Dynamic Trend of Myocardial Edema in Takotsubo Syndrome: A Serial Cardiac Magnetic Resonance Study
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Cardiovascular Magnetic Resonance
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Baseline Patient Characteristics
3.2. CMR Imaging
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sato, H.; Tateishi, H.; Uchida, T.; Dote, K.; Ishihara, M. Tako-tsubo-like left ventricular dysfunction due to multivessel coronary spasm. In Clinical Aspect of Myocardial Injury: From Ischemia to Heart Failure Tokyo; Kodama, K., Haze, K., Hori, M., Eds.; Kagakuhyoronsha Publishing Co.: Tokyo, Japan, 1990; pp. 56–64. [Google Scholar]
- Akashi, Y.J.; Goldstein, D.S.; Barbaro, G.; Ueyama, T. Takotsubo cardiomyopathy: A new form of acute, reversible heart failure. Circulation 2008, 118, 2754–2762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, D.G.; Cho, I.-J.; Shim, C.Y.; Ryu, S.K.; Chang, H.-J.; Hong, G.-R.; Ha, J.-W.; Chung, N. Transient apical wall thickening in patients with stress cardiomyopathy: Prevalence, profile, and impact on clinical course. Int. J. Cardiol. 2015, 194, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Lyon, A.R.; Ghadri, J.-R.; Templin, C. Takotsubo syndrome: Aetiology, presentation and treatment. Heart 2017, 103, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Eitel, I.; Von Knobelsdorff-Brenkenhoff, F.; Bernhardt, P.; Carbone, I.; Muellerleile, K.; Aldrovandi, A.; Francone, M.; Desch, S.; Gutberlet, M.; Strohm, O.; et al. Clinical Characteristics and Cardiovascular Magnetic Resonance Findings in Stress (Takotsubo) Cardiomyopathy. JAMA 2011, 306, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.B.; Chao, T.; Herzka, D.A.; Zeman, P.R.; Cooper, H.A.; Lindsay, J.; Fuisz, A.R. Cardiovascular magnetic resonance T2 signal abnormalities in left ventricular ballooning syndrome. Int. J. Cardiovasc. Imaging 2009, 26, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Rolf, A.; Nef, H.M.; Möllmann, H.; Troidl, C.; Voss, S.; Conradi, G.; Rixe, J.; Steiger, H.; Beiring, K.; Hamm, C.W.; et al. Immunohistological basis of the late gadolinium enhancement phenomenon in tako-tsubo cardiomyopathy. Eur. Heart J. 2009, 30, 1635–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naruse, Y.; Sato, A.; Kasahara, K.; Makino, K.; Sano, M.; Takeuchi, Y.; Nagasaka, S.; Wakabayashi, Y.; Katoh, H.; Satoh, H.; et al. The clinical impact of late gadolinium enhancement in Takotsubo cardiomyopathy: Serial analysis of cardiovascular magnetic resonance images. J. Cardiovasc. Magn. Reson. 2011, 13, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamori, S.; Matsuoka, K.; Onishi, K.; Kurita, T.; Ichikawa, Y.; Nakajima, H.; Ishida, M.; Kitagawa, K.; Tanigawa, T.; Nakamura, T.; et al. Prevalence and Signal Characteristics of Late Gadolinium Enhancement on Contrast-Enhanced Magnetic Resonance Imaging in Patients with Takotsubo Cardiomyopathy. Circ. J. 2012, 76, 914–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, A.; Lerman, A.; Rihal, C.S. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am. Heart J. 2008, 155, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Templin, C.; Ghadri, J.R.; Diekmann, J.; Napp, L.C.; Bataiosu, D.R.; Jaguszewski, M.; Cammann, V.L.; Sarcon, A.; Geyer, V.; Neumann, C.A.; et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N. Engl. J. Med. 2015, 373, 929–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S.; et al. Cardiovascular Magnetic Resonance in Myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, K.; Daimon, M.; Ishibashi, I.; Kobayashi, Y. Myocardial Edema in Takotsubo Syndrome—Serial Cardiovascular Magnetic Resonance Imaging of the Natural Course. Circ. J. 2017, 81, 1368–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchihashi, K.; Ueshima, K.; Uchida, T.; Oh-Mura, N.; Kimura, K.; Owa, M.; Yoshiyama, M.; Miyazaki, S.; Haze, K.; Ogawa, H.; et al. Transient left ventricular apical ballooning without coronary artery stenosis: A novel heart syndrome mimicking acute myocardial infarction. J. Am. Coll. Cardiol. 2001, 38, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Ahtarovski, K.A.; Iversen, K.K.; Christensen, T.E.; Andersson, H.; Grande, P.; Holmvang, L.; Bang, L.; Hasbak, P.; Lønborg, J.T.; Madsen, P.L.; et al. Takotsubo cardiomyopathy, a two-stage recovery of left ventricular systolic and diastolic function as determined by cardiac magnetic resonance imaging. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 855–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marra, M.P.; Zorzi, A.; Corbetti, F.; De Lazzari, M.; Migliore, F.; Tona, F.; Tarantini, G.; Iliceto, S.; Corrado, D. Apicobasal gradient of left ventricular myocardial edema underlies transient T-wave inversion and QT interval prolongation (Wellens’ ECG pattern) in Tako-Tsubo cardiomyopathy. Heart Rhythm. 2013, 10, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Akashi, Y.; Nef, H.M.; Lyon, A.R. Epidemiology and pathophysiology of Takotsubo syndrome. Nat. Rev. Cardiol. 2015, 12, 387–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Jimenez, R.; Martin-García, A.; Barreiro-Perez, M.; Sánchez-González, J.; Fuster, V.; Sanchez, P.L.; Ibanez, B. Dynamic Edematous Response of the Human Heart to Myocardial Infarction: Implications for Assessing Myocardial Area at Risk and Salvage. Circulation 2017, 136, 1288–1300. [Google Scholar] [CrossRef] [PubMed]
- Wittstein, I.S.; Thiemann, D.R.; Lima, J.A.; Baughman, K.L.; Schulman, S.P.; Gerstenblith, G.; Wu, K.C.; Rade, J.J.; Bivalacqua, T.J.; Champion, H.C. Neurohumoral Features of Myocardial Stunning Due to Sudden Emotional Stress. N. Engl. J. Med. 2005, 352, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Hibino, T.; Kako, N.; Murai, S.; Oguri, M.; Kato, K.; Yajima, K.; Ohte, N.; Yokoi, K.; Kimura, G. A pathophysiologic study of tako-tsubo cardiomyopathy with F-18 fluorodeoxyglucose positron emission tomography. Eur. Heart J. 2007, 28, 2598–2604. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.; Fijalkowska, M.; Gilis-Malinowska, N.; Jaguszewski, M.; Galaska, R.; Rojek, A.; Narkiewicz, K.; Gruchala, M.; Fijalkowski, M. Left ventricular function after takotsubo is not fully recovered in long-term follow-up: A speckle tracking echocardiography study. Cardiol. J. 2017, 24, 57–64. [Google Scholar] [CrossRef] [Green Version]

| Variable | All |
|---|---|
| (n = 15) | |
| Age, years | 71 ± 5 |
| Female | 15 (100%) |
| BMI, kg/m2 | 22.5 ± 3.3 |
| Coronary risk factors | |
| Hypertension | 9 (60%) |
| Dyslipidemia | 7 (47%) |
| Diabetes mellitus | 3 (20%) |
| Smoking | 1 (7%) |
| Symptoms | |
| Chest pain | 10 (67%) |
| Dyspnea | 2 (13%) |
| Triggers | |
| Emotional stress | 6 (40%) |
| Physical stress | 3 (20%) |
| No apparent trigger | 6 (40%) |
| ECG findings at presentation | |
| ST elevation | 9 (60%) |
| T wave inversion | 12 (80%) |
| QTc, msec | 493 ± 72 |
| Troponin elevation | 14 (93%) |
| Maximal CK myocardial band, U/L | 18.0 (11.0–22.9) |
| LV ejection fraction (LVG), % | 48 ± 12 |
| Ballooning pattern | |
| Apical ballooning | 10 (66%) |
| Atypical variants | 5 (33%) |
| Acute | Subacute | Chronic | p Value | |
|---|---|---|---|---|
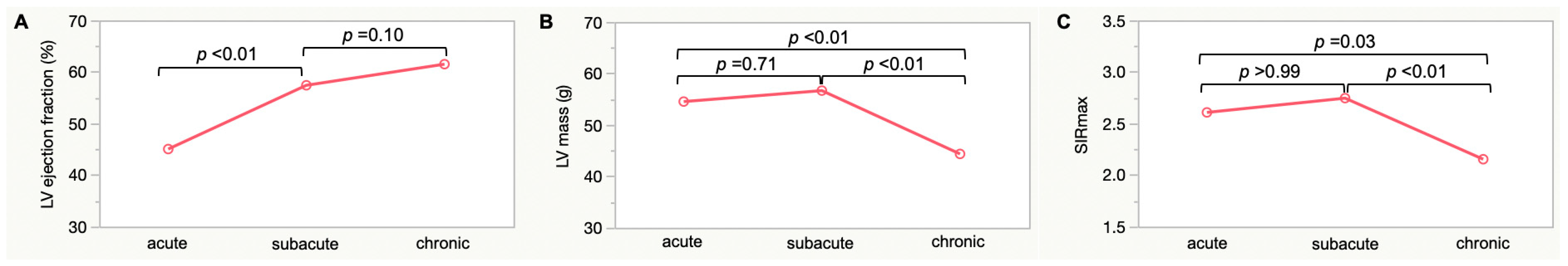
| LV ejection fraction, % | 42 ± 13 | 56 ± 10 | 62 ± 6 | <0.01 |
| LVEDV, mL | 93 ± 20 | 88 ± 19 | 88 ± 21 | 0.20 |
| LVESV, mL | 54 ± 17 | 39 ± 12 | 34 ± 11 | 0.01 |
| LV stroke volume, mL | 39 ± 14 | 49 ± 13 | 54 ± 13 | <0.01 |
| LV mass, g | 53 ± 16 | 56 ± 16 | 44 ± 14 | <0.01 |
| SIRmax | 2.7 ± 0.6 | 2.8 ± 0.6 | 2.2 ± 0.4 | <0.01 |
| LGE, n/total n (%) | 3/14 (21) | 3/12 (25) | 5/12 (42) | 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, K.; Daimon, M.; Sano, M.; Matsuno, K.; Sakai, Y.; Ishibashi, I.; Kadohira, T.; Matsumoto, K.; Masuda, Y.; Uno, T.; et al. Dynamic Trend of Myocardial Edema in Takotsubo Syndrome: A Serial Cardiac Magnetic Resonance Study. J. Clin. Med. 2022, 11, 987. https://doi.org/10.3390/jcm11040987
Kato K, Daimon M, Sano M, Matsuno K, Sakai Y, Ishibashi I, Kadohira T, Matsumoto K, Masuda Y, Uno T, et al. Dynamic Trend of Myocardial Edema in Takotsubo Syndrome: A Serial Cardiac Magnetic Resonance Study. Journal of Clinical Medicine. 2022; 11(4):987. https://doi.org/10.3390/jcm11040987
Chicago/Turabian StyleKato, Ken, Michiko Daimon, Masanori Sano, Koki Matsuno, Yoshiaki Sakai, Iwao Ishibashi, Tadayuki Kadohira, Koji Matsumoto, Yoshitada Masuda, Takashi Uno, and et al. 2022. "Dynamic Trend of Myocardial Edema in Takotsubo Syndrome: A Serial Cardiac Magnetic Resonance Study" Journal of Clinical Medicine 11, no. 4: 987. https://doi.org/10.3390/jcm11040987
APA StyleKato, K., Daimon, M., Sano, M., Matsuno, K., Sakai, Y., Ishibashi, I., Kadohira, T., Matsumoto, K., Masuda, Y., Uno, T., Ghadri, J.-R., Templin, C., & Kobayashi, Y. (2022). Dynamic Trend of Myocardial Edema in Takotsubo Syndrome: A Serial Cardiac Magnetic Resonance Study. Journal of Clinical Medicine, 11(4), 987. https://doi.org/10.3390/jcm11040987






