Current and Emerging Medical Therapies in Pituitary Tumors
Abstract
1. Introduction
- ▪
- Prolactin-secreting PT resistant to dopamine agonist therapy. This situation is often encountered in young men and associated with a higher risk of aggressive tumors [6]. Surgery and radiotherapy are not efficient enough to control the tumor and, ultimately, temozolomide, or even check-point inhibitors, can represent therapeutic options [5,7]. Overcoming resistance-to-Dopamine Agonist (DA) currently represents the most relevant challenge in the medical treatment of prolactin-secreting PT.
- ▪
- Growth hormone secreting PT (GH-secreting PT) that are resistant or insufficiently controlled by first-generation somatostatin receptor ligands (SRLs). Second-line treatments are discussed as well as radiotherapy and/or second surgery.
- ▪
- Adrenocorticotropic hormone secreting PT (ACTH-secreting PT, formerly Cushing’s disease) that are not visualized by pituitary MRI raise the question of whether surgery should be performed in first intention. If not, the choice to begin a steroidogenesis inhibitor and how to titrate it, regarding the induced risk of adrenal insufficiency, implies a close follow-up.
- ▪
- Eventually, non-functioning PT represent one of the most represented phenotypes of PT; however, few medical therapies are available so far. We will discuss potential targets of interest in this review.
2. Material and Methods
- Prolactinoma section: “Prolactinoma” OR “Prolactin-secreting pituitary tumor” OR “Lactotroph tumor” OR “Lactotroph adenoma”;
- Acromegaly section: “Acromegaly” OR “GH-secreting pituitary tumor” OR “Somatotroph tumor” OR “Somatotroph adenoma”;
- Cushing’s disease section: “Cushing’s disease” OR “ACTH-secreting pituitary tumor” OR “Corticotroph tumor” OR “Corticotroph adenoma” OR “Silent Corticotropinomas”;
- Non-Functioning Pituitary Adenoma section: “Non-Functioning Pituitary Adenoma” OR “NFPA” or “Gonadotroph Pituitary Adenoma”;
- Thyrotroph Pituitary Adenomas: “Thyrotroph Pituitary Adenoma” OR “Thyrotropinoma” OR “TSH-secreting Pituitary Tumor” OR “TSH-secreting Pituitary Adenoma”.
3. Current Medical Therapies Approved in the Treatment of PT
3.1. Prolactin-Secreting PT
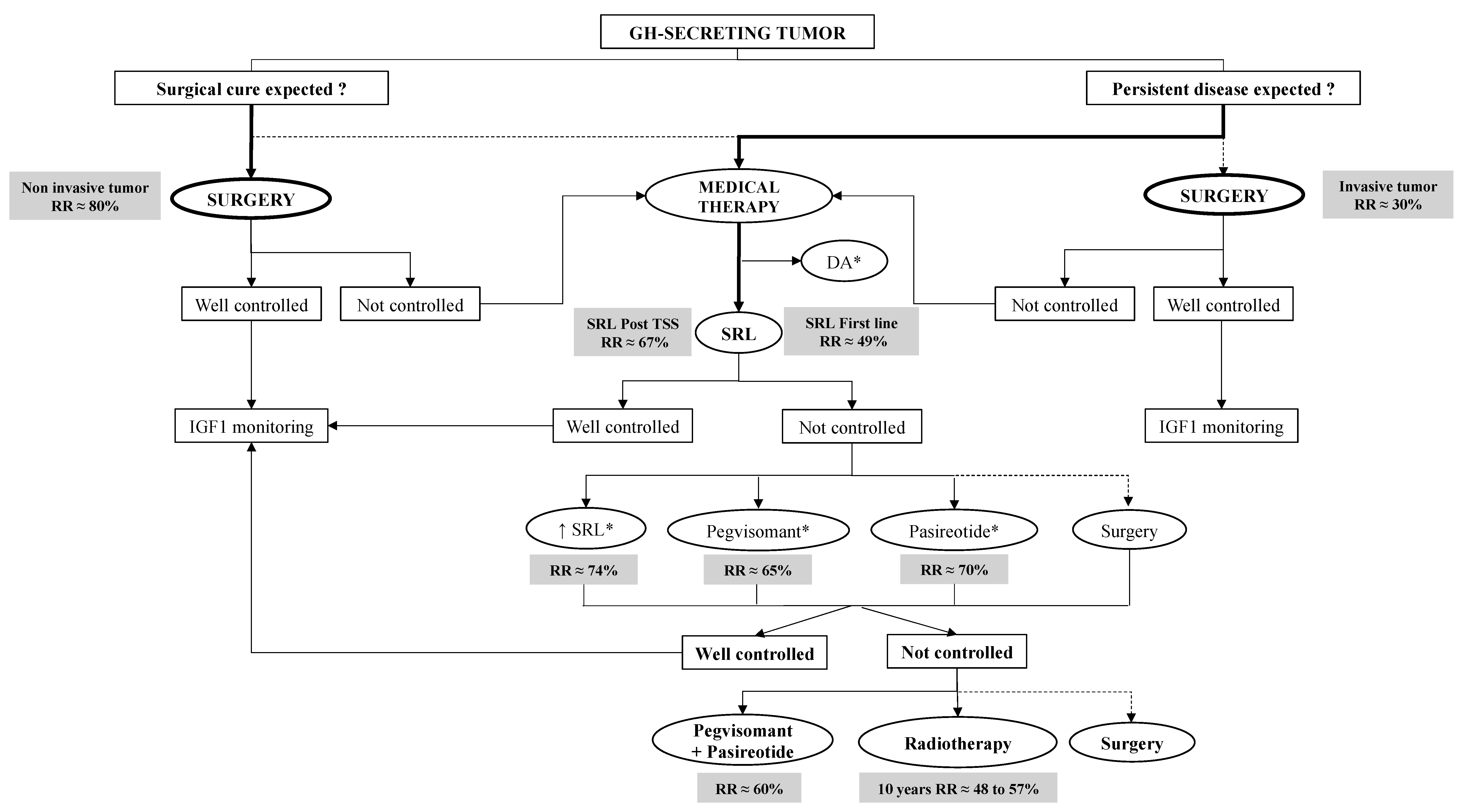
3.2. GH-Secreting PT (Acromegaly)
3.3. ACTH-Secreting PT (Cushing’s Disease)
3.3.1. Pituitary-Targeting Drugs
3.3.2. Adrenal-Directed Steroidogenesis Inhibitors
3.4. TSH-Secreting PT (TSHomas)
3.5. Non-Functioning PT
4. New and Alternative Therapies for the Treatment of PT
4.1. Prolactin-Secreting Pituitary Adenomas
4.1.1. Switching to Another Dopamine Agonist
4.1.2. Somatostatin Receptors Ligands
4.1.3. Metformin
4.1.4. Inhibition of EGF Receptor
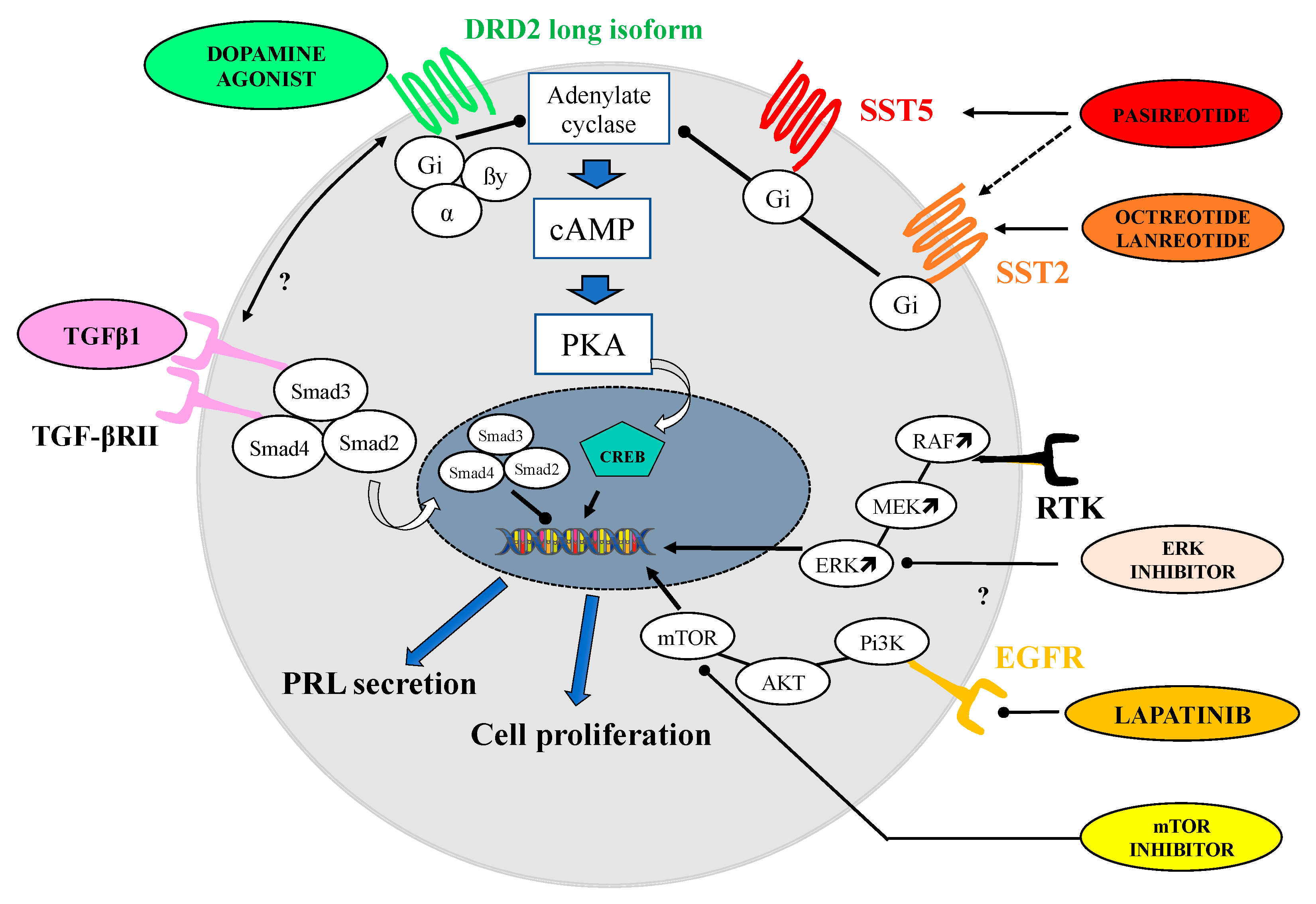
4.1.5. TGF-Beta Signaling Pathway
4.1.6. MAPK Kinases and Pi3K/AKT/mTOR Signaling Pathways
4.2. GH-Secreting Pituitary Adenomas
4.2.1. New Formulations of Somatostatin Receptor Ligands
Oral Octreotide
Octreotide Subcutaneous (SC) Depot
Nasal Octreotide (DP1038)
4.2.2. New Drugs Targeting GH Axis
4.2.3. Dopastatin
4.3. ACTH-Secreting PT (Cushing’s Disease)
4.3.1. Retinoic Acid
4.3.2. EGFR and BRAF Inhibitors
4.3.3. CDK Inhibitor
4.3.4. New Compounds in Cushing’s Disease
4.4. Non-Functioning and TSH-Secreting PT
5. Summarizing Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
| PA | Pituitary adenomas |
| PT | Pituitary tumors |
| PitNETs | Pituitary neuroendocrine tumors |
| DA | Dopamine Agonist |
| SRL | Somatostatin Receptor Ligand |
| PRL | Prolactin |
| DRD2 | Dopamine receptor subtype 2 |
| BRC | Bromocriptine |
| CAB | Cabergoline |
| QNA | Quinagolide |
| GH | growth hormone |
| GHRH | Growth Hormone releasing hormone |
| IGF-1 | Insulin-like growth-factor 1 |
| SST (1–5) | Somatostatin receptor (subtypes 1–5) |
| SG | Sparsely granulated |
| AIP | Arylhydrocarbon receptor interacting protein |
| FIPA | Familial Isolated Pituitary Adenoma |
| X-LAG | X-linked acrogigantism |
| PAS | Pasireotide |
| PEG | Pegvisomant |
| CS | Cushing’s syndrome |
| CD | Cushing’s disease |
| ACTH | Adrenocorticotropic hormone |
| NFPT | Non-functioning pituitary tumor |
| SI | Steroidogenesis inhibitors |
| GR | Glucocorticoid receptor |
| UFC | Urinary free cortisol |
| LNSC | Late night salivary cortisol |
| VEGF | vascular endothelial growth factor |
| APMK | AMP-activated protein kinase |
| mTOR | Mammalian target of rapamycin |
| EGF | Epidermal growth factor |
| TGF | Transforming Growth Factor |
| LAR | Long action release |
| GPCR | G-protein coupled receptor |
| USP8 | Ubiquitin-specific protease 8 |
| HSP-90 | Heat Shock protein 90 |
| CDK | Cyclin-dependent kinases |
| NTR4 | Nuclear testicular orphan receptor 4 |
References
- Ho, K.; Fleseriu, M.; Kaiser, U.; Salvatori, R.; Brue, T.; Lopes, M.B.; Kunz, P.; Molitch, M.; Camper, S.A.; Gadelha, M.; et al. Pituitary Neoplasm Nomenclature Workshop: Does Adenoma Stand the Test of Time? J. Endocr. Soc. 2021, 5, bvaa205. [Google Scholar] [CrossRef] [PubMed]
- Melmed, S. Pituitary-Tumor Endocrinopathies. N. Engl. J. Med. 2020, 382, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Brue, T.; Castinetti, F. The risks of overlooking the diagnosis of secreting pituitary adenomas. Orphanet J. Rare Dis. 2016, 11, 135. [Google Scholar] [CrossRef]
- Molitch, M.E. Diagnosis and treatment of pituitary adenomas: A review. JAMA J. Am. Med. Assoc. 2017, 317, 516–524. [Google Scholar] [CrossRef]
- Raverot, G.; Ilie, M.D.; Lasolle, H.; Amodru, V.; Trouillas, J.; Castinetti, F.; Brue, T. Aggressive pituitary tumours and pituitary carcinomas. Nat. Rev. Endocrinol. 2021, 17, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Vroonen, L.; Jaffrain-Rea, M.-L.; Petrossians, P.; Tamagno, G.; Chanson, P.; Vilar, L.; Borson-Chazot, F.; Naves, L.A.; Brue, T.; Gatta, B.; et al. Prolactinomas resistant to standard doses of cabergoline: A multicenter study of 92 patients. Eur. J. Endocrinol. 2012, 167, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Colao, A.; Savastano, S. Medical treatment of prolactinomas. Nat. Rev. Endocrinol. 2011, 7, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Melmed, S.; Casanueva, F.F.; Hoffman, A.R.; Kleinberg, D.L.; Montori, V.M.; Schlechte, J.A.; Wass, J.A.H. Endocrine Society Diagnosis and treatment of hyperprolactinemia: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 273–288. [Google Scholar] [CrossRef]
- Stefaneanu, L.; Kovacs, K.; Horvath, E.; Buchfelder, M.; Fahlbusch, R.; Lancranjan, I. Dopamine D2 receptor gene expression in human adenohypophysial adenomas. Endocrine 2001, 14, 329–336. [Google Scholar] [CrossRef]
- Peverelli, E.; Treppiedi, D.; Mangili, F.; Catalano, R.; Spada, A.; Mantovani, G. Drug resistance in pituitary tumours: From cell membrane to intracellular signalling. Nat. Rev. Endocrinol. 2021, 17, 560–571. [Google Scholar] [CrossRef]
- Tang, C.; Sun, R.; Wen, G.; Zhong, C.; Yang, J.; Zhu, J.; Cong, Z.; Luo, X.; Ma, C. Bromocriptine and cabergoline induce cell death in prolactinoma cells via the ERK/EGR1 and AKT/mTOR pathway respectively. Cell Death Dis. 2019, 10, 335. [Google Scholar] [CrossRef] [PubMed]
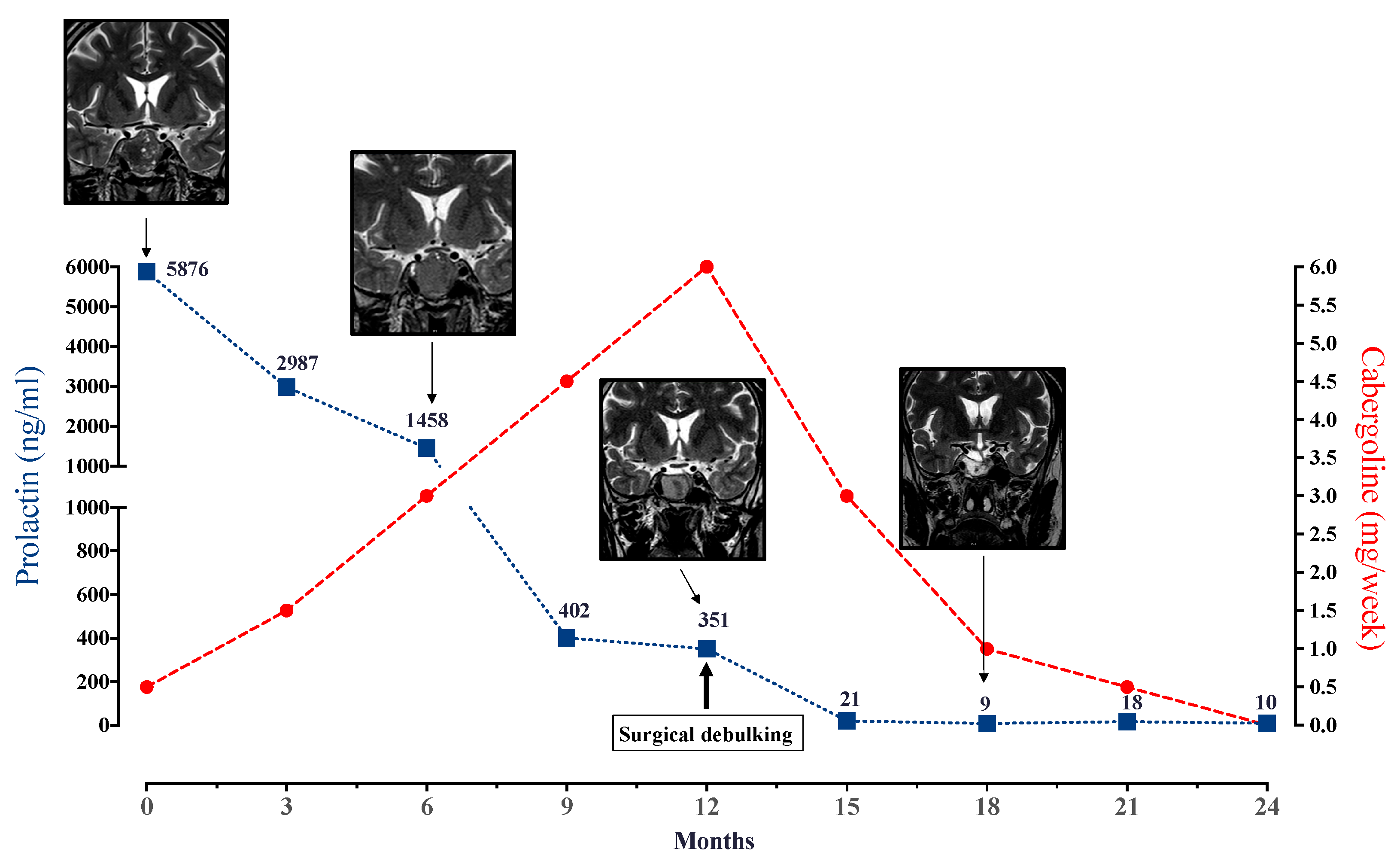
- Sahakian, N.; Castinetti, F.; Dufour, H.; Graillon, T.; Romanet, P.; Barlier, A.; Brue, T.; Cuny, T. Clinical management of difficult to treat macroprolactinomas. Expert Rev. Endocrinol. Metab. 2019, 14, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Molitch, M.E.; Elton, R.L.; Blackwell, R.E.; Caldwell, B.; Chang, R.J.; Jaffe, R.; Joplin, G.; Robbins, R.J.; Tyson, J.; Thorner, M.O. Bromocriptine as primary therapy for prolactin-secreting macroadenomas: Results of a prospective multicenter study. Obstet. Gynecol. Surv. 1986, 41, 48–50. [Google Scholar] [CrossRef]
- Dallabonzana, D.; Liuzzi, A.; Oppizzi, G.; Cozzi, R.; Verde, G.; Chiodini, P.; Rainer, E.; Dorow, R.; Horowski, R. Chronic treatment of pathological hyperprolactinemia and acromegaly with the new ergot derivative terguride. J. Clin. Endocrinol. Metab. 1986, 63, 1002–1007. [Google Scholar] [CrossRef]
- Colao, A.; Di Sarno, A.; Guerra, E.; De Leo, M.; Mentone, A.; Lombardi, G. Drug Insight: Cabergoline and bromocriptine in the treatment of hyperprolactinemia in men and women. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Rains, C.P.; Bryson, H.M.; Fitton, A. Cabergoline: A Review of its Pharmacological Properties and Therapeutic Potential in the Treatment of Hyperprolactinaemia and Inhibition of Lactation. Drugs 1995, 49, 255–279. [Google Scholar] [CrossRef]
- Webster, J.; Piscitelli, G.; Polli, A.; Ferrari, C.I.; Ismail, I.; Scanlon, M.F. A Comparison of Cabergoline and Bromocriptine in the Treatment of Hyperprolactinemic Amenorrhea. N. Engl. J. Med. 1994, 331, 904–909. [Google Scholar] [CrossRef]
- Verhelst, J.; Abs, R.; Maiter, D.; van den Bruel, A.; Vandeweghe, M.; Velkeniers, B.; Mockel, J.; Lamberigts, G.; Petrossians, P.; Coremans, P.; et al. Cabergoline in the treatment of hyperprolactinemia: A study in 455 patients. J. Clin. Endocrinol. Metab. 1999, 84, 2518–2522. [Google Scholar] [CrossRef]
- Colao, A.; Di Sarno, A.; Landi, M.L.; Cirillo, S.; Sarnacchiaro, F.; Facciolli, G.; Pivonello, R.; Cataldi, M.; Merola, B.; Annunziato, L.; et al. Long-term and low-dose treatment with Cabergoline induces macroprolactinoma shrinkage. J. Clin. Endocrinol. Metab. 1997, 82, 3574–3579. [Google Scholar] [CrossRef]
- Pontikides, N.; Krassas, G.E.; Nikopoulou, E.; Kaltsas, T. Cabergoline as a first-line treatment in newly diagnosed macroprolactinomas. Pituitary 2000, 2, 277–281. [Google Scholar] [CrossRef]
- Maiter, D.; Delgrange, E. Therapy of endocrine disease: The challenges in managing giant prolactinomas. Eur. J. Endocrinol. 2014, 170, R213–R227. [Google Scholar] [CrossRef] [PubMed]
- Biagetti, B.; Sarria-Estrada, S.; Ng-Wong, Y.K.; Martinez-Saez, E.; Casteràs, A.; Cordero Asanza, E.; Hernandez, I.; Giralt-Arnaiz, M.; Simò, R. Shrinkage by the third month predicts long-term response of macroprolactinoma after cabergoline. Eur. J. Endocrinol. 2021, 185, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Molitch, M.E. Pharmacologic Resistance in Prolactinoma Patients. Pituitary 2005, 8, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Paepegaey, A.-C.; Salenave, S.; Kamenicky, P.; Maione, L.; Brailly-Tabard, S.; Young, J.; Chanson, P. Cabergoline Tapering Is Almost Always Successful in Patients with Macroprolactinomas. J. Endocr. Soc. 2017, 1, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Colao, A.; Vitale, G.; Cappabianca, P.; Briganti, F.; Ciccarelli, A.; De Rosa, M.; Zarrilli, S.; Lombardi, G. Outcome of Cabergoline Treatment in Men with Prolactinoma: Effects of a 24-Month Treatment on Prolactin Levels, Tumor Mass, Recovery of Pituitary Function, and Semen Analysis. J. Clin. Endocrinol. Metab. 2004, 89, 1704–1711. [Google Scholar] [CrossRef]
- Colao, A.; Di Sarno, A.; Landi, M.L.; Scavuzzo, F.; Cappabianca, P.; Pivonello, R.; Volpe, R.; Di Salle, F.; Cirillo, S.; Annunziato, L.; et al. Macroprolactinoma shrinkage during cabergoline treatment is greater in naive patients than in patients pretreated with other dopamine agonists: A prospective study in 110 patients. J. Clin. Endocrinol. Metab. 2000, 85, 2247–2252. [Google Scholar] [CrossRef]
- Ono, M.; Miki, N.; Kawamata, T.; Makino, R.; Amano, K.; Seki, T.; Kubo, O.; Hori, T.; Takano, K. Prospective study of high-dose cabergoline treatment of prolactinomas in 150 patients. J. Clin. Endocrinol. Metab. 2008, 93, 4721–4727. [Google Scholar] [CrossRef]
- Hamidi, O.; Van Gompel, J.; Gruber, L.; Kittah, N.E.; Donegan, D.; Philbrick, K.A.; Koeller, K.K.; Erickson, D.; Natt, N.; Nippoldt, T.B.; et al. Management and outcomes of giant prolactinoma: A series of 71 patients. Endocr. Pract. 2019, 25, 340–352. [Google Scholar] [CrossRef]
- Daly, A.F.; Beckers, A. A Hard Look at Cardiac Safety with Dopamine Agonists in Endocrinology. J. Clin. Endocrinol. Metab. 2021, 106, E2452–E2454. [Google Scholar] [CrossRef] [PubMed]
- Beccuti, G.; Guaraldi, F.; Natta, G.; Cambria, V.; Prencipe, N.; Cicolin, A.; Montanaro, E.; Lopiano, L.; Ghigo, E.; Zibetti, M.; et al. Increased prevalence of impulse control disorder symptoms in endocrine diseases treated with dopamine agonists: A cross-sectional study. J. Endocrinol. Investig. 2021, 44, 1699–1706. [Google Scholar] [CrossRef]
- Noronha, S.; Stokes, V.; Karavitaki, N.; Grossman, A. Treating prolactinomas with dopamine agonists: Always worth the gamble? Endocrine 2016, 51, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Saleh, Z.; Koshy, S.; Sidhu, V.; Opgenorth, A.; Senaratne, J. Spontaneous coronary artery dissection in association with cabergoline therapy. BMJ Case Rep. 2021, 14, e240022. [Google Scholar] [CrossRef]
- Schultz, P.N.; Ginsberg, L.; McCutcheon, I.E.; Samaan, N.; Leavens, M.; Gagel, R.F. Quinagolide in the management of prolactinoma. Pituitary 2000, 3, 239–249. [Google Scholar] [CrossRef]
- Brue, T.H.; Pellegrini, I.S.; Gunz, G.I.; Morange, I.S.; Dewailly, D.I.; Brownell, J.U.; Enjalbert, A.L.; Jaquet, P.H. Effects of the dopamine agonist CV 205-502 in human prolactinomas resistant to bromocriptine. J. Clin. Endocrinol. Metab. 1992, 74, 577–584. [Google Scholar] [CrossRef]
- Colao, A.; Grasso, L.F.S.; Giustina, A.; Melmed, S.; Chanson, P.; Pereira, A.M.; Pivonello, R. Acromegaly. Nat. Rev. Dis. Prim. 2019, 5, 20. [Google Scholar] [CrossRef]
- Asa, S.L.; Ezzat, S. An Update on Pituitary Neuroendocrine Tumors Leading to Acromegaly and Gigantism. J. Clin. Med. 2021, 10, 2254. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Barkhoudarian, G.; Beckers, A.; Ben-Shlomo, A.; Biermasz, N.; Biller, B.; Boguszewski, C.; Bolanowski, M.; Bollerslev, J.; Bonert, V.; et al. Multidisciplinary management of acromegaly: A consensus. Rev. Endocr. Metab. Disord. 2020, 21, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, J.D.; Bonert, V.S.; Nuño, M.; Ly, D.; Melmed, S. Acromegaly clinical trial methodology impact on reported biochemical efficacy rates of somatostatin receptor ligand treatments: A meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 1825–1833. [Google Scholar] [CrossRef]
- Chanson, P.; Borson-Chazot, F.; Kuhn, J.M.; Blumberg, J.; Maisonobe, P.; Delemer, B.; Archambeaud, F.; Arlot, S.; Bringer, J.; Caron, P.; et al. Control of IGF-I levels with titrated dosing of lanreotide Autogel over 48 weeks in patients with acromegaly. Clin. Endocrinol. 2008, 69, 299–305. [Google Scholar] [CrossRef]
- Annamalai, A.K.; Webb, A.; Kandasamy, N.; Elkhawad, M.; Moir, S.; Khan, F.; Maki-Petaja, K.; Gayton, E.L.; Strey, C.H.; O’Toole, S.; et al. A comprehensive study of clinical, biochemical, radiological, vascular, cardiac, and sleep parameters in an unselected cohort of patients with acromegaly undergoing presurgical somatostatin receptor ligand therapy. J. Clin. Endocrinol. Metab. 2013, 98, 1040–1050. [Google Scholar] [CrossRef]
- Brown, M.; Rivier, J.; Vale, W. Somatostatin: Analogs with selected biological activities. Science 1977, 196, 1467–1469. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, R.; Gerich, J.E. Somatostatin: Physiological and clinical significance. Annu. Rev. Med. 1976, 27, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Casar-Borota, O.; Heck, A.; Schulz, S.; Nesland, J.M.; Ramm-Pettersen, J.; Lekva, T.; Alafuzoff, I.; Bollerslev, J. Expression of SSTR2a, but not of SSTRs 1, 3, or 5 in somatotroph adenomas assessed by monoclonal antibodies was reduced by octreotide and correlated with the acute and long-term effects of octreotide. J. Clin. Endocrinol. Metab. 2013, 98, E1730–E1739. [Google Scholar] [CrossRef] [PubMed]
- Albarel, F.; Castinetti, F.; Morange, I.; Guibert, N.; Graillon, T.; Dufour, H.; Brue, T. Pre-surgical medical treatment, a major prognostic factor for long-term remission in acromegaly. Pituitary 2018, 21, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Mazziotti, G.; Torri, V.; Spinello, M.; Floriani, I.; Melmed, S. Meta-analysis on the effects of octreotide on tumor mass in acromegaly. PLoS ONE 2012, 7, e0036411. [Google Scholar] [CrossRef]
- Caron, P.J.; Bevan, J.S.; Petersenn, S.; Flanagan, D.; Tabarin, A.; Prévost, G.; Maisonobe, P.; Clermont, A. Tumor shrinkage with lanreotide autogel 120 mg as primary therapy in acromegaly: Results of a prospective multicenter clinical trial. J. Clin. Endocrinol. Metab. 2014, 99, 1282–1290. [Google Scholar] [CrossRef]
- Colao, A.; Auriemma, R.S.; Pivonello, R. The effects of somatostatin analogue therapy on pituitary tumor volume in patients with acromegaly. Pituitary 2016, 19, 210–221. [Google Scholar] [CrossRef]
- Gadelha, M.R.; Kasuki, L.; Korbonits, M. Novel pathway for somatostatin analogs in patients with acromegaly. Trends Endocrinol. Metab. 2013, 24, 238–246. [Google Scholar] [CrossRef]
- Potorac, I.; Petrossians, P.; Daly, A.F.; Alexopoulou, O.; Borot, S.; Sahnoun-Fathallah, M.; Castinetti, F.; Devuyst, F.; Jaffrain-Rea, M.L.; Briet, C.; et al. T2-weighted MRI signal predicts hormone and tumor responses to somatostatin analogs in acromegaly. Endocr. Relat. Cancer 2016, 23, 871–881. [Google Scholar] [CrossRef]
- Heck, A.; Emblem, K.E.; Casar-Borota, O.; Bollerslev, J.; Ringstad, G. Quantitative analyses of T2-weighted MRI as a potential marker for response to somatostatin analogs in newly diagnosed acromegaly. Endocrine 2016, 52, 333–343. [Google Scholar] [CrossRef]
- Wildemberg, L.E.; Henriques, D.; Elias, P.C.; Lima, C.H.; Musolino, N.R.; Camacho, A.H.; Faria, O.; Nazato, D.; Abucham, J.; Vilar, L.; et al. gsp Mutation Is Not a Molecular Biomarker of Long-Term Response to First-Generation Somatostatin Receptor Ligands in Acromegaly. Cancers 2021, 13, 4857. [Google Scholar] [CrossRef] [PubMed]
- Verloes, A.; Stevenaert, A.; Teh, B.T.; Petrossians, P.; Beckers, A. Familial acromegaly: Case report and review of the literature. Pituitary 1999, 1, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Vierimaa, O.; Georgitsi, M.; Lehtonen, R.; Vahteristo, P.; Kokko, A.; Raitila, A.; Tuppurainen, K.; Ebeling, T.M.L.; Salmela, P.I.; Paschke, R.; et al. Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science 2006, 312, 1228–1230. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.F.; Tichomirowa, M.A.; Petrossians, P.; Heliövaara, E.; Jaffrain-Rea, M.L.; Barlier, A.; Naves, L.A.; Ebeling, T.; Karhu, A.; Raappana, A.; et al. Clinical characteristics and therapeutic responses in patients with germ-line AIP mutations and pituitary adenomas: An international collaborative study. J. Clin. Endocrinol. Metab. 2010, 95, E373–E383. [Google Scholar] [CrossRef] [PubMed]
- de Pinho, L.K.J.; Neto, L.V.; Wildemberg, L.E.A.; Moraes, A.B.; Takiya, C.M.; Frohman, L.A.; Korbonits, M.; Gadelha, M.R. Familial isolated pituitary adenomas experience at a single center: Clinical importance of AIP mutation screening. Arq. Bras. Endocrinol. Metabol. 2010, 54, 698–704. [Google Scholar] [CrossRef][Green Version]
- Daly, A.F.; Rostomyan, L.; Betea, D.; Bonneville, J.F.; Villa, C.; Pellegata, N.S.; Waser, B.; Reubi, J.C.; Stephan, C.W.; Christ, E.; et al. AIP-mutated acromegaly resistant to first-generation somatostatin analogs: Long-term control with pasireotide LAR in two patients. Endocr. Connect. 2019, 8, 367–377. [Google Scholar] [CrossRef]
- Iacovazzo, D.; Carlsen, E.; Lugli, F.; Chiloiro, S.; Piacentini, S.; Bianchi, A.; Giampietro, A.; Mormando, M.; Clear, A.J.; Doglietto, F.; et al. Factors predicting pasireotide responsiveness in somatotroph pituitary adenomas resistant to first-generation somatostatin analogues: An immunohistochemical study. Eur. J. Endocrinol. 2016, 174, 241–250. [Google Scholar] [CrossRef]
- Ferraù, F.; Romeo, P.D.; Puglisi, S.; Ragonese, M.; Spagnolo, F.; Salpietro, C.; Ientile, R.; Currò, M.; Visalli, G.; Alibrandi, A.; et al. GSTP1 gene methylation and AHR rs2066853 variant predict resistance to first generation somatostatin analogs in patients with acromegaly. J. Endocrinol. Investig. 2019, 42, 825–831. [Google Scholar] [CrossRef]
- Trivellin, G.; Daly, A.F.; Faucz, F.R.; Yuan, B.; Rostomyan, L.; Larco, D.O.; Schernthaner-Reiter, M.H.; Szarek, E.; Leal, L.F.; Caberg, J.-H.; et al. Gigantism and acromegaly due to Xq26 microduplications and GPR101 mutation. N. Engl. J. Med. 2014, 371, 2363–2374. [Google Scholar] [CrossRef]
- Beckers, A.; Lodish, M.B.; Trivellin, G.; Rostomyan, L.; Lee, M.; Faucz, F.R.; Yuan, B.; Choong, C.S.; Caberg, J.H.; Verrua, E.; et al. X-linked acrogigantism syndrome: Clinical profile and therapeutic responses. Endocr. Relat. Cancer 2015, 22, 353–367. [Google Scholar] [CrossRef]
- Colao, A.; Pivonello, R.; Auriemma, R.S.; Galdiero, M.; Savastano, S.; Lombardi, G. Beneficial effect of dose escalation of Octreotide-LAR as first-line therapy in patients with acromegaly. Eur. J. Endocrinol. 2007, 157, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Bonadonna, S.; Bugari, G.; Colao, A.; Cozzi, R.; Cannavo, S.; De Marinis, L.; Degli Uberti, E.; Bogazzi, F.; Mazziotti, G.; et al. High-dose intramuscular octreotide in patients with acromegaly inadequately controlled on conventional somatostatin analogue therapy: A randomised controlled trial. Eur. J. Endocrinol. 2009, 161, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Mazziotti, G.; Cannavò, S.; Castello, R.; Arnaldi, G.; Bugari, G.; Cozzi, R.; Ferone, D.; Formenti, A.M.; Gatti, E.; et al. High-dose and high-frequency lanreotide autogel in acromegaly: A randomized, multicenter study. J. Clin. Endocrinol. Metab. 2017, 102, 2454–2464. [Google Scholar] [CrossRef] [PubMed]
- Bruns, C.; Lewis, I.; Briner, U.; Meno-Tetang, G.; Weckbecker, G. SOM230: A novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur. J. Endocrinol. 2002, 146, 707–716. [Google Scholar] [CrossRef]
- Gadelha, M.R.; Wildemberg, L.E.; Kasuki, L. The future of somatostatin receptor ligands in acromegaly. J. Clin. Endocrinol. Metab. 2021, 107, 297–308. [Google Scholar] [CrossRef]
- Gadelha, M.R.; Bronstein, M.D.; Brue, T.; Coculescu, M.; Fleseriu, M.; Guitelman, M.; Pronin, V.; Raverot, G.; Shimon, I.; Lievre, K.K.; et al. Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): A randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2014, 2, 875–884. [Google Scholar] [CrossRef]
- Fleseriu, M.; Rusch, E.; Geer, E.B. Safety and tolerability of pasireotide long-acting release in acromegaly—Results from the acromegaly, open-label, multicenter, safety monitoring program for treating patients who have a need to receive medical therapy (ACCESS) study. Endocrine 2017, 55, 247–255. [Google Scholar] [CrossRef]
- Gadelha, M.R.; Gu, F.; Bronstein, M.D.; Brue, T.C.; Fleseriu, M.; Shimon, I.; van der Lely, A.J.; Ravichandran, S.; Kandra, A.; Pedroncelli, A.M.; et al. Risk factors and management of pasireotide-associated hyperglycemia in acromegaly. Endocr. Connect. 2020, 9, 1178–1190. [Google Scholar] [CrossRef]
- Samson, S.L.; Gu, F.; Feldt-Rasmussen, U.; Zhang, S.; Yu, Y.; Witek, P.; Kalra, P.; Pedroncelli, A.M.; Pultar, P.; Jabbour, N.; et al. Managing pasireotide-associated hyperglycemia: A randomized, open-label, Phase IV study. Pituitary 2021, 24, 887–903. [Google Scholar] [CrossRef]
- Ferone, D.; Pivonello, R.; Resmini, E.; Boschetti, M.; Rebora, A.; Albertelli, M.; Albanese, V.; Colao, A.; Culler, M.D.; Minuto, F. Preclinical and clinical experiences with the role of dopamine receptors in the treatment of pituitary adenomas. Eur. J. Endocrinol. Suppl. 2007, 156, 37–43. [Google Scholar] [CrossRef]
- Ferone, D.; De Herder, W.W.; Pivonello, R.; Kros, J.M.; Van Koetsveld, P.M.; De Jong, T.; Minuto, F.; Colao, A.; Lamberts, S.W.J.; Hofland, L.J. Correlation of in vitro and in vivo somatotropic adenoma responsiveness to somatostatin analogs and dopamine agonists with immunohistochemical evaluation of somatostatin and dopamine receptors and electron microscopy. J. Clin. Endocrinol. Metab. 2008, 93, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Sandret, L.; Maison, P.; Chanson, P. Place of cabergoline in acromegaly: A meta-analysis. J. Clin. Endocrinol. Metab. 2011, 96, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Kopchick, J.J.; List, E.O.; Kelder, B.; Gosney, E.S.; Berryman, D.E. Evaluation of growth hormone (GH) action in mice: Discovery of GH receptor antagonists and clinical indications. Mol. Cell. Endocrinol. 2014, 386, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Van Der Lely, A.J.; Kuhn, E.; Muhammad, A.; Coopmans, E.C.; Neggers, S.J.; Chanson, P. Pegvisomant and not somatostatin receptor ligands (SRLs) is first-line medical therapy for acromegaly. Eur. J. Endocrinol. 2020, 182, D17–D29. [Google Scholar] [CrossRef]
- Barraud, S.; Caron, P.; Raingeard, I.; Lefebvre, H.; Raverot, G.; Cortet-Rudelli, C.; Desailloud, R.; Henocque, R.; Brault, Y.; Brue, T.; et al. Pegvisomant treatment in acromegaly in clinical practice: Final results of the French ACROSTUDY (312 patients). Ann. Endocrinol. 2021, 82, 582–589. [Google Scholar] [CrossRef]
- Neggers, S.J.C.M.M.; Franck, S.E.; De Rooij, F.W.M.; Dallenga, A.H.G.; Poublon, R.M.L.; Feelders, R.A.; Janssen, J.A.M.J.L.; Buchfelder, M.; Hofland, L.J.; Jørgensen, J.O.L.; et al. Long-term efficacy and safety of pegvisomant in combination with long-acting somatostatin analogs in acromegaly. J. Clin. Endocrinol. Metab. 2014, 99, 3644–3652. [Google Scholar] [CrossRef]
- Lacroix, A.; Feelders, R.A.; Stratakis, C.A.; Nieman, L.K. Cushing’s syndrome. Lancet 2015, 386, 913–927. [Google Scholar] [CrossRef]
- Feelders, R.A.; Newell-Price, J.; Pivonello, R.; Nieman, L.K.; Hofland, L.J.; Lacroix, A. Advances in the medical treatment of Cushing’s syndrome. Lancet Diabetes Endocrinol. 2019, 7, 300–312. [Google Scholar] [CrossRef]
- De Bruin, C.; Feelders, R.A.; Lamberts, S.W.J.; Hofland, L.J. Somatostatin and dopamine receptors as targets for medical treatment of Cushing’s Syndrome. Rev. Endocr. Metab. Disord. 2009, 10, 91–102. [Google Scholar] [CrossRef]
- Colao, A.; Petersenn, S.; Newell-Price, J.; Findling, J.W.; Gu, F.; Maldonado, M.; Schoenherr, U.; Mills, D.; Salgado, L.R.; Biller, B.M. A 12-month phase 3 study of pasireotide in Cushing’s disease. N. Engl. J. Med. 2012, 366, 914–924. [Google Scholar] [CrossRef]
- Schopohl, J.; Gu, F.; Rubens, R.; Van Gaal, L.; Bertherat, J.; Ligueros-Saylan, M.; Trovato, A.; Hughes, G.; Salgado, L.R.; Boscaro, M.; et al. Pasireotide can induce sustained decreases in urinary cortisol and provide clinical benefit in patients with Cushing’s disease: Results from an open-ended, open-label extension trial. Pituitary 2015, 18, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Simeoli, C.; Auriemma, R.S.; Tortora, F.; De Leo, M.; Iacuaniello, D.; Cozzolino, A.; De Martino, M.C.; Pivonello, C.; Mainolfi, C.G.; Rossi, R.; et al. The treatment with pasireotide in Cushing’s disease: Effects of long-term treatment on tumor mass in the experience of a single center. Endocrine 2015, 50, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, A.; Gu, F.; Gallardo, W.; Pivonello, R.; Yu, Y.; Witek, P.; Boscaro, M.; Salvatori, R.; Yamada, M.; Tauchmanova, L.; et al. Efficacy and safety of once-monthly pasireotide in Cushing’s disease: A 12 month clinical trial. Lancet Diabetes Endocrinol. 2018, 6, 17–26. [Google Scholar] [CrossRef]
- Fleseriu, M.; Petersenn, S.; Biller, B.M.K.; Kadioglu, P.; De Block, C.; T’Sjoen, G.; Vantyghem, M.C.; Tauchmanova, L.; Wojna, J.; Roughton, M.; et al. Long-term efficacy and safety of once-monthly pasireotide in Cushing’s disease: A Phase III extension study. Clin. Endocrinol. 2019, 91, 776–785. [Google Scholar] [CrossRef] [PubMed]
- De Bruin, C.; Pereira, A.M.; Feelders, R.A.; Romijn, J.A.; Roelfsema, F.; Sprij-Mooij, D.M.; Van Aken, M.O.; Der Van Lelij, A.J.; De Herder, W.W.; Lamberts, S.W.J.; et al. Coexpression of dopamine and somatostatin receptor subtypes in corticotroph adenomas. J. Clin. Endocrinol. Metab. 2009, 94, 1118–1124. [Google Scholar] [CrossRef]
- Pivonello, R.; Ferone, D.; De Herder, W.W.; Kros, J.M.; Del Basso De Caro, M.L.; Arvigo, M.; Annunziato, L.; Lombardi, G.; Colao, A.; Hofland, L.J.; et al. Dopamine Receptor Expression and Function in Corticotroph Pituitary Tumors. J. Clin. Endocrinol. Metab. 2004, 89, 2452–2462. [Google Scholar] [CrossRef]
- Godbout, A.; Manavela, M.; Danilowicz, K.; Beauregard, H.; Bruno, O.D.; Lacroix, A. Cabergoline monotherapy in the long-term treatment of Cushing’s disease. Eur. J. Endocrinol. 2010, 163, 709–716. [Google Scholar] [CrossRef]
- Ferriere, A.; Cortet, C.; Chanson, P.; Delemer, B.; Caron, P.; Chabre, O.; Reznik, Y.; Bertherat, J.; Rohmer, V.; Briet, C.; et al. Cabergoline for Cushing’s disease: A large retrospective multicenter study. Eur. J. Endocrinol. 2017, 176, 305–314. [Google Scholar] [CrossRef]
- Burman, P.; Edén-Engström, B.; Ekman, B.; Karlsson, F.A.; Schwarcz, E.; Wahlberg, J. Limited value of cabergoline in Cushing’s disease: A prospective study of a 6-week treatment in 20 patients. Eur. J. Endocrinol. 2016, 174, 17–24. [Google Scholar] [CrossRef]
- Thakkar, K.; Lila, A.; Sarathi, V.; Ramteke-Jadhav, S.; Goroshi, M.; Memon, S.S.; Krishnatry, R.; Gupta, T.; Jalali, R.; Goel, A.; et al. Cabergoline may act as a radioprotective agent in Cushing’s disease. Clin. Endocrinol. 2020, 92, 55–62. [Google Scholar] [CrossRef]
- McCullagh, E.P.; Tretbar, H.A. Treatment of Cushing’s syndrome with amphenone: Report of two cases, one with probable thymoma. J. Clin. Endocrinol. Metab. 1958, 18, 134–148. [Google Scholar] [CrossRef]
- Castinetti, F.; Nieman, L.K.; Reincke, M.; Newell-Price, J. Approach to the Patient Treated with Steroidogenesis Inhibitors. J. Clin. Endocrinol. Metab. 2021, 106, 2114–2123. [Google Scholar] [CrossRef] [PubMed]
- Fleseriu, M.; Auchus, R.; Bancos, I.; Ben-Shlomo, A.; Bertherat, J.; Biermasz, N.R.; Boguszewski, C.L.; Bronstein, M.D.; Buchfelder, M.; Carmichael, J.D.; et al. Consensus on diagnosis and management of Cushing’s disease: A guideline update. Lancet Diabetes Endocrinol. 2021, 9, 847–875. [Google Scholar] [CrossRef]
- Castinetti, F.; Guignat, L.; Giraud, P.; Muller, M.; Kamenicky, P.; Drui, D.; Caron, P.; Luca, F.; Donadille, B.; Vantyghem, M.C.; et al. Ketoconazole in cushing’s disease: Is it worth a try. J. Clin. Endocrinol. Metab. 2014, 99, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Pivonello, R.; De Leo, M.; Cozzolino, A.; Colao, A. The treatment of cushing’s disease. Endocr. Rev. 2015, 36, 385–486. [Google Scholar] [CrossRef] [PubMed]
- Young, J.; Bertherat, J.; Vantyghem, M.C.; Chabre, O.; Senoussi, S.; Chadarevian, R.; Castinetti, F. Hepatic safety of ketoconazole in Cushing’s syndrome: Results of a Compassionate Use Programme in France. Eur. J. Endocrinol. 2018, 178, 447–458. [Google Scholar] [CrossRef]
- Daniel, E.; Aylwin, S.; Mustafa, O.; Ball, S.; Munir, A.; Boelaert, K.; Chortis, V.; Cuthbertson, D.J.; Daousi, C.; Rajeev, S.P.; et al. Effectiveness of Metyrapone in Treating Cushing’s Syndrome: A Retrospective Multicenter Study in 195 Patients. J. Clin. Endocrinol. Metab. 2015, 100, 4146–4154. [Google Scholar] [CrossRef]
- Ceccato, F.; Zilio, M.; Barbot, M.; Albiger, N.; Antonelli, G.; Plebani, M.; Watutantrige-Fernando, S.; Sabbadin, C.; Boscaro, M.; Scaroni, C. Metyrapone treatment in Cushing’s syndrome: A real-life study. Endocrine 2018, 62, 701–711. [Google Scholar] [CrossRef]
- Bertagna, X.; Pivonello, R.; Fleseriu, M.; Zhang, Y.; Robinson, P.; Taylor, A.; Watson, C.E.; Maldonado, M.; Hamrahian, A.H.; Boscaro, M.; et al. LCI699, a Potent 11β-hydroxylase Inhibitor, normalizes urinary cortisol in patients with Cushing’s disease: Results from a multicenter, proof-of-concept study. J. Clin. Endocrinol. Metab. 2014, 99, 1375–1383. [Google Scholar] [CrossRef]
- Fleseriu, M.; Pivonello, R.; Young, J.; Hamrahian, A.H.; Molitch, M.E.; Shimizu, C.; Tanaka, T.; Shimatsu, A.; White, T.; Hilliard, A.; et al. Osilodrostat, a potent oral 11β-hydroxylase inhibitor: 22-week, prospective, Phase II study in Cushing’s disease. Pituitary 2016, 19, 138–148. [Google Scholar] [CrossRef]
- Pivonello, R.; Fleseriu, M.; Newell-Price, J.; Bertagna, X.; Findling, J.; Shimatsu, A.; Gu, F.; Auchus, R.; Leelawattana, R.; Lee, E.J.; et al. Efficacy and safety of osilodrostat in patients with Cushing’s disease (LINC 3): A multicentre phase III study with a double-blind, randomised withdrawal phase. Lancet Diabetes Endocrinol. 2020, 8, 748–761. [Google Scholar] [CrossRef]
- Fleseriu, M.; Biller, B.M.K.; Bertherat, J.; Young, J.; Arnaldi, G.; O’Connell, P.; Izquierdo, M.; Pedroncelli, A.M.; Pivonello, R. Long-Term Control of Urinary Free Cortisol with Osilodrostat in Patients with Cushing’s Disease: Final Results from the LINC 2 Study. J. Endocr. Soc. 2021, 5, A521–A522. [Google Scholar] [CrossRef]
- Gadelha, M.; Bex, M.; Feelders, R.A.; Heaney, A.P.; Auchus, R.J.; Gilis-Januszewska, A.; Witek, P.; Belaya, Z.; Liao, Z.; Ku, C.H.C.; et al. Abstract 7065—Osilodrostat is an effective and well-tolerated treatment for Cushing’s disease (CD): Results from a Phase III study with an upfront, randomized, double-blind, placebo-controlled phase (LINC 4). Abstr. Present. Endo. Soc. 2021, 5 (Suppl. S1), A516–A517. [Google Scholar]
- Castinetti, F.; Amodru, V.; Brue, T. Osilodrostat in Cushing’s disease: The risk of delayed adrenal insufficiency should be carefully monitored. Clin. Endocrinol. 2021. [Google Scholar] [CrossRef]
- Fleseriu, M.; Pivonello, R.; Elenkova, A.; Salvatori, R.; Auchus, R.J.; Feelders, R.A.; Geer, E.B.; Greenman, Y.; Witek, P.; Cohen, F.; et al. Efficacy and safety of levoketoconazole in the treatment of endogenous Cushing’s syndrome (SONICS): A phase 3, multicentre, open-label, single-arm trial. Lancet Diabetes Endocrinol. 2019, 7, 855–865. [Google Scholar] [CrossRef]
- Preda, V.A.; Sen, J.; Karavitaki, N.; Grossman, A.B. Etomidate in the management of hypercortisolaemia in Cushing’s syndrome: A review. Eur. J. Endocrinol. 2012, 167, 137–143. [Google Scholar] [CrossRef]
- McGrath, M.; Ma, C.; Raines, D.E. Dimethoxy-etomidate: A Nonhypnotic Etomidate Analog that Potently Inhibits Steroidogenesis. J. Pharmacol. Exp. Ther. 2018, 364, 229–237. [Google Scholar] [CrossRef]
- McGrath, M.; Hofmann, A.; Raines, D.E. Behavioral and steroidogenic pharmacology of phenyl ring substituted etomidate analogs in rats. BMC Pharmacol. Toxicol. 2019, 20, 48. [Google Scholar] [CrossRef]
- Fleseriu, M.; Biller, B.M.K.; Findling, J.W.; Molitch, M.E.; Schteingart, D.E.; Gross, C. Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing’s syndrome. J. Clin. Endocrinol. Metab. 2012, 97, 2039–2049. [Google Scholar] [CrossRef]
- Önnestam, L.; Berinder, K.; Burman, P.; Dahlqvist, P.; Engström, B.E.; Wahlberg, J.; Nyström, H.F. National incidence and prevalence of TSH-secreting pituitary adenomas in Sweden. J. Clin. Endocrinol. Metab. 2013, 98, 626–635. [Google Scholar] [CrossRef]
- Beck-Peccoz, P.; Persani, L.; Mannavola, D.; Campi, I. Pituitary tumours: TSH-secreting adenomas. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 597–606. [Google Scholar] [CrossRef] [PubMed]
- de Herdt, C.; Philipse, E.; de Block, C. ENDOCRINE TUMOURS: Thyrotropin-secreting pituitary adenoma: A structured review of 535 adult cases. Eur. J. Endocrinol. 2021, 185, R65–R74. [Google Scholar] [CrossRef] [PubMed]
- Gatto, F.; Barbieri, F.; Gatti, M.; Wurth, R.; Schulz, S.; Ravetti, J.L.; Zona, G.; Culler, M.D.; Saveanu, A.; Giusti, M.; et al. Balance between somatostatin and D2 receptor expression drives TSH-secreting adenoma response to somatostatin analogues and dopastatins. Clin. Endocrinol. 2012, 76, 407–414. [Google Scholar] [CrossRef]
- Illouz, F.; Chanson, P.; Sonnet, E.; Brue, T.; Ferriere, A.; Sanson, M.L.R.; Vantyghem, M.C.; Raverot, G.; Munier, M.; Rodien, P.; et al. Somatostatin receptor ligands induce TSH deficiency in thyrotropin-secreting pituitary adenoma. Eur. J. Endocrinol. 2021, 184, 1–8. [Google Scholar] [CrossRef]
- Teramoto, A.; Sanno, N.; Tahara, S.; Osamura, Y.R. Pathological study of thyrotropin-secreting pituitary adenoma: Plurihormonality and medical treatment. Acta Neuropathol. 2004, 108, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Gatto, F.; Grasso, L.F.; Nazzari, E.; Cuny, T.; Anania, P.; Di Somma, C.; Colao, A.; Zona, G.; Weryha, G.; Pivonello, R.; et al. Clinical outcome and evidence of high rate post-surgical anterior hypopituitarism in a cohort of TSH-secreting adenoma patients: Might somatostatin analogs have a role as first-line therapy? Pituitary 2015, 18, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, J. In search of a prognostic classification of endocrine pituitary tumors. Endocr. Pathol. 2014, 25, 124–132. [Google Scholar] [CrossRef]
- Lamback, E.B.; Wildemberg, L.E.; Gadelha, M.R. Current opinion on the diagnosis and management of non-functioning pituitary adenomas. Expert Rev. Endocrinol. Metab. 2021, 16, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Neto, L.V.; Machado, E.D.O.; Luque, R.M.; Taboada, G.F.; Marcondes, J.B.; Chimelli, L.M.C.; Quintella, L.P.; Niemeyer, P.; De Carvalho, D.P.; Kineman, R.D.; et al. Expression analysis of dopamine receptor subtypes in normal human pituitaries, nonfunctioning pituitary adenomas and somatotropinomas, and the association between dopamine and somatostatin receptors with clinical response to octreotide-LAR in acromegaly. J. Clin. Endocrinol. Metab. 2009, 94, 1931–1937. [Google Scholar] [CrossRef]
- Colao, A.; di Somma, C.; Pivonello, R.; Faggiano, A.; Lombardi, G.; Savastano, S. Medical therapy for clinically non-functioning pituitary adenomas. Endocr. Relat. Cancer 2008, 15, 905–915. [Google Scholar] [CrossRef]
- Gagliano, T.; Filieri, C.; Minoia, M.; Buratto, M.; Tagliati, F.; Ambrosio, M.R.; Lapparelli, M.; Zoli, M.; Frank, G.; Degli Uberti, E.; et al. Cabergoline reduces cell viability in non functioning pituitary adenomas by inhibiting vascular endothelial growth factor secretion. Pituitary 2013, 16, 91–100. [Google Scholar] [CrossRef]
- Batista, R.L.; Musolino, N.R.C.; Cescato, V.A.S.; Da Silva, G.O.; Medeiros, R.S.S.; Herkenhoff, C.G.B.; Trarbach, E.B.; Cunha-Neto, M.B. Cabergoline in the Management of Residual Nonfunctioning Pituitary Adenoma. Am. J. Clin. Oncol. Cancer Clin. Trials 2019, 42, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Greenman, Y.; Bronstein, M.D. Cabergoline should be attempted in progressing non-functioning pituitary macroadenoma. Eur. J. Endocrinol. 2021, 185, D11–D20. [Google Scholar] [CrossRef]
- Vieria Neto, L.; Wildemberg, L.E.; Colli, L.M.; Kasuki, L.; Marques, N.V.; Moraes, A.B.; Gasparetto, E.L.; Takiya, C.M.; Castro, M.; Gadelha, M.R. ZAC1 and SSTR2 Are Downregulated in Non-Functioning Pituitary Adenomas but Not in somatotropinomas. PLoS ONE 2013, 8, e0077406. [Google Scholar] [CrossRef]
- Even-Zohar, N.; Greenman, Y. Management of NFAs: Medical treatment. Pituitary 2018, 21, 168–175. [Google Scholar] [CrossRef]
- Zatelli, M.C.; Piccin, D.; Vignali, C.; Tagliati, F.; Ambrosio, M.R.; Bondanelli, M.; Cimino, V.; Bianchi, A.; Schmid, H.A.; Scanarini, M.; et al. Pasireotide, a multiple somatostatin receptor subtypes ligand, reduces cell viability in non-functioning pituitary adenomas by inhibiting vascular endothelial growth factor secretion. Endocr. Relat. Cancer 2007, 14, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Florio, T.; Barbieri, F.; Spaziante, R.; Zona, G.; Hofland, L.J.; Van Koetsveld, P.M.; Feelders, R.A.; Stella, G.K.; Theodoropoulou, M.; Culler, M.D.; et al. Efficacy of a dopamine-somatostatin chimeric molecule, BIM-23A760, in the control of cell growth from primary cultures of human non-functioning pituitary adenomas: A multi-center study. Endocr. Relat. Cancer 2008, 15, 583–596. [Google Scholar] [CrossRef]
- Halem, H.A.; Hochgeschwender, U.; Rih, J.K.; Nelson, R.; Allan Johnson, G.; Thiagalingam, A.; Culler, M.D. TBR-760, a Dopamine-Somatostatin Compound, Arrests Growth of Aggressive Nonfunctioning Pituitary Adenomas in Mice. Endocrinology 2020, 161, bqaa101. [Google Scholar] [CrossRef]
- Primeau, V.; Raftopoulos, C.; Maiter, D. Outcomes of transsphenoidal surgery in prolactinomas: Improvement of hormonal control in dopamine agonist-resistant patients. Eur. J. Endocrinol. 2012, 166, 779–786. [Google Scholar] [CrossRef]
- Pouratian, N.; Sheehan, J.; Jagannathan, J.; Laws, E.R.; Steiner, L.; Vance, M.L. Gamma Knife Radiosurgery for Medically and Surgically Refractory Prolactinomas. Neurosurgery 2006, 59, 255–266. [Google Scholar] [CrossRef]
- Iyer, P.; Molitch, M.E. Positive prolactin response to bromocriptine in 2 patients with cabergoline-resistant prolactinomas. Endocr. Pract. 2011, 17, E55–E58. [Google Scholar] [CrossRef] [PubMed]
- Fusco, A.; Gunz, G.; Jaquet, P.; Dufour, H.; Germanetti, A.L.; Guller, M.D.; Barlier, A.; Saveanu, A. Somatostatinergic ligands in dopamine-sensitive and -resistant prolactinomas. Eur. J. Endocrinol. 2008, 158, 595–603. [Google Scholar] [CrossRef]
- Sosa-Eroza, E.; Espinosa, E.; Ramírez-Rentería, C.; Mendoza, V.; Arreola, R.; Mercado, M. Treatment of multiresistant prolactinomas with a combination of cabergoline and octreotide LAR. Endocrine 2018, 61, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Fusco, A.; Lugli, F.; Sacco, E.; Tilaro, L.; Bianchi, A.; Angelini, F.; Tofani, A.; Barini, A.; Lauriola, L.; Maira, G.; et al. Efficacy of the combined cabergoline and octreotide treatment in a case of a dopamine-agonist resistant macroprolactinoma. Pituitary 2011, 14, 351–357. [Google Scholar] [CrossRef]
- Lasolle, H.; Vasiljevic, A.; Borson-Chazot, F.; Raverot, G. Pasireotide: A potential therapeutic alternative for resistant prolactinoma. Ann. Endocrinol. 2019, 80, 84–88. [Google Scholar] [CrossRef]
- Coopmans, E.C.; Van Meyel, S.W.F.; Pieterman, K.J.; Van Ipenburg, J.A.; Hofland, L.J.; Donga, E.; Daly, A.F.; Beckers, A.; Van Der Lely, A.J.; Neggers, S.J.C.M.M. Excellent response to pasireotide therapy in an aggressive and dopamine-resistant prolactinoma. Eur. J. Endocrinol. 2019, 181, K21–K27. [Google Scholar] [CrossRef]
- Raverot, G.; Vasiljevic, A.; Jouanneau, E.; Lasolle, H. Confirmation of a new therapeutic option for aggressive or dopamine agonist-resistant prolactin pituitary neuroendocrine tumors. Eur. J. Endocrinol. 2019, 181, C1–C3. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.J.; Leng, Z.G.; Guo, Y.H.; Cai, L.; Cai, Y.; Li, N.; Shang, H.B.; Le, W.D.; Zhao, W.G.; Wu, Z.B. Suppression of mTOR pathway and induction of autophagy-dependent cell death by cabergoline. Oncotarget 2015, 6, 39329–39341. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Y.; Han, G.; Deng, K.; Liu, X.; Bao, X.; Feng, M.; Yao, Y.; Lian, W.; Xing, B.; et al. Metformin inhibits growth and prolactin secretion of pituitary prolactinoma cells and xenografts. J. Cell. Mol. Med. 2018, 22, 6368–6379. [Google Scholar] [CrossRef]
- Vázquez-Borrego, M.C.; Fuentes-Fayos, A.C.; Herrera-Martínez, A.D.; L-López, F.; Ibáñez-Costa, A.; Moreno-Moreno, P.; Alhambra-Expósito, M.R.; Barrera-Martín, A.; Blanco-Acevedo, C.; Dios, E.; et al. Biguanides Exert Antitumoral Actions in Pituitary Tumor Cells through AMPK-Dependent and -Independent Mechanisms. J. Clin. Endocrinol. Metab. 2019, 104, 3501–3513. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Gao, J.; Feng, M.; Bao, X.; Deng, K.; Yao, Y.; Wang, R. Combination Treatment with Bromocriptine and Metformin in Patients with Bromocriptine-Resistant Prolactinomas: Pilot Study. World Neurosurg. 2018, 115, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Portari, L.H.C.; Correa-Silva, S.R.; Abucham, J. Prolactin response to metformin in cabergoline-resistant prolactinomas: A pilot study. Neuroendocrinology 2022, 112, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Esparís-Ogando, A.; Montero, J.; Arribas, J.; Ocaña, A.; Pandiella, A. Targeting the EGF/HER Ligand-Receptor System in Cancer. Curr. Pharm. Des. 2016, 22, 5887–5898. [Google Scholar] [CrossRef]
- Kontogeorgos, G.; Stefaneanu, L.; Kovacs, K.; Cheng, Z. Localization of Epidermal Growth Factor (EGF) and Epidermal Growth Factor Receptor (EGFr) in Human Pituitary Adenomas and Nontumorous Pituitaries: An Immunocytochemical Study. Endocr. Pathol. 1996, 7, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Cooper, O.; Mamelak, A.; Bannykh, S.; Carmichael, J.; Bonert, V.; Lim, S.; Cook-Wiens, G.; Ben-Shlomo, A. Prolactinoma ErbB receptor expression and targeted therapy for aggressive tumors. Endocrine 2014, 46, 318–327. [Google Scholar] [CrossRef]
- Cooper, O.; Vlotides, G.; Fukuoka, H.; Greene, M.I.; Melmed, S. Expression and function of ErbB receptors and ligands in the pituitary. Endocr. Relat. Cancer 2011, 18, R197–R211. [Google Scholar] [CrossRef] [PubMed]
- Vlotides, G.; Siegel, E.; Donangelo, I.; Gutman, S.; Ren, S.G.; Melmed, S. Rat prolactinoma cell growth regulation by epidermal growth factor receptor ligands. Cancer Res. 2008, 68, 6377–6386. [Google Scholar] [CrossRef] [PubMed]
- Recouvreux, M.V.; Camilletti, M.A.; Rifkin, D.B.; Díaz-Torga, G. The pituitary TGFβ1 system as a novel target for the treatment of resistant prolactinomas. J. Endocrinol. 2016, 228, R73–R83. [Google Scholar] [CrossRef]
- Massagué, J. TGF-beta signal transduction. Annu. Rev. Biochem. 1998, 67, 753–791. [Google Scholar] [CrossRef]
- Heldin, C.-H.; Miyazono, K.; ten Dijke, P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature 1997, 390, 465–471. [Google Scholar] [CrossRef]
- Sarkar, D.K.; Kim, K.H.; Minami, S. Transforming growth factor-beta 1 messenger RNA and protein expression in the pituitary gland: Its action on prolactin secretion and lactotropic growth. Mol. Endocrinol. 1992, 6, 1825–1833. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sarkar, D.K.; Pastorcic, M.; De, A.; Engel, M.; Moses, H.; Ghasemzadeh, M.B. Role of transforming growth factor (TGF)-beta Type I and TGF-beta type II receptors in the TGF-beta1-regulated gene expression in pituitary prolactin-secreting lactotropes. Endocrinology 1998, 139, 3620–3628. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Recouvreux, M.V.; Guida, M.C.; Rifkin, D.B.; Becu-Villalobos, D.; Díaz-Torga, G. Active and Total Transforming Growth Factor-β1 Are Differentially Regulated by Dopamine and Estradiol in the Pituitary. Endocrinology 2011, 152, 2722–2730. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Q.; Li, C.; Zong, X.; Bai, J.; Wu, Y.; Lan, X.; Yu, G.; Zhang, Y. The role of TGF-β/Smad signaling in dopamine agonist-resistant prolactinomas. Mol. Cell. Endocrinol. 2015, 402, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Leslie, N.R.; Biondi, R.M.; Alessi, D.R. Phosphoinositide-regulated kinases and phosphoinositide phosphatases. Chem. Rev. 2001, 101, 2365–2380. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Muşat, M.; Korbonits, M.; Kola, B.; Borboli, N.; Hanson, M.R.; Nanzer, A.M.; Grigson, J.; Jordan, S.; Morris, D.G.; Gueorguiev, M.; et al. Enhanced protein kinase B/Akt signalling in pituitary tumours. Endocr. Relat. Cancer 2005, 12, 423–433. [Google Scholar] [CrossRef]
- Dworakowska, D.; Wlodek, E.; Leontiou, C.A.; Igreja, S.; Cakir, M.; Teng, M.; Prodromou, N.; Góth, M.I.; Grozinsky-Glasberg, S.; Gueorguiev, M.; et al. Activation of RAF/MEK/ERK and PI3K/AKT/mTOR pathways in pituitary adenomas and their effects on downstream effectors. Endocr. Relat. Cancer 2009, 16, 1329–1338. [Google Scholar] [CrossRef]
- Roof, A.K.; Jirawatnotai, S.; Trudeau, T.; Kuzyk, C.; Wierman, M.E.; Kiyokawa, H.; Gutierrez-Hartmann, A. The Balance of PI3K and ERK Signaling Is Dysregulated in Prolactinoma and Modulated by Dopamine. Endocrinology 2018, 159, 2421–2434. [Google Scholar] [CrossRef]
- Chen, R.; Duan, J.; Li, L.; Ma, Q.; Sun, Q.; Ma, J.; Li, C.; Zhou, X.; Chen, H.; Jing, Y.; et al. MTOR promotes pituitary tumor development through activation of PTTG1. Oncogene 2017, 36, 979–988. [Google Scholar] [CrossRef]
- Geng, X.; Ma, L.; Li, Z.; Li, Z.; Li, J.; Li, M.; Wang, Q.; Chen, Z.; Sun, Q. Bromocriptine Induces Autophagy-Dependent Cell Death in Pituitary Adenomas. World Neurosurg. 2017, 100, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Way, J.S.; Zhang, X.; Sergey, M.; Bergsneider, M.; Wang, M.B.; Yong, W.H.; Heaney, A.P. Effect of Everolimus in Treatment of Aggressive Prolactin-Secreting Pituitary Adenomas. J. Clin. Endocrinol. Metab. 2019, 104, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Tuvia, S.; Pelled, D.; Marom, K.; Salama, P.; Levin-Arama, M.; Karmeli, I.; Idelson, G.H.; Landau, I.; Mamluk, R. A novel suspension formulation enhances intestinal absorption of macromolecules via transient and reversible transport mechanisms. Pharm. Res. 2014, 31, 2010–2021. [Google Scholar] [CrossRef]
- Tuvia, S.; Atsmon, J.; Teichman, S.L.; Katz, S.; Salama, P.; Pelled, D.; Landau, I.; Karmeli, I.; Bidlingmaier, M.; Strasburger, C.J.; et al. Oral octreotide absorption in human subjects: Comparable pharmacokinetics to parenteral octreotide and effective growth hormone suppression. J. Clin. Endocrinol. Metab. 2012, 97, 2362–2369. [Google Scholar] [CrossRef] [PubMed]
- Melmed, S.; Popovic, V.; Bidlingmaier, M.; Mercado, M.; Van Der Lely, A.J.; Biermasz, N.; Bolanowski, M.; Coculescu, M.; Schopohl, J.; Racz, K.; et al. Safety and efficacy of oral octreotide in acromegaly: Results of a multicenter phase III trial. J. Clin. Endocrinol. Metab. 2015, 100, 1699–1708. [Google Scholar] [CrossRef]
- Samson, S.L.; Nachtigall, L.B.; Fleseriu, M.; Gordon, M.B.; Bolanowski, M.; Labadzhyan, A.; Ur, E.; Molitch, M.; Ludlam, W.H.; Patou, G.; et al. Maintenance of Acromegaly Control in Patients Switching from Injectable Somatostatin Receptor Ligands to Oral Octreotide. J. Clin. Endocrinol. Metab. 2020, 105, e3785–e3797. [Google Scholar] [CrossRef] [PubMed]
- Labadzhyan, A.; Nachtigall, L.B.; Fleseriu, M.; Gordon, M.B.; Molitch, M.; Kennedy, L.; Samson, S.L.; Greenman, Y.; Biermasz, N.; Bolanowski, M.; et al. Oral octreotide capsules for the treatment of acromegaly: Comparison of 2 phase 3 trial results. Pituitary 2021, 24, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Tiberg, F.; Roberts, J.; Cervin, C.; Johnsson, M.; Sarp, S.; Tripathi, A.P.; Linden, M. Octreotide s.c. depot provides sustained octreotide bioavailability and similar IGF-1 suppression to octreotide LAR in healthy volunteers. Br. J. Clin. Pharmacol. 2015, 80, 460–472. [Google Scholar] [CrossRef]
- Pavel, M.; Borson-Chazot, F.; Cailleux, A.; Hörsch, D.; Lahner, H.; Pivonello, R.; Tauchmanova, L.; Darstein, C.; Olsson, H.; Tiberg, F.; et al. Octreotide SC depot in patients with acromegaly and functioning neuroendocrine tumors: A phase 2, multicenter study. Cancer Chemother. Pharmacol. 2019, 83, 375–385. [Google Scholar] [CrossRef]
- Harris, A.G.; Weeke, J.; Christensen, S.E.; Kaal, A.; Illum, P.; Ørskov, H. Preliminary results with Sandostatin nasal powder in acromegalic patients. Metabolism 1992, 41, 72–75. [Google Scholar] [CrossRef]
- Weeke, J.; Christensen, S.E.; ØRskov, H.; Kaal, A.; Mau Pedersen, M.; Illum, P.; Harris, A.G. A randomized comparison of intranasal and injectable octreotide administration in patients with acromegaly. J. Clin. Endocrinol. Metab. 1992, 75, 163–169. [Google Scholar] [CrossRef]
- Levisetti, M.; Laurent, O.; Daniels, M.; Milad, M.; Mazzoni, M.; Martin, J. Pharmacokinetics, Pharmacodynamics, Safety, and Tolerability of DP1038, an Intranasal Formulation of Octreotide Acetate, in Healthy Volunteers. Endo. 2019 2012, 1038, 1200. [Google Scholar]
- Antunes, X.; Kasuki, L.; Gadelha, M.R. New and emerging pharmacological treatment options for acromegaly. Expert Opin. Pharmacother. 2021, 22, 1615–1623. [Google Scholar] [CrossRef]
- Madan, A.; Zhu, Y.F.; Markison, S.; Betz, S.; Krasner, A.; Luo, R.; Oltersdorf, T.; Jochelson, T.; Lickliter, J.; Struthers, S. SAT-429 Final Results from the First in Man Phase 1 Clinical Trial of CRN00808, an Orally Bioavailable sst2-Selective, Nonpeptide Somatostatin Biased Agonist, for the Treatment of Acromegaly: Safety, Pharmacokinetics, Pharmacodynamics, and Midazolam Drug Interaction in Healthy Volunteers. J. Endocr. Soc. 2019, 3, SAT-429. [Google Scholar] [CrossRef]
- Afargan, M.; Janson, E.T.; Gelerman, G.; Rosenfeld, R.; Ziv, O.; Karpov, O.; Wolf, A.; Bracha, M.; Shohat, D.; Liapakis, G.; et al. Novel long-acting somatostatin analog with endocrine selectivity: Potent suppression of growth hormone but not of insulin. Endocrinology 2001, 142, 477–486. [Google Scholar] [CrossRef]
- Shimon, I.; Rubinek, T.; Hadani, M.; Alhadef, N. PTR-3173 (somatoprim), a novel somatostatin analog with affinity for somatostatin receptors 2, 4 and 5 is a potent inhibitor of human GH secretion. J. Endocrinol. Investig. 2004, 27, 721–727. [Google Scholar] [CrossRef]
- Plöckinger, U.; Hoffmann, U.; Geese, M.; Lupp, A.; Buchfelder, M.; Flitsch, J.; Vajkoczy, P.; Jakob, W.; Saeger, W.; Schulz, S.; et al. DG3173 (somatoprim), a unique somatostatin receptor subtypes 2-, 4- and 5-selective analogue, effectively reduces GH secretion in human GH-secreting pituitary adenomas even in Octreotide non-responsive tumours. Eur. J. Endocrinol. 2012, 166, 223–234. [Google Scholar] [CrossRef]
- Single Dose Pharmacology Study of DG3173 and Octreotide in Acromegalic Patients.—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02235987 (accessed on 12 December 2021).
- Zhou, C.; Bonert, V.; Mamelak, A.; Kelly, D.; Barkhoudarian, G.; Hoon, D.; Komagata, T.; Shinozaki, K.; Melmed, S. MON-459 ONO-5788, a Novel Oral Small Molecule Somatostatin Receptor Type-2 (SST2) Agonist, Attenuates GH Hypersecretion in Human GH-Secreting, Pituitary Adenoma-Derived Cells. J. Endocr. Soc. 2019, 3, MON-459. [Google Scholar] [CrossRef]
- Tanaka, H.; Komagata, T.; Nishio, T.; Ishida, A.; Okada, H.; Katsumata, S.; Shinozaki, K. MON-477 Octreotide and ONO-ST-468, a Novel and Potent Somatostatin Receptor Type-2 (SST2) Agonist, Suppress GH Hypersecretion in the Monkey. J. Endocr. Soc. 2019, 3, MON-477. [Google Scholar] [CrossRef]
- Trainer, P.J.; Newell-Price, J.D.C.; Ayuk, J.; Aylwin, S.J.B.; Rees, A.; Drake, W.; Chanson, P.; Brue, T.; Webb, S.M.; Fajardo, C.; et al. A randomised, open-label, parallel group phase 2 study of antisense oligonucleotide therapy in acromegaly. Eur. J. Endocrinol. 2018, 179, 97–108. [Google Scholar] [CrossRef]
- Rocheville, M.; Lange, D.C.; Kumar, U.; Patel, S.C.; Patel, R.C.; Patel, Y.C. Receptors for dopamine and somatostatin: Formation of hetero-oligomers with enhanced functional activity. Science 2000, 288, 154–157. [Google Scholar] [CrossRef]
- Rocheville, M.; Lange, D.C.; Kumar, U.; Sasi, R.; Patel, R.C.; Patel, Y.C. Subtypes of the somatostatin receptor assemble as functional homo- and heterodimers. J. Biol. Chem. 2000, 275, 7862–7869. [Google Scholar] [CrossRef]
- Saveanu, A.; Lavaque, E.; Gunz, G.; Barlier, A.; Kim, S.; Taylor, J.E.; Culler, M.D.; Enjalbert, A.; Jaquet, P. Demonstration of enhanced potency of a chimeric somatostatin-dopamine molecule, BIM-23A387, in suppressing growth hormone and prolactin secretion from human pituitary somatotroph adenoma cells. J. Clin. Endocrinol. Metab. 2002, 87, 5545–5552. [Google Scholar] [CrossRef]
- Jaquet, P.; Gunz, G.; Saveanu, A.; Dufour, H.; Taylor, J.; Dong, J.; Kim, S.; Moreau, J.P.; Enjalbert, A.; Culler, M.D. Efficacy of chimeric molecules directed towards multiple somatostatin and dopamine receptors on inhibition of GH and prolactin secretion from GH-secreting pituitary adenomas classified as partially responsive to somatostatin analog therapy. Eur. J. Endocrinol. 2005, 153, 135–141. [Google Scholar] [CrossRef]
- Culler, M.D. Somatostatin-dopamine chimeras: A novel approach to treatment of neuroendocrine tumors. Horm. Metab. Res. 2011, 43, 854–857. [Google Scholar] [CrossRef]
- Hill, J.; Kim, S.; Tsomaia, N.; Dong, J.Z.; Culler, M. Chimeric Somatostatin–Dopamine Compounds (Dopastatins) for the Treatment of Neuroendocrine Disease, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 6, ISBN 9780124095472. [Google Scholar]
- Vázquez-Borrego, M.C.; L-López, F.; Gálvez-Moreno, M.A.; Fuentes-Fayos, A.C.; Venegas-Moreno, E.; Herrera-Martínez, A.D.; Blanco-Acevedo, C.; Solivera, J.; Landsman, T.; Gahete, M.D.; et al. A New Generation Somatostatin-Dopamine Analogue Exerts Potent Antitumoral Actions on Pituitary Neuroendocrine Tumor Cells. Neuroendocrinology 2020, 110, 70–82. [Google Scholar] [CrossRef]
- Cuny, T.; Graillon, T.; Defilles, C.; Datta, R.; Zhang, S.; Figarella-Branger, D.; Dufour, H.; Mougel, G.; Brue, T.; Landsman, T.; et al. Characterization of the ability of a, second-generation SST-DA chimeric molecule, TBR-065, to suppress GH secretion from human GH-secreting adenoma cells. Pituitary 2021, 24, 351–358. [Google Scholar] [CrossRef]
- Kim, J.; Oh, J.H.; Harlem, H.; Culler, M.D.; Ku, C.R.; Lee, E.J. Therapeutic effect of a novel chimeric molecule targeting both somatostatin and dopamine receptors on growth hormone-secreting pituitary adenomas. Endocrinol. Metab. 2020, 35, 177–187. [Google Scholar] [CrossRef]
- De Boon, W.M.I.; Van Esdonk, M.J.; Stuurman, F.E.; Biermasz, N.R.; Pons, L.; Paty, I.; Burggraaf, J. A Novel Somatostatin-Dopamine Chimera (BIM23B065) Reduced GH Secretion in a First-in-Human Clinical Trial. J. Clin. Endocrinol. Metab. 2018, 104, 883–891. [Google Scholar] [CrossRef]
- Theodoropoulou, M.; Reincke, M. Tumor-Directed Therapeutic Targets in Cushing Disease. J. Clin. Endocrinol. Metab. 2019, 104, 925–933. [Google Scholar] [CrossRef]
- Castinetti, F.; Graillon, T.; Dufour, H.; Brue, T. Letter to the Editor: “Why We Should Still Treat by Neurosurgery Patients with Cushing Disease and a Normal or Inconclusive Pituitary MRI”. J. Clin. Endocrinol. Metab. 2019, 104, 5791–5792. [Google Scholar] [CrossRef]
- Chabre, O.; Cristante, J.; Lefournier, V.; Gay, E. Response to Letter to the Editor: “Why We Should Still Treat by Neurosurgery Patients With Cushing Disease and a Normal or Inconclusive Pituitary MRI”. J. Clin. Endocrinol. Metab. 2019, 104, 5793–5794. [Google Scholar] [CrossRef]
- Páez-Pereda, M.; Kovalovsky, D.; Hopfner, U.; Theodoropoulou, M.; Pagotto, U.; Uhl, E.; Losa, M.; Stalla, J.; Grübler, Y.; Missale, C.; et al. Retinoic acid prevents experimental Cushing syndrome. J. Clin. Investig. 2001, 108, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Pecori Giraldi, F.; Sesta, A.; Tapella, L.; Cassarino, M.F.; Castelli, L. Dual effects of 9-cis retinoic acid on ACTH-dependent hyperplastic adrenal tissues. Sci. Rep. 2021, 11, 14315. [Google Scholar] [CrossRef]
- Sesta, A.; Cassarino, M.F.; Tapella, L.; Castelli, L.; Cavagnini, F.; Pecori Giraldi, F. Effect of retinoic acid on human adrenal corticosteroid synthesis. Life Sci. 2016, 151, 277–280. [Google Scholar] [CrossRef][Green Version]
- Occhi, G.; Regazzo, D.; Albiger, N.M.; Ceccato, F.; Ferasin, S.; Scanarini, M.; Denaro, L.; Cosma, C.; Plebani, M.; Cassarino, M.F.; et al. Activation of the dopamine receptor type-2 (DRD2) promoter by 9-cis retinoic acid in a cellular model of Cushing’s disease mediates the inhibition of cell proliferation and ACTH secretion without a complete corticotroph-to-melanotroph transdifferentiation. Endocrinology 2014, 155, 3538–3549. [Google Scholar] [CrossRef]
- Giraldi, F.P.; Ambrogio, A.G.; Andrioli, M.; Sanguin, F.; Karamouzis, I.; Corsello, S.M.; Scaroni, C.; Arvat, E.; Pontercorvi, A.; Cavagnini, F. Potential role for retinoic acid in patients with Cushing’s disease. J. Clin. Endocrinol. Metab. 2012, 97, 3577–3583. [Google Scholar] [CrossRef]
- Vilar, L.; Albuquerque, J.L.; Lyra, R.; Trovão Diniz, E.; Rangel Filho, F.; Gadelha, P.; Thé, A.C.; Ibiapina, G.R.; Gomes, B.S.; Santos, V.; et al. The Role of Isotretinoin Therapy for Cushing’s Disease: Results of a Prospective Study. Int. J. Endocrinol. 2016, 2016, 8173182. [Google Scholar] [CrossRef][Green Version]
- Reincke, M.; Sbiera, S.; Hayakawa, A.; Theodoropoulou, M.; Osswald, A.; Beuschlein, F.; Meitinger, T.; Mizuno-Yamasaki, E.; Kawaguchi, K.; Saeki, Y.; et al. Mutations in the deubiquitinase gene USP8 cause Cushing’s disease. Nat. Genet. 2015, 47, 31–38. [Google Scholar] [CrossRef]
- Castellnou, S.; Vasiljevic, A.; Lapras, V.; Raverot, V.; Alix, E.; Borson-Chazot, F.; Jouanneau, E.; Raverot, G.; Lasolle, H. SST5 expression and USP8 mutation in functioning and silent corticotroph pituitary tumors. Endocr. Connect. 2020, 9, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Meijer, I.M.J.; Van Leeuwen, J.E.M. ERBB2 is a target for USP8-mediated deubiquitination. Cell. Signal. 2011, 23, 458–467. [Google Scholar] [CrossRef]
- Araki, T.; Liu, X.; Kameda, H.; Tone, Y.; Fukuoka, H.; Tone, M.; Melmed, S. EGFR Induces E2F1-Mediated Corticotroph Tumorigenesis. J. Endocr. Soc. 2017, 1, 127–143. [Google Scholar] [CrossRef]
- Fukuoka, H.; Cooper, O.; Ben-Shlomo, A.; Mamelak, A.; Ren, S.G.; Bruyette, D.; Melmed, S. EGFR as a therapeutic target for human, canine, and mouse ACTH-secreting pituitary adenomas. J. Clin. Investig. 2011, 121, 4712–4721. [Google Scholar] [CrossRef]
- Asari, Y.; Kageyama, K.; Sugiyama, A.; Kogawa, H.; Niioka, K.; Daimon, M. Lapatinib decreases the ACTH production and proliferation of corticotroph tumor cells. Endocr. J. 2019, 66, 515–522. [Google Scholar] [CrossRef]
- Shen, Y.; Ji, C.; Jian, X.; Zhou, J.; Zhang, Q.; Qiao, N.; Zhang, Y.; Shou, X.; Zhou, X.; Ma, Z. Regulation of the EGFR Pathway by HSP90 Is Involved in the Pathogenesis of Cushing’s Disease. Front. Endocrinol. 2021, 11, 1057. [Google Scholar] [CrossRef]
- Riebold, M.; Kozany, C.; Freiburger, L.; Sattler, M.; Buchfelder, M.; Hausch, F.; Stalla, G.K.; Paez-Pereda, M. A C-terminal HSP90 inhibitor restores glucocorticoid sensitivity and relieves a mouse allograft model of Cushing disease. Nat. Med. 2015, 21, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Ciato, D.; Albani, A. Molecular Mechanisms of Glucocorticoid Resistance in Corticotropinomas: New Developments and Drug Targets. Front. Endocrinol. 2020, 11, 21. [Google Scholar] [CrossRef]
- Hinojosa-Amaya, J.M.; Lam-Chung, C.E.; Cuevas-Ramos, D. Recent Understanding and Future Directions of Recurrent Corticotroph Tumors. Front. Endocrinol. 2021, 12, 392. [Google Scholar] [CrossRef]
- Chen, J.; Jian, X.; Deng, S.; Ma, Z.; Shou, X.; Shen, Y.; Zhang, Q.; Song, Z.; Li, Z.; Peng, H.; et al. Identification of recurrent USP48 and BRAF mutations in Cushing’s disease. Nat. Commun. 2018, 9, 3171. [Google Scholar] [CrossRef]
- Liu, N.A.; Jiang, H.; Ben-Shlomo, A.; Wawrowsky, K.; Fan, X.M.; Lin, S.; Melmed, S. Targeting zebrafish and murine pituitary corticotroph tumors with a cyclin-dependent kinase (CDK) inhibitor. Proc. Natl. Acad. Sci. USA 2011, 108, 8414–8419. [Google Scholar] [CrossRef]
- Treatment of Cushing’s Disease with R-Roscovitine. Available online: https://clinicaltrials.gov/ct2/show/NCT02160730 (accessed on 12 December 2021).
- Luque, R.M.; Ibáñez-Costa, A.; López-Sánchez, L.M.; Jiménez-Reina, L.; Venegas-Moreno, E.; Gálvez, M.A.; Villa-Osaba, A.; Madrazo-Atutxa, A.M.; Japón, M.A.; De La Riva, A.; et al. A cellular and molecular basis for the selective desmopressin-induced ACTH release in Cushing disease patients: Key role of AVPR1b receptor and potential therapeutic implications. J. Clin. Endocrinol. Metab. 2013, 98, 4160–4169. [Google Scholar] [CrossRef]
- Lu, J.; Chatain, G.P.; Bugarini, A.; Wang, X.; Maric, D.; Walbridge, S.; Zhuang, Z.; Chittiboina, P. Histone Deacetylase Inhibitor SAHA Is a Promising Treatment of Cushing Disease. J. Clin. Endocrinol. Metab. 2017, 102, 2825–2835. [Google Scholar] [CrossRef]
- Du, L.; Bergsneider, M.; Mirsadraei, L.; Young, S.H.; Jonker, J.W.; Downes, M.; Yong, W.H.; Evans, R.M.; Heaney, A.P. Evidence for orphan nuclear receptor TR4 in the etiology of Cushing disease. Proc. Natl. Acad. Sci. USA 2013, 110, 8555–8560. [Google Scholar] [CrossRef]
- A Study of the Efficacy and Safety of Relacorilant in Patients with Endogenous Cushing Syndrome. Available online: https://clinicaltrials.gov/ct2/show/NCT03697109 (accessed on 13 December 2021).
- Daly, A.F.; Beckers, A. The Epidemiology of Pituitary Adenomas. Endocrinol. Metab. Clin. N. Am. 2020, 49, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Flores-Martinez, Á.; Venegas-Moreno, E.; Dios, E.; Remón-Ruiz, P.; Gros-Herguido, N.; Vázquez-Borrego, M.C.; Madrazo-Atutxa, A.; Japón, M.A.; Kaen, A.; Cárdenas-Valdepeñas, E.; et al. Quantitative Analysis of Somatostatin and Dopamine Receptors Gene Expression Levels in Non-functioning Pituitary Tumors and Association with Clinical and Molecular Aggressiveness Features. J. Clin. Med. 2020, 9, 3052. [Google Scholar] [CrossRef]
- Vazquez-Borrego, M.C.; Gupta, V.; Ibañez-Costa, A.; Gahete, M.D.; Venegas-Moreno, E.; Toledano-Delgado, Á.; Cano, D.A.; Blanco-Acevedo, C.; Ortega-Salas, R.; Japon, M.A.; et al. A somatostatin receptor subtype-3 (SST3) peptide agonist shows antitumor effects in experimental models of nonfunctioning pituitary tumors. Clin. Cancer Res. 2020, 26, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Costa, A.; López-Sánchez, L.M.; Gahete, M.D.; Rivero-Cortés, E.; Vázquez-Borrego, M.C.; Gálvez, M.A.; De La Riva, A.; Venegas-Moreno, E.; Jiménez-Reina, L.; Moreno-Carazo, A.; et al. BIM-23A760 influences key functional endpoints in pituitary adenomas and normal pituitaries: Molecular mechanisms underlying the differential response in adenomas. Sci. Rep. 2017, 7, 42002. [Google Scholar] [CrossRef]
- Principe, M.; Chanal, M.; Ilie, M.D.; Ziverec, A.; Vasiljevic, A.; Jouanneau, E.; Hennino, A.; Raverot, G.; Bertolino, P. Immune Landscape of Pituitary Tumors Reveals Association Between Macrophages and Gonadotroph Tumor Invasion. J. Clin. Endocrinol. Metab. 2020, 105, 3459–3473. [Google Scholar] [CrossRef]
- Marques, P.; Barry, S.; Carlsen, E.; Collier, D.; Ronaldson, A.; Awad, S.; Dorward, N.; Grieve, J.; Mendoza, N.; Muquit, S.; et al. Chemokines modulate the tumour microenvironment in pituitary neuroendocrine tumours. Acta Neuropathol. Commun. 2019, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- van Eersel, M.E.A.; Meeuwisse–Pasterkamp, S.H.; Muller Kobold, A.C.; Meiners, L.C.; den Dunnen, W.F.; Hofland, L.J.; van den Berg, G. Treatment of a thyrotropin-secreting pituitary adenoma (TSH-oma) with pasireotide LAR. Clin. Endocrinol. 2017, 87, 877–879. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahakian, N.; Castinetti, F.; Brue, T.; Cuny, T. Current and Emerging Medical Therapies in Pituitary Tumors. J. Clin. Med. 2022, 11, 955. https://doi.org/10.3390/jcm11040955
Sahakian N, Castinetti F, Brue T, Cuny T. Current and Emerging Medical Therapies in Pituitary Tumors. Journal of Clinical Medicine. 2022; 11(4):955. https://doi.org/10.3390/jcm11040955
Chicago/Turabian StyleSahakian, Nicolas, Frédéric Castinetti, Thierry Brue, and Thomas Cuny. 2022. "Current and Emerging Medical Therapies in Pituitary Tumors" Journal of Clinical Medicine 11, no. 4: 955. https://doi.org/10.3390/jcm11040955
APA StyleSahakian, N., Castinetti, F., Brue, T., & Cuny, T. (2022). Current and Emerging Medical Therapies in Pituitary Tumors. Journal of Clinical Medicine, 11(4), 955. https://doi.org/10.3390/jcm11040955





