blood cells, or hemorrhage into a critical anatomical site (e.g., intracranial, retroperitoneal) [15].
- Ischemic major adverse cardiovascular events (stroke, systemic embolism, transient ischemic attack, myocardial infarction) at 30 days.
- Length of in-hospital stay.
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Patti, G.; Cavallari, I.; Hanon, O.; De Caterina, R. The safety and efficacy of non-vitamin K antagonist oral anticoagulants in atrial fibrillation in the elderly. Int. J. Cardiol. 2018, 265, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, I. Efficacy and safety of oral anticoagulation in elderly patients with atrial fibrillation. Anatol. J. Cardiol. 2018, 19, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- Blomström-Lundqvist, C.; Traykov, V.; Erba, P.A.; Burri, H.; Nielsen, J.C.; Bongiorni, M.G.; Poole, J.; Boriani, G.; Costa, R.; Deharo, J.-C.; et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections—Endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Europace 2019, 22, 515–549. [Google Scholar] [CrossRef]
- Burri, H.; Starck, C.; Auricchio, A.; Biffi, M.; Burri, M.; D’Avila, A.; Deharo, J.C.; Glikson, M.; Israel, C.; Lau, C.P.; et al. EHRA expert consensus statement and practical guide on optimal implantation technique for conventional pacemakers and implantable cardioverter-defibrillators: Endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), and the Latin-American Heart Rhythm Society (LAHRS). Europace 2021, 23, 983–1008. [Google Scholar] [CrossRef]
- Birnie, D.H.; Healey, J.S.; Wells, G.A.; Verma, A.; Tang, A.S.; Krahn, A.D.; Simpson, C.S.; Ayala-Paredes, F.; Coutu, B.; Leiria, T.L.L.; et al. Pacemaker or Defibrillator Surgery without Interruption of Anticoagulation. N. Engl. J. Med. 2013, 368, 2084–2093. [Google Scholar] [CrossRef] [Green Version]
- Birnie, D.H.; Healey, J.S.; Wells, G.A.; Ayala-Paredes, F.; Coutu, B.; Sumner, G.L.; Becker, G.; Verma, A.; Philippon, F.; Kalfon, E.; et al. Continued vs. interrupted direct oral anticoagulants at the time of device surgery, in patients with moderate to high risk of arterial thrombo-embolic events (BRUISE CONTROL-2). Eur. Heart J. 2018, 39, 3973–3979. [Google Scholar] [CrossRef]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [Green Version]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.; Piccini, J.P.; et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Ave-zum, A.; et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace 2021, 23, 1612–1676. [Google Scholar] [CrossRef]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized bleed-ing definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef] [Green Version]
- Garrido, M.; Kelley, A.S.; Paris, J.; Roza, K.; Meier, D.E.; Morrison, R.S.; Aldridge, M.D. Methods for Constructing and Assessing Propensity Scores. Health Serv. Res. 2014, 49, 1701–1720. [Google Scholar] [CrossRef] [Green Version]
- Eikelboom, J.W.; Connolly, S.J.; Brueckmann, M.; Granger, C.B.; Kappetein, A.P.; Mack, M.J.; Blatchford, J.; Devenny, K.; Friedman, J.; Guiver, K.; et al. Dabigatran versus Warfarin in Patients with Mechanical Heart Valves. N. Engl. J. Med. 2013, 369, 1206–1214. [Google Scholar] [CrossRef] [Green Version]
- Cappato, R.; Marchlinski, F.E.; Hohnloser, S.H.; Naccarelli, G.V.; Xiang, J.; Wilber, D.J.; Ma, C.-S.; Hess, S.; Wells, D.S.; Juang, G.; et al. Uninterrupted rivaroxaban vs. uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur. Heart J. 2015, 36, 1805–1811. [Google Scholar] [CrossRef] [Green Version]
- Navarese, E.P.; Grisafi, L.; Spinoni, E.G.; Mennuni, M.G.; Rognoni, A.; Ratajczak, J.; Podhajski, P.; Koni, E.; Kubica, J.; Patti, G. Safety and Efficacy of Different Antithrombotic Strategies after Transcatheter Aortic Valve Implantation: A Network Meta-Analysis. Thromb. Haemost. 2021, 122, 216–225. [Google Scholar] [CrossRef]
- Renda, G.; Zimarino, M.; Ricci, F.; Piccini, J.P.; Ezekowitz, M.D.; Patel, M.R.; Cappato, R.; Giugliano, R.P.; De Caterina, R. Efficacy and Safety of Non-Vitamin K Antagonist Oral Anticoagulants After Cardioversion for Nonvalvular Atrial Fibrillation. Am. J. Med. 2016, 129, 1117–1123.e2. [Google Scholar] [CrossRef]
- He, H.; Ke, B.; Li, Y.; Han, F.; Li, X.; Zeng, Y. Novel oral anticoagulants in the preoperative period: A meta-analysis. J. Thromb. Thrombolysis 2018, 45, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Essebag, V.; Verma, A.; Healey, J.S.; Krahn, A.D.; Kalfon, E.; Coutu, B.; Ayala-Paredes, F.; Tang, A.S.; Sapp, J.; Sturmer, M.; et al. BRUISE CONTROL Investigators. Clinically Significant Pocket Hematoma Increases Long-Term Risk of De-vice Infection: BRUISE CONTROL INFECTION Study. J. Am. Coll. Cardiol. 2016, 67, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
| Variables | NOACs | VKAs | p Value |
|---|---|---|---|
| (n = 146) | (n = 165) | ||
| Age (years) | 78.4 ± 7.3 | 78.6 ± 9.5 | 0.84 |
| Male gender | 99 (67.8%) | 94 (57.0%) | 0.049 |
| Body mass index (Kg/m2) | 27.3 ± 5.0 | 26.3 ± 4.9 | 0.08 |
| Device type | 0.020 | ||
| Pace-maker | 120 (82.2%) | 117 (70.9%) | |
| ICD | 26 (17.8%) | 48 (29.1%) | |
| AF type | 0.006 | ||
| Paroxysmal | 35 (24.0%) | 20 (12.1%) | |
| Persistent/permanent | 111 (76.0%) | 145 (87.9%) | |
| CHA2DS2-VASC score | 0.40 | ||
| 1 | 3 (2.1%) | 1 (0.6%) | |
| 2 | 11 (7.5%) | 9 (5.5%) | |
| 3 | 27 (18.5%) | 27 (16.4%) | |
| 4 | 46 (31.5%) | 47 (28.5%) | |
| 5 | 41 (28.1%) | 42 (25.5%) | |
| 6 | 11 (7.5%) | 22 (13.3%) | |
| 7 | 6 (4.1%) | 13 (7.9%) | |
| 8 | 1 (0.7%) | 3 (1.7%) | |
| 9 | - | 1 (0.6%) | |
| Diabetes mellitus | 38 (26.0%) | 39 (23.6%) | 0.53 |
| Diabetes on insulin | 21 (14.4%) | 19 (11.5%) | 0.45 |
| Arterial hypertension | 122 (83.6%) | 132 (80.0%) | 0.42 |
| Peripheral artery disease | 52 (35.6%) | 63 (38.2%) | 0.64 |
| Previous MI | 19 (13.0%) | 28 (17.0%) | 0.33 |
| Previous stroke | 11 (7.5%) | 27 (16.4%) | 0.018 |
| Heart failure | 76 (52.0%) | 118 (71.5%) | <0.001 |
| LVEF (%) | 50.0 ± 13.3 | 45.2 ± 13.3 | 0.002 |
| Chronic renal failure | 52 (35.6%) | 67 (40.6%) | 0.37 |
| COPD | 21 (14.4%) | 24 (14.5%) | 0.97 |
| HAS-BLED score | 0.08 | ||
| 0 | 6 (4.1%) | 8 (4.8%) | |
| 1 | 96 (65.8%) | 89 (54.0%) | |
| 2 | 40 (27.4%) | 52 (31.5%) | |
| 3 | 4 (2.7%) | 15 (9.1%) | |
| 4 | - | 1 (0.6%) | |
| Liver disease | 8 (5.5%) | 11 (6.7%) | 0.67 |
| Previous major bleeding | 12 (8.2%) | 19 (11.5%) | 0.33 |
| OAC type | |||
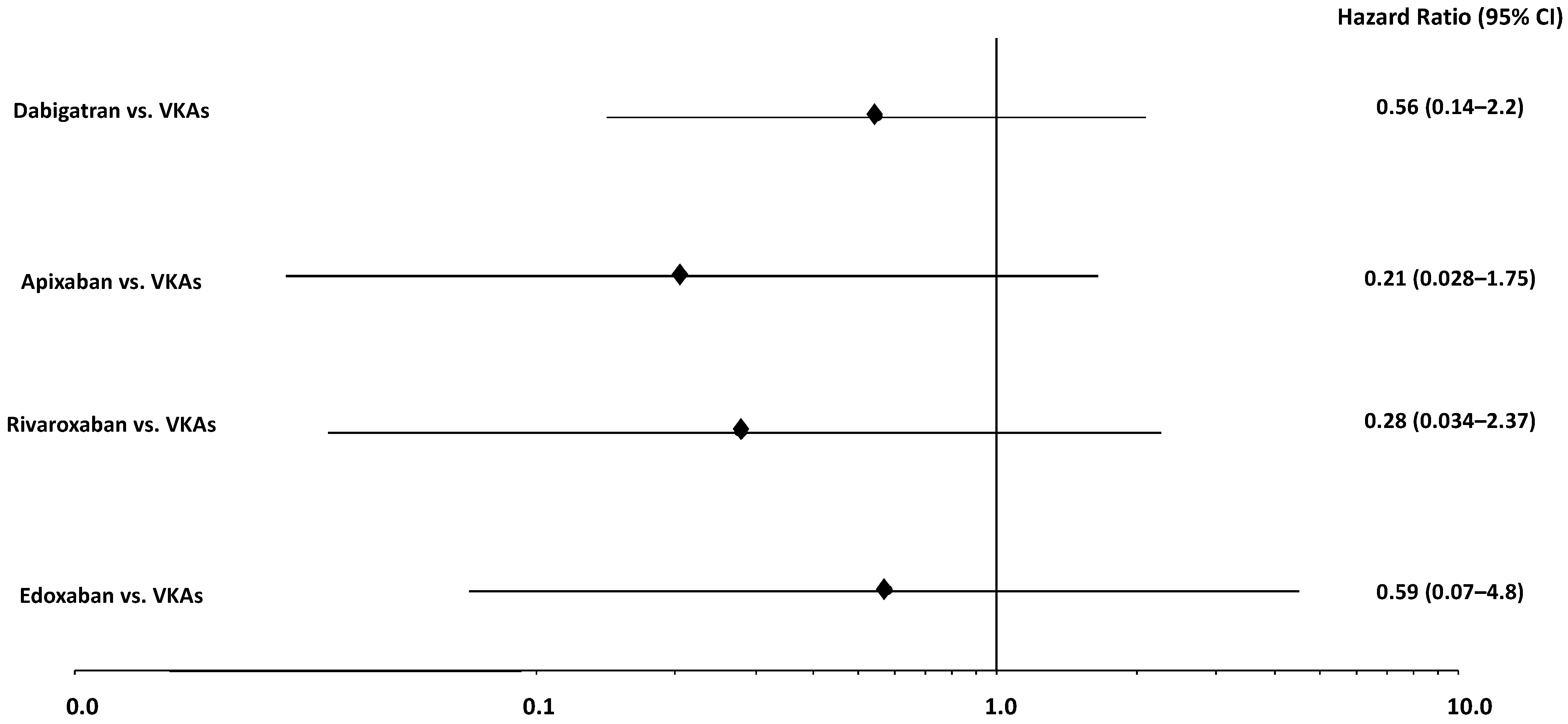
| Dabigatran | 49 (33.6%) | - | |
| Rivaroxaban | 39 (26.7%) | - | |
| Apixaban | 45 (30.8%) | - | |
| Edoxaban | 13 (8.9%) | - | |
| Warfarin | - | 145 (87.9%) | |
| Acenocoumarin | - | 20 (12.1%) | |
| Concomitant antiplatelet therapy | 13 (8.9%) | 14 (8.5%) | 0.90 |
| Peri-procedural LMWH bridging | 4 (2.7%) | 54 (32.7%) | <0.001 |
| Hours from last OAC administration | 35.1 ± 17.6 | 18.2 ± 10.6 | <0.001 |
| INR at the time of the procedure | - | 2.09 (1.8–2.4) | |
| Serum creatinine (mg/dL) | 1.1 ± 0.4 | 1.2 ± 0.5 | 0.15 |
| eGFR (mL/min) | 56.8 ± 17.5 | 56.1 ± 19.4 | 0.74 |
| Hemoglobin (g/dL) | 13.2 ± 1.9 | 12.8 ± 1.8 | 0.042 |
| Platelet count (per microliter) | 205,164.4 ± 60,194.5 | 191,606.1 ± 58,982.4 | 0.046 |
| Variables | NOACs | VKAs | p Value |
|---|---|---|---|
| New implants | n = 109 | n = 82 | |
| Device type | 0.033 | ||
| Pace-maker | 89 (81.7%) | 56 (68.3%) | |
| ICD | 20 (18.3%) | 26 (31.7%) | |
| Leads number | 0.05 | ||
| One lead | 44 (40.4%) | 47 (57.3%) | |
| Two leads | 44 (40.4%) | 21 (25.6%) | |
| CRT | 21 (19.3%) | 14 (17.1%) | |
| Venous access | 0.09 | ||
| Cephalic vein | 60 (55.1%) | 36 (43.9%) | |
| Subclavian vein | 24 (22.0%) | 30 (36.6%) | |
| Axillary vein | 25 (22.9%) | 16 (19.5%) | |
| Device side | 0.52 | ||
| Left | 100 (91.7%) | 73 (89.0%) | |
| Right | 9 (8.3%) | 9 (11.0%) | |
| Generator replacement/downgrading | n = 30 | n = 73 | |
| Device type | 0.32 | ||
| Pacemaker | 27 (90.0%) | 60 (82.2%) | |
| ICD | 3 (10.0%) | 13 (17.8%) | |
| Device side | 0.06 | ||
| Left | 21 (70.0%) | 36 (49.3%) | |
| Right | 9 (30.0%) | 37 (50.7%) | |
| Upgrading | n = 7 | n = 10 | |
| Device type | 0.036 | ||
| Pacemaker | 4 (57.1%) | 1 (10.0%) | |
| ICD | 3 (42.9%) | 9 (90.0%) | |
| Device side | 0.036 | ||
| Left | 3 (42.9%) | 9 (90.0%) | |
| Right | 4 (57.1%) | 1 (10.0%) |
| Variables | Hematoma (n = 27) | No Hematoma (n = 284) | p Value |
|---|---|---|---|
| Age (years) | 73.7 ± 11.4 | 78.9 ± 8.1 | 0.002 |
| Male gender | 17 (63.0%) | 176 (62.0%) | 0.92 |
| Body mass index (Kg/m2) | 27 ± 5.6 | 26.7 ± 4.9 | 0.77 |
| Device type | <0.001 | ||
| Pace-maker | 10 (37.0%) | 227 (80.0%) | |
| ICD | 17 (63.0%) | 57 (20.0%) | |
| AF type | 0.14 | ||
| Paroxysmal | 2 (7.4%) | 53 (18.7%) | |
| Persistent/permanent | 25 (92.6%) | 231 (1.3%) | |
| CHA2DS2-VASC score | <0.001 | ||
| 1 | 1 (3.7%) | 3 (1.1%) | |
| 2 | 1 (3.7%) | 19 (6.7%) | |
| 3 | 2 (7.4%) | 52 (18.2%) | |
| 4 | 8 (29.6%) | 85 (29.9%) | |
| 5 | 10 (37.0%) | 73 (25.7%) | |
| 6 | 2 (7.4%) | 31 (10.9%) | |
| 7 | - | 19 (6.7%) | |
| 8 | 3 (11.2%) | 1 (0.4%) | |
| 9 | - | 1 (0.4%) | |
| Diabetes mellitus | 11 (40.7%) | 66 (23.2%) | 0.12 |
| Diabetes on insulin | 5 (18.5%) | 35 (12.3%) | 0.36 |
| Arterial hypertension | 22 (81.5%) | 232 (81.7%) | 0.98 |
| Peripheral artery disease | 13 (48.1%) | 102 (35.9%) | 0.21 |
| Previous MI | 9 (33.3%) | 38 (13.3%) | 0.006 |
| Previous stroke | 7 (25.9%) | 31 (10.9%) | 0.023 |
| Chronic heart failure | 24 (88.9%) | 170 (59.9%) | 0.003 |
| LVEF (%) | 48.5 ± 13.2 | 36.7 ± 11.5 | <0.001 |
| Chronic renal failure | 13 (48.1%) | 106 (37.3%) | 0.27 |
| COPD | 3 (11.1%) | 42 (14.8%) | 0.60 |
| HAS-BLED score | <0.001 | ||
| 0 | 2 (7.4%) | 12 (4.2%) | |
| 1 | 8 (29.6%) | 177 (62.3%) | |
| 2 | 9 (33.3%) | 83 (29.2%) | |
| 3 | 7 (25.9%) | 12 (4.2%) | |
| 4 | 1 (3.7%) | - | |
| Liver disease | - | 19 (6.7%) | 0.17 |
| Previous major bleeding | 5 (18.5%) | 26 (9.2%) | 0.12 |
| OAC type | 0.05 | ||
| Dabigatran | 3 (11.1%) | 46 (16.2%) | |
| Rivaroxaban | 1 (3.7%) | 38 (13.4%) | |
| Apixaban | 0 (0) | 45 (15.8%) | |
| Edoxaban | 1 (3.7%) | 12 (4.2%) | |
| Warfarin | 19 (70.4%) | 126 (44.4%) | |
| Acenocoumarin | 3 (11.1%) | 17 (6.0%) | |
| Concomitant antiplatelet therapy | 7 (25.9%) | 20 (7.0%) | <0.001 |
| Peri-procedural LMWH bridging | 11 (40.7%) | 47 (16.5%) | 0.002 |
| Hours from last OAC administration | 20.7 ± 12.1 | 26.7 ± 16.9 | 0.07 |
| INR at the time of the procedure | 2.3 ± 0.5 | 2.1 ± 0.7 | 0.47 |
| Serum creatinine (mg/dL) | 1.4 ± 0.8 | 1.1 ± 0.4 | <0.001 |
| eGFR (mL/min) | 50.5 ± 20 | 57.0 ± 18.3 | 0.08 |
| Hemoglobin (g/dL) | 12.9 ± 1.6 | 13 ± 1.8 | 0.71 |
| Platelet count (per microliter) | 173,592.0 ± 48,069.1 | 200,288.7 ± 60,402.8 | 0.027 |
| Primary Endpoint | NOACs | VKAs | p Value |
|---|---|---|---|
| (n = 146) | (n = 165) | ||
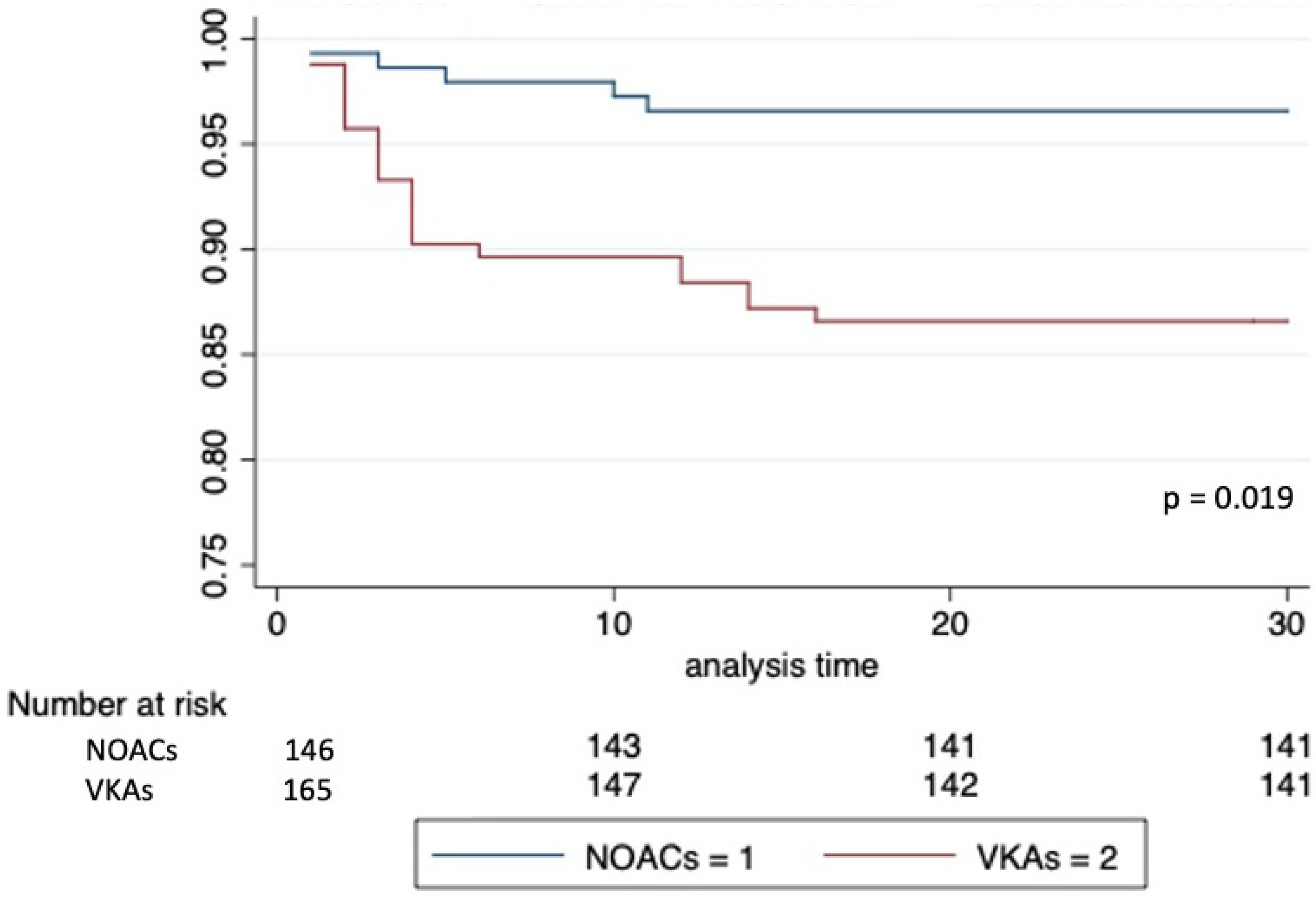
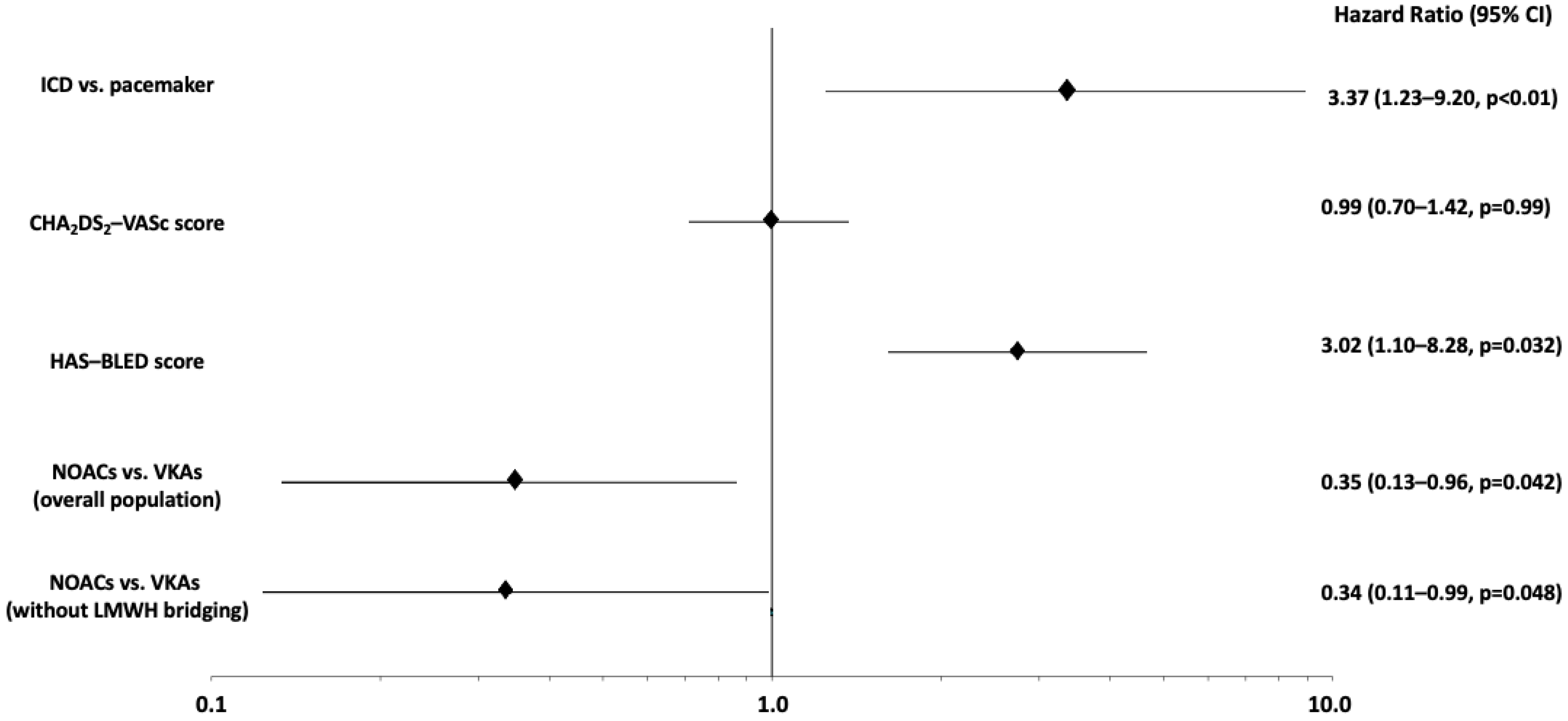
| Pocket hematoma | 5 (3.4%) | 22 (13.3%) | 0.002 |
| Secondary Endpoints | NOACs | VKAs | p Value |
| (n = 146) | (n = 165) | ||
| Need for surgical evacuation of pocket hematoma | 1 (0.7%) | 7 (4.3%) | 0.06 |
| Pocket infection | 3 (1.8%) | 2 (1.2%) | 0.17 |
| In-hospital length of stay | 2 (1–5) | 2 (0–5) | 0.50 |
| Non procedure-related major bleeding | 1 (0.7%) | 3 (1.8%) | 0.37 |
| Stroke/myocardial infarction/TIA/systemic embolism | 0 | 0 | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spinoni, E.G.; Ghiglieno, C.; Costantino, S.; Battistini, E.; Dell’Era, G.; Porcellini, S.; Santagostino, M.; De Vecchi, F.; Renda, G.; Patti, G. Access Site Bleeding Complications with NOACs versus VKAs in Patients with Atrial Fibrillation Undergoing Cardiac Implantable Device Intervention. J. Clin. Med. 2022, 11, 986. https://doi.org/10.3390/jcm11040986
Spinoni EG, Ghiglieno C, Costantino S, Battistini E, Dell’Era G, Porcellini S, Santagostino M, De Vecchi F, Renda G, Patti G. Access Site Bleeding Complications with NOACs versus VKAs in Patients with Atrial Fibrillation Undergoing Cardiac Implantable Device Intervention. Journal of Clinical Medicine. 2022; 11(4):986. https://doi.org/10.3390/jcm11040986
Chicago/Turabian StyleSpinoni, Enrico Guido, Chiara Ghiglieno, Simona Costantino, Eleonora Battistini, Gabriele Dell’Era, Stefano Porcellini, Matteo Santagostino, Federica De Vecchi, Giulia Renda, and Giuseppe Patti. 2022. "Access Site Bleeding Complications with NOACs versus VKAs in Patients with Atrial Fibrillation Undergoing Cardiac Implantable Device Intervention" Journal of Clinical Medicine 11, no. 4: 986. https://doi.org/10.3390/jcm11040986
APA StyleSpinoni, E. G., Ghiglieno, C., Costantino, S., Battistini, E., Dell’Era, G., Porcellini, S., Santagostino, M., De Vecchi, F., Renda, G., & Patti, G. (2022). Access Site Bleeding Complications with NOACs versus VKAs in Patients with Atrial Fibrillation Undergoing Cardiac Implantable Device Intervention. Journal of Clinical Medicine, 11(4), 986. https://doi.org/10.3390/jcm11040986







