Estimation of Gross Motor Functions in Children with Cerebral Palsy Using Zebris FDM-T Treadmill
Abstract
1. Introduction
2. Materials and Methods
2.1. Gross Motor Function Measure (GMFM)
- lying and rolling (17 measurements),
- sitting (20 measurements),
- crawling and kneeling (14 measurements),
- standing (13 measurements),
- 0 points—does not initiate movement,
- 1 point—activity performed in the range below 10% (initiates movement),
- 2 points—activity executed in the range between 10–100%,
- 3 points—activity performed in 100%,
- NT—not tested [66].
2.2. Zebris FDM-T Treadmill Device
2.3. Study Population
2.4. Zebris FDM-T Treadmill Diagnostics Scheme
2.5. Data Analysis
- training set—80% of the data,
- testing set—remaining 20% of the data.
- Mean Squared Error (MSE),
- Root Mean Squared Error (RMSE),
- Mean Absolute Error (MAE),
- Mean Absolute Percentage Error (MAPE),
- Coefficient of Determination () [71].
- min—minimal value,
- q1—first quartile,
- median—second quartile,
- q3—third quartile,
- max—maximal value,
- q3 − q1—difference between third and first quartile,
- range—difference between maximal and minimal value.
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CP | Cerebral palsy |
| BDS | Big Data Analytics |
| ML | Machine Learning |
| FV | Focal Vibration |
| CV | Computer Vision |
| GCSs | Gait Classification Systems |
| TD | Typically developing |
| 3DIGA | Three-dimensional Instrumented Gait Analysis |
| GGI | Gillette Gait Index |
| GDI | Gait Deviation Index |
| GPS | Gait Profile Score |
| GMFM | Gross Motor Function Measure |
| GMFCS | Gross Motor Function Classification System |
| 6MWT | 6-m Walk Test |
| GOAL | Gait Outcomes Assessment List |
| RF | Random Forest |
| SVM | Support Vector Machine |
| CFCS | Communication Function Classification System |
| FTSST | Five-Times-Sit-to-Stand Test |
| MACS | Manual Ability Classification System |
| CNN | Convolutional Neural Network |
| RR | Ridge Regression |
| GCNN | Graph Convolutional Neural Network |
| GMAE | Gross Motor Ability Estimator |
| TWEC | Technologicznie Wspomagana Edukacja Chodu |
| COP | Center of Pressure |
| GLM | Generalized Linear Model |
| MSE | Mean Squared Error |
| RMSE | Root Mean Squared Error |
| MAE | Mean Absolute Error |
| MAPE | Mean Absolute Percentage Error |
| Coefficient of Determination |
References
- Sadowska, M.; Sarecka-Hujar, B.; Kopyta, I. Cerebral palsy: Current opinions on definition, epidemiology, risk factors, classification and treatment options. Neuropsychiatr. Dis. Treat. 2020, 16, 1505. [Google Scholar] [CrossRef] [PubMed]
- Afzali, M.; Etemad, K.; Kazemi, A.; Rabiei, R. Cerebral palsy information system with an approach to information architecture: A systematic review. BMJ Health Care Inform. 2019, 26, e100055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Oskoui, M.; Shevell, M. A population-based study of communication impairment in cerebral palsy. J. Child Neurol. 2015, 30, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.M.; Meehan, E.M.; Arnup, S.J.; Reddihough, D.S. Intellectual disability in cerebral palsy: A population-based retrospective study. Dev. Med. Child Neurol. 2018, 60, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Whitney, D.G.; Warschausky, S.A.; Peterson, M.D. Mental health disorders and physical risk factors in children with cerebral palsy: A cross-sectional study. Dev. Med. Child Neurol. 2019, 61, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S.; Bini, F.; Del Percio, C.; Marinozzi, F.; Celletti, C.; Suppa, A.; Ferri, R.; Staltari, E.; Camerota, F.; Babiloni, C. Electroencephalographic sensorimotor rhythms are modulated in the acute phase following focal vibration in healthy subjects. Neuroscience 2017, 352, 236–248. [Google Scholar] [CrossRef]
- Coker-Bolt, P.; Downey, R.J.; Connolly, J.; Hoover, R.; Shelton, D.; Seo, N.J. Exploring the feasibility and use of accelerometers before, during, and after a camp-based CIMT program for children with cerebral palsy. J. Pediatr. Rehabil. Med. 2017, 10, 27–36. [Google Scholar] [CrossRef]
- Sartori, M.; Fernandez, J.; Modenese, L.; Carty, C.; Barber, L.; Oberhofer, K.; Zhang, J.; Handsfield, G.; Stott, N.; Besier, T.; et al. Toward modeling locomotion using electromyography-informed 3D models: Application to cerebral palsy. Wiley Interdiscip. Rev. Syst. Biol. Med. 2017, 9, e1368. [Google Scholar] [CrossRef]
- Zhang, J. Multivariate analysis and machine learning in cerebral palsy research. Front. Neurol. 2017, 8, 715. [Google Scholar] [CrossRef]
- Ku, J.P.; Hicks, J.L.; Hastie, T.; Leskovec, J.; Ré, C.; Delp, S.L. The mobilize center: An NIH big data to knowledge center to advance human movement research and improve mobility. J. Am. Med. Inform. Assoc. 2015, 22, 1120–1125. [Google Scholar] [CrossRef]
- Bergamini, L.; Calderara, S.; Bicocchi, N.; Ferrari, A.; Vitetta, G. Signal Processing and Machine Learning for Diplegia Classification. In Proceedings of the International Conference on Image Analysis and Processing, Catania, Italy, 11–15 September 2017; Springer: Berlin/Heidelberg, Germany, 2017; pp. 97–108. [Google Scholar]
- Kuntze, G.; Nettel-Aguirre, A.; Ursulak, G.; Robu, I.; Bowal, N.; Goldstein, S.; Emery, C.A. Multi-joint gait clustering for children and youth with diplegic cerebral palsy. PLoS ONE 2018, 13, e0205174. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Bergamini, L.; Guerzoni, G.; Calderara, S.; Bicocchi, N.; Vitetta, G.; Borghi, C.; Neviani, R.; Ferrari, A. Gait-based diplegia classification using lsmt networks. J. Healthc. Eng. 2019, 2019, 3796898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, Y. Application of supervised machine learning algorithms in the classification of sagittal gait patterns of cerebral palsy children with spastic diplegia. Comput. Biol. Med. 2019, 106, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Ihlen, E.A.; Støen, R.; Boswell, L.; de Regnier, R.A.; Fjørtoft, T.; Gaebler-Spira, D.; Labori, C.; Loennecken, M.C.; Msall, M.E.; Möinichen, U.I.; et al. Machine learning of infant spontaneous movements for the early prediction of cerebral palsy: A multi-site cohort study. J. Clin. Med. 2020, 9, 5. [Google Scholar] [CrossRef]
- Choisne, J.; Fourrier, N.; Handsfield, G.; Signal, N.; Taylor, D.; Wilson, N.; Stott, S.; Besier, T.F. An unsupervised data-driven model to classify gait patterns in children with cerebral palsy. J. Clin. Med. 2020, 9, 1432. [Google Scholar] [CrossRef]
- Sukhadia, N.; Kamboj, P. Detection of Spastic Cerebral Palsy Using Different Techniques in Infants. In ICT Analysis and Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 57–71. [Google Scholar]
- Kurowski, B.G.; Greve, K.; Bailes, A.F.; Zahner, J.; Vargus-Adams, J.; Mcmahon, M.A.; Aronow, B.J.; Mitelpunkt, A. Electronic health record and patterns of care for children with cerebral palsy. Dev. Med. Child Neurol. 2021, 63, 1337–1343. [Google Scholar] [CrossRef]
- Harris, C.M.; Wright, S.M. Malnutrition in hospitalized adults with cerebral palsy. J. Parenter. Enter. Nutr. 2021, 45, 1749–1754. [Google Scholar] [CrossRef]
- Sakkos, D.; Mccay, K.D.; Marcroft, C.; Embleton, N.D.; Chattopadhyay, S.; Ho, E.S. Identification of Abnormal Movements in Infants: A Deep Neural Network for Body Part-Based Prediction of Cerebral Palsy. IEEE Access 2021, 9, 94281–94292. [Google Scholar] [CrossRef]
- Phinyomark, A.; Petri, G.; Ibáñez-Marcelo, E.; Osis, S.T.; Ferber, R. Analysis of big data in gait biomechanics: Current trends and future directions. J. Med. Biol. Eng. 2018, 38, 244–260. [Google Scholar] [CrossRef]
- Silva, N.; Zhang, D.; Kulvicius, T.; Gail, A.; Barreiros, C.; Lindstaedt, S.; Kraft, M.; Bölte, S.; Poustka, L.; Nielsen-Saines, K.; et al. The future of General Movement Assessment: The role of computer vision and machine learning–A scoping review. Res. Dev. Disabil. 2021, 110, 103854. [Google Scholar] [CrossRef]
- Papageorgiou, E.; Nieuwenhuys, A.; Vandekerckhove, I.; Van Campenhout, A.; Ortibus, E.; Desloovere, K. Systematic review on gait classifications in children with cerebral palsy: An update. Gait Posture 2019, 69, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Tsitlakidis, S.; Schwarze, M.; Westhauser, F.; Heubisch, K.; Horsch, A.; Hagmann, S.; Wolf, S.I.; Götze, M. Gait Indices for Characterization of Patients with Unilateral Cerebral Palsy. J. Clin. Med. 2020, 9, 3888. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, H.M.; Nielsen, D.B.; Pedersen, N.W.; Overgaard, S.; Holsgaard-Larsen, A. Gait Deviation Index, Gait Profile Score and Gait Variable Score in children with spastic cerebral palsy: Intra-rater reliability and agreement across two repeated sessions. Gait Posture 2015, 42, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.; McGinley, J.L.; Schwartz, M.H.; Beynon, S.; Rozumalski, A.; Graham, H.K.; Tirosh, O. The gait profile score and movement analysis profile. Gait Posture 2009, 30, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, D.; Hickey, C.; Delahunt, E.; Walsh, M.; O’Brien, T. Six-minute walk test in children with spastic cerebral palsy and children developing typically. Pediatr. Phys. Ther. 2016, 28, 192–199. [Google Scholar] [CrossRef]
- Guinet, A.; Desailly, E. Six-minute walk test (6MWT) in children with cerebral palsy. Systematic review and proposal of an adapted version. Ann. Phys. Rehabil. Med. 2018, 61, e304. [Google Scholar] [CrossRef]
- Thomason, P.; Tan, A.; Donnan, A.; Rodda, J.; Graham, H.K.; Narayanan, U. The Gait Outcomes Assessment List (GOAL): Validation of a new assessment of gait function for children with cerebral palsy. Dev. Med. Child Neurol. 2018, 60, 618–623. [Google Scholar] [CrossRef]
- Palisano, R.J.; Hanna, S.E.; Rosenbaum, P.L.; Russell, D.J.; Walter, S.D.; Wood, E.P.; Raina, P.S.; Galuppi, B.E. Validation of a model of gross motor function for children with cerebral palsy. Phys. Ther. 2000, 80, 974–985. [Google Scholar] [CrossRef]
- Duran, I.; Stark, C.; Saglam, A.; Semmelweis, A.; Lioba Wunram, H.; Spiess, K.; Schoenau, E. Artificial intelligence to improve efficiency of administration of gross motor function assessment in children with cerebral palsy. Dev. Med. Child Neurol. 2021, 64, 228–234. [Google Scholar] [CrossRef]
- Brunton, L.K.; Bartlett, D.J. Validity and reliability of two abbreviated versions of the Gross Motor Function Measure. Phys. Ther. 2011, 91, 577–588. [Google Scholar] [CrossRef]
- Margaretha, V.; Prananta, M.S.; Alam, A. Correlation between gross motor function classification system and communication function classification system in children with cerebral palsy. Althea Med. J. 2017, 4, 221–227. [Google Scholar] [CrossRef]
- Mutlu, A.; Kara, Ö.K.; Livanelioğlu, A.; Karahan, S.; Alkan, H.; Yardımcı, B.N.; Hidecker, M.J.C. Agreement between parents and clinicians on the communication function levels and relationship of classification systems of children with cerebral palsy. Disabil. Health J. 2018, 11, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Noritake, K.; Sugiura, H.; Kamiya, Y.; Tomita, H.; Ito, Y.; Sugiura, H.; Ochi, N.; Yoshihashi, Y. Association between gait deviation index and physical function in children with bilateral spastic cerebral palsy: A cross-sectional study. J. Clin. Med. 2020, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Malt, M.A.; Aarli, Å.; Bogen, B.; Fevang, J.M. Correlation between the Gait Deviation Index and gross motor function (GMFCS level) in children with cerebral palsy. J. Child. Orthop. 2016, 10, 261–266. [Google Scholar] [CrossRef]
- Matsunaga, N.; Ito, T.; Noritake, K.; Sugiura, H.; Kamiya, Y.; Ito, Y.; Mizusawa, J.; Sugiura, H. Correlation between the Gait Deviation Index and skeletal muscle mass in children with spastic cerebral palsy. J. Phys. Ther. Sci. 2018, 30, 1176–1179. [Google Scholar] [CrossRef]
- Nicholson, K.; Lennon, N.; Church, C.; Miller, F. Gait analysis parameters and walking activity pre-and postoperatively in children with cerebral palsy. Pediatr. Phys. Ther. 2018, 30, 203–207. [Google Scholar] [CrossRef]
- Goudriaan, M.; Nieuwenhuys, A.; Schless, S.H.; Goemans, N.; Molenaers, G.; Desloovere, K. A new strength assessment to evaluate the association between muscle weakness and gait pathology in children with cerebral palsy. PLoS ONE 2018, 13, e0191097. [Google Scholar] [CrossRef]
- Guinet, A.L.; Néjib, K.; Eric, D. Clinical gait analysis and physical examination don’t correlate with physical activity of children with cerebral palsy. Cross-sectional study. Int. Biomech. 2020, 7, 88–96. [Google Scholar] [CrossRef]
- Shevell, M.I.; Dagenais, L.; Hall, N.; Consortium, R. The relationship of cerebral palsy subtype and functional motor impairment: A population-based study. Dev. Med. Child Neurol. 2009, 51, 872–877. [Google Scholar] [CrossRef]
- Jeon, H.; Jung, J.H.; Yoon, J.A.; Choi, H. Strabismus is correlated with gross motor function in children with spastic cerebral palsy. Curr. Eye Res. 2019, 44, 1258–1263. [Google Scholar] [CrossRef]
- Al-Nemr, A.; Abdelazeim, F. Relationship of cognitive functions and gross motor abilities in children with spastic diplegic cerebral palsy. Appl. Neuropsychol. Child 2018, 7, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Cha, Y.H.; Chun, Y.S.; Shin, H.S. Correlation of the torsion values measured by rotational profile, kinematics, and CT study in CP patients. Gait Posture 2017, 57, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Panibatla, S.; Kumar, V.; Narayan, A. Relationship between trunk control and balance in children with spastic cerebral palsy: A cross-sectional study. J. Clin. Diagn. Res. JCDR 2017, 11, YC05. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.L.; Wu, Y.Q.; Liu, X.M.; Li, A.M. Correlation of the predisposition of Chinese children to cerebral palsy with nucleotide variation in pri-miR-124 that alters the non-canonical apoptosis pathway. Acta Pharmacol. Sin. 2018, 39, 1453–1462. [Google Scholar] [CrossRef]
- Kallem Seyyar, G.; Aras, B.; Aras, O. Trunk control and functionality in children with spastic cerebral palsy. Dev. Neurorehabilit. 2019, 22, 120–125. [Google Scholar] [CrossRef]
- van Gorp, M.; Dallmeijer, A.J.; van Wely, L.; de Groot, V.; Terwee, C.B.; Flens, G.; Stam, H.J.; van der Slot, W.; Roebroeck, M.E.; on behalf of the PERRIN DECADE Study Group. Pain, fatigue, depressive symptoms and sleep disturbance in young adults with cerebral palsy. Disabil. Rehabil. 2021, 43, 2164–2171. [Google Scholar] [CrossRef]
- Monica, S.; Nayak, A.; Joshua, A.M.; Mithra, P.; Amaravadi, S.K.; Misri, Z.; Unnikrishnan, B. Relationship between Trunk Position Sense and Trunk Control in Children with Spastic Cerebral Palsy: A Cross-Sectional Study. Rehabil. Res. Pract. 2021, 2021, 9758640. [Google Scholar] [CrossRef]
- O’Sullivan, R.; French, H.P.; Van Rossom, S.; Jonkers, I.; Horgan, F. The association between gait analysis measures associated with crouch gait, functional health status and daily activity levels in cerebral palsy. J. Pediatr. Rehabil. Med. 2021, 14, 227–235. [Google Scholar] [CrossRef]
- Ries, A.J.; Novacheck, T.F.; Schwartz, M.H. A data driven model for optimal orthosis selection in children with cerebral palsy. Gait Posture 2014, 40, 539–544. [Google Scholar] [CrossRef]
- Galarraga, C.O.A.; Vigneron, V.; Dorizzi, B.; Khouri, N.; Desailly, E. Predicting postoperative gait in cerebral palsy. Gait Posture 2017, 52, 45–51. [Google Scholar] [CrossRef]
- Galarraga, O.; Vigneron, V.; Khouri, N.; Dorizzi, B.; Desailly, E. Predictive simulation of surgery effect on cerebral palsy gait. Comput. Methods Biomech. Biomed. Eng. 2017, 20, S85–S86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rosenberg, M.; Steele, K.M. Simulated impacts of ankle foot orthoses on muscle demand and recruitment in typically-developing children and children with cerebral palsy and crouch gait. PLoS ONE 2017, 12, e0180219. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, A.; Kidziński, Ł.; McGlaughlin, A.S.; Hicks, J.L.; Delp, S.L.; Schwartz, M.H. Estimating the effect size of surgery to improve walking in children with cerebral palsy from retrospective observational clinical data. Sci. Rep. 2018, 8, 16344. [Google Scholar] [CrossRef] [PubMed]
- Duran, I.; Stark, C.; Martakis, K.; Hamacher, S.; Semler, O.; Schoenau, E. Reference centiles for the gross motor function measure and identification of therapeutic effects in children with cerebral palsy. J. Eval. Clin. Pract. 2019, 25, 78–87. [Google Scholar] [CrossRef]
- Pitto, L.; Kainz, H.; Falisse, A.; Wesseling, M.; Van Rossom, S.; Hoang, H.; Papageorgiou, E.; Hallemans, A.; Desloovere, K.; Molenaers, G.; et al. SimCP: A simulation platform to predict gait performance following orthopedic intervention in children with cerebral palsy. Front. Neurorobot. 2019, 13, 54. [Google Scholar] [CrossRef]
- Kidziński, Ł.; Yang, B.; Hicks, J.L.; Rajagopal, A.; Delp, S.L.; Schwartz, M.H. Deep neural networks enable quantitative movement analysis using single-camera videos. Nat. Commun. 2020, 11, 4054. [Google Scholar] [CrossRef]
- Jalata, I.K.; Truong, T.D.; Allen, J.L.; Seo, H.S.; Luu, K. Movement Analysis for Neurological and Musculoskeletal Disorders Using Graph Convolutional Neural Network. Future Internet 2021, 13, 194. [Google Scholar] [CrossRef]
- Azhand, A.; Rabe, S.; Müller, S.; Sattler, I.; Heimann-Steinert, A. Algorithm based on one monocular video delivers highly valid and reliable gait parameters. Sci. Rep. 2021, 11, 14065. [Google Scholar] [CrossRef]
- Afifi, J. Prediction of Cerebral Palsy in Very Preterm Infants. Ph.D. Thesis, Dalhousie University Halifax, Halifax, NS, Canada, 2021. [Google Scholar]
- Russell, D.J.; Avery, L.M.; Rosenbaum, P.L.; Raina, P.S.; Walter, S.D.; Palisano, R.J. Improved scaling of the gross motor function measure for children with cerebral palsy: Evidence of reliability and validity. Phys. Ther. 2000, 80, 873–885. [Google Scholar] [CrossRef]
- Pietrzak, S.; Jóźwiak, M. Subjective and objective scales to assess the development of children cerebral palsy. Ortop. Traumatol. Rehabil. 2001, 3, 487–489. [Google Scholar]
- Russell, D.J.; Rosenbaum, P.L.; Cadman, D.T.; Gowland, C.; Hardy, S.; Jarvis, S. The gross motor function measure: A means to evaluate the effects of physical therapy. Dev. Med. Child Neurol. 1989, 31, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Kim, M. Reliability and responsiveness of the gross motor function measure-88 in children with cerebral palsy. Phys. Ther. 2013, 93, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, M.; Long, T.; Kennedy, E.; Bavishi, S. The efficacy of GMFM-88 and GMFM-66 to detect changes in gross motor function in children with cerebral palsy (CP): A literature review. Disabil. Rehabil. 2014, 36, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Engelen, V.; Ketelaar, M.; Gorter, J.W. Selecting the appropriate outcome in paediatric physical therapy: How individual treatment goals for children with cerebral palsy are reflected in GMFM-88 and PEDI. J. Rehabil. Med. 2007, 39, 225–231. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zebris Medical GmbH. Zebris FDM-T System Specifications and Operating Instructions. Available online: https://www.zebris.de/fileadmin/Editoren/zebris-PDF-Manuals/Medizin/Hardware/Alte_Versionen/FDM-T_Hardware-Manual_160119_en.pdf (accessed on 19 October 2021).
- Zebris Medical GmbH. The Zebris FDM-T System for Stance and Gait Analysis. Available online: https://www.zebris.de/fileadmin/Editoren/zebris-PDF/zebris-Prospekte-EN/FDM-T_Prospekt_en_120901_72dpi.pdf (accessed on 20 October 2021).
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning with Applications in R, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- MLlib: Main Guide. Classification and Regression, Version 3.2.0. Available online: https://spark.apache.org/docs/3.2.0/ml-guide.html (accessed on 20 October 2021).
- Barten, A.P. The coefficient of determination for regression without a constant term. In The Practice of Econometrics: Studies on Demand, Forecasting, Money and Income; Springer: Dordrecht, The Netherlands, 1987; pp. 181–189. [Google Scholar] [CrossRef]
- Chicco, D.; Warrens, M.J.; Jurman, G. The coefficient of determination R-squared is more informative than SMAPE, MAE, MAPE, MSE and RMSE in regression analysis evaluation. PeerJ Comput. Sci. 2021, 7, e623. [Google Scholar] [CrossRef] [PubMed]
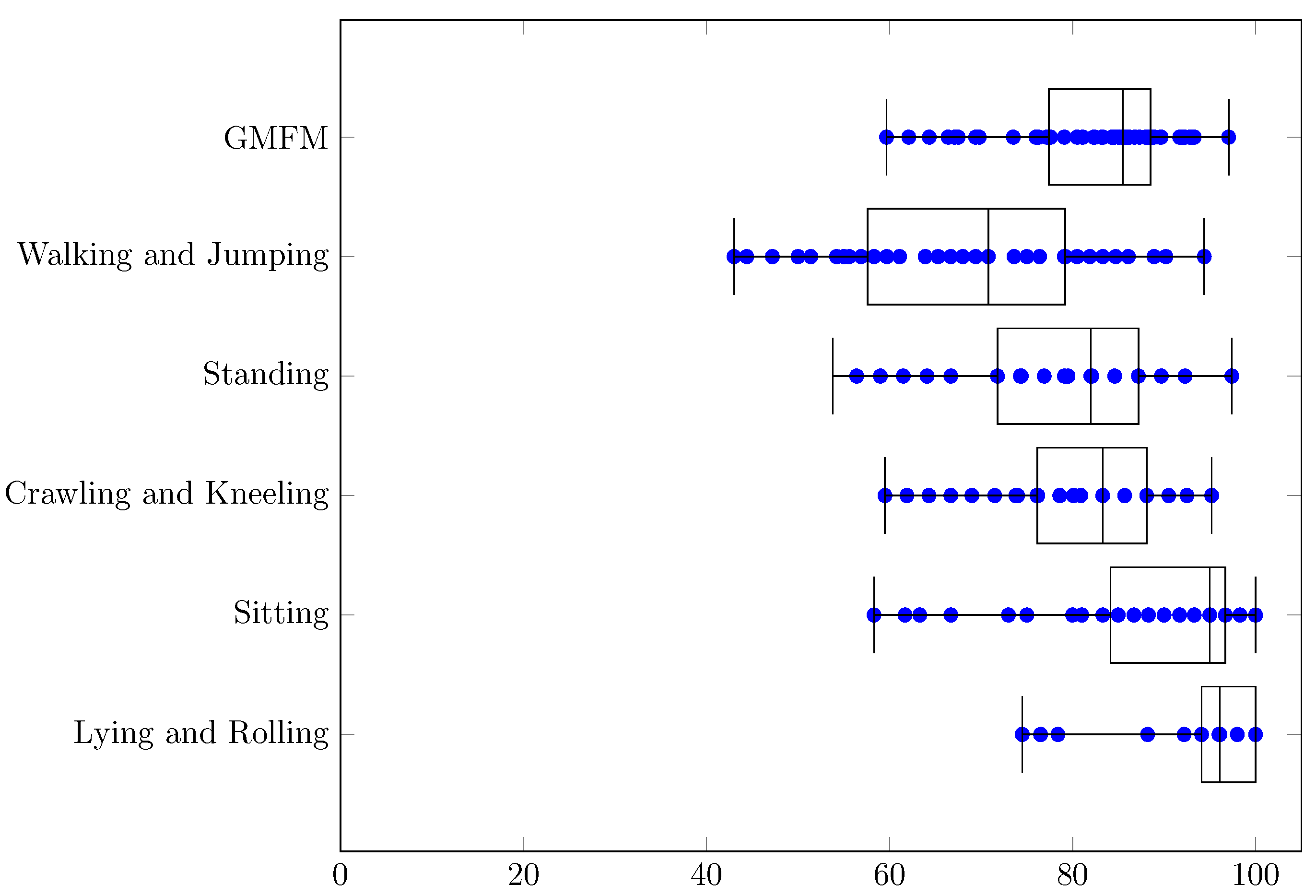
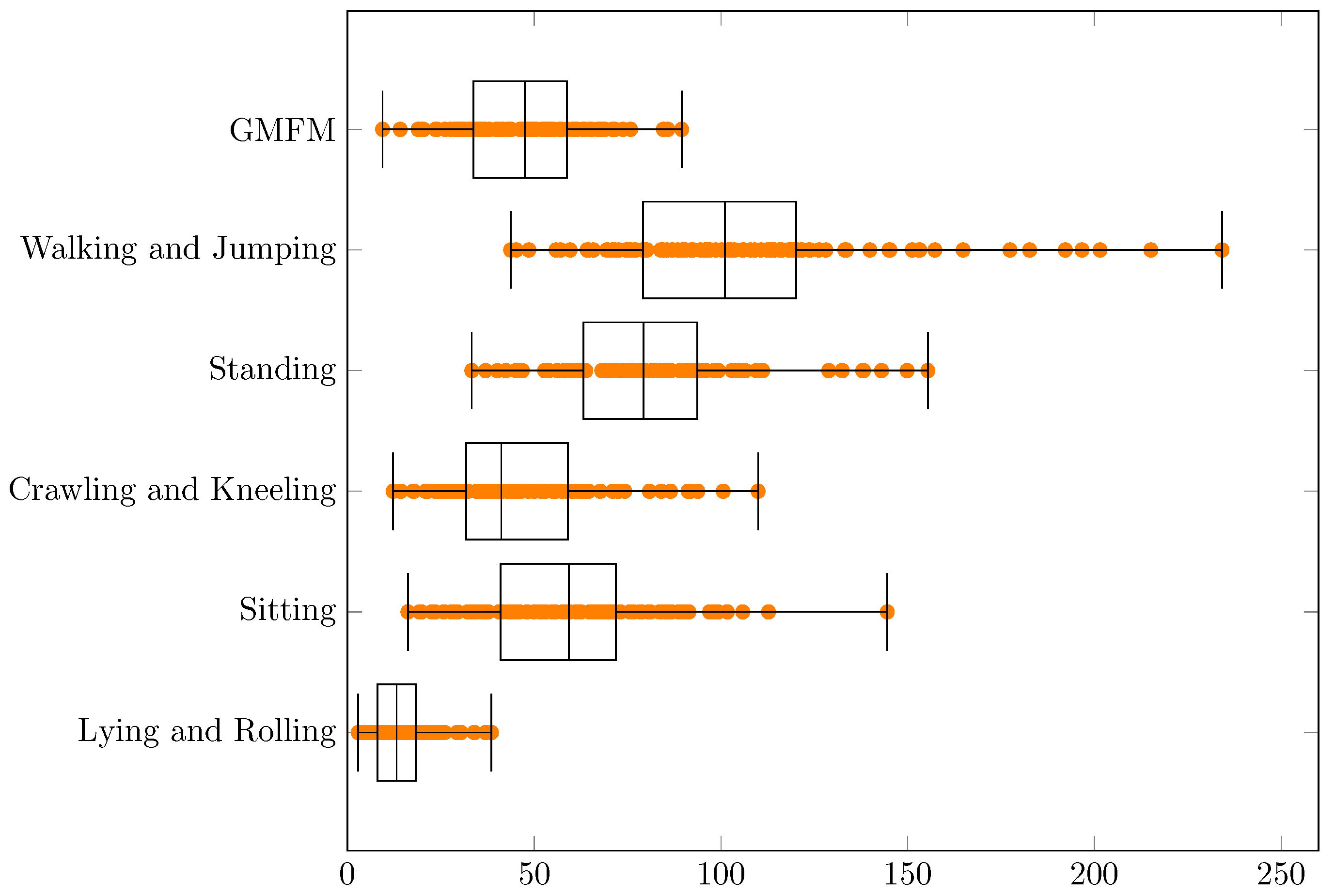

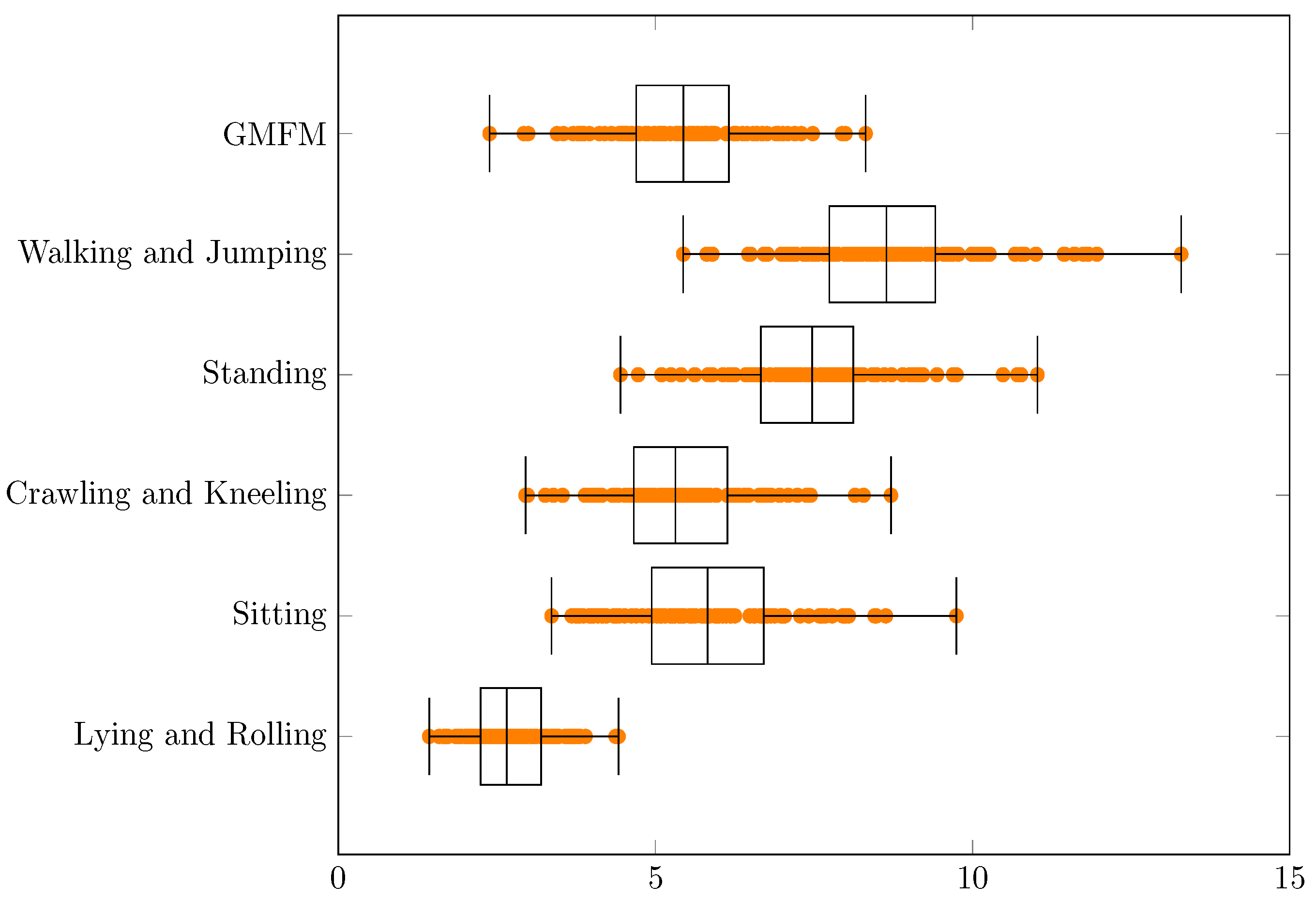
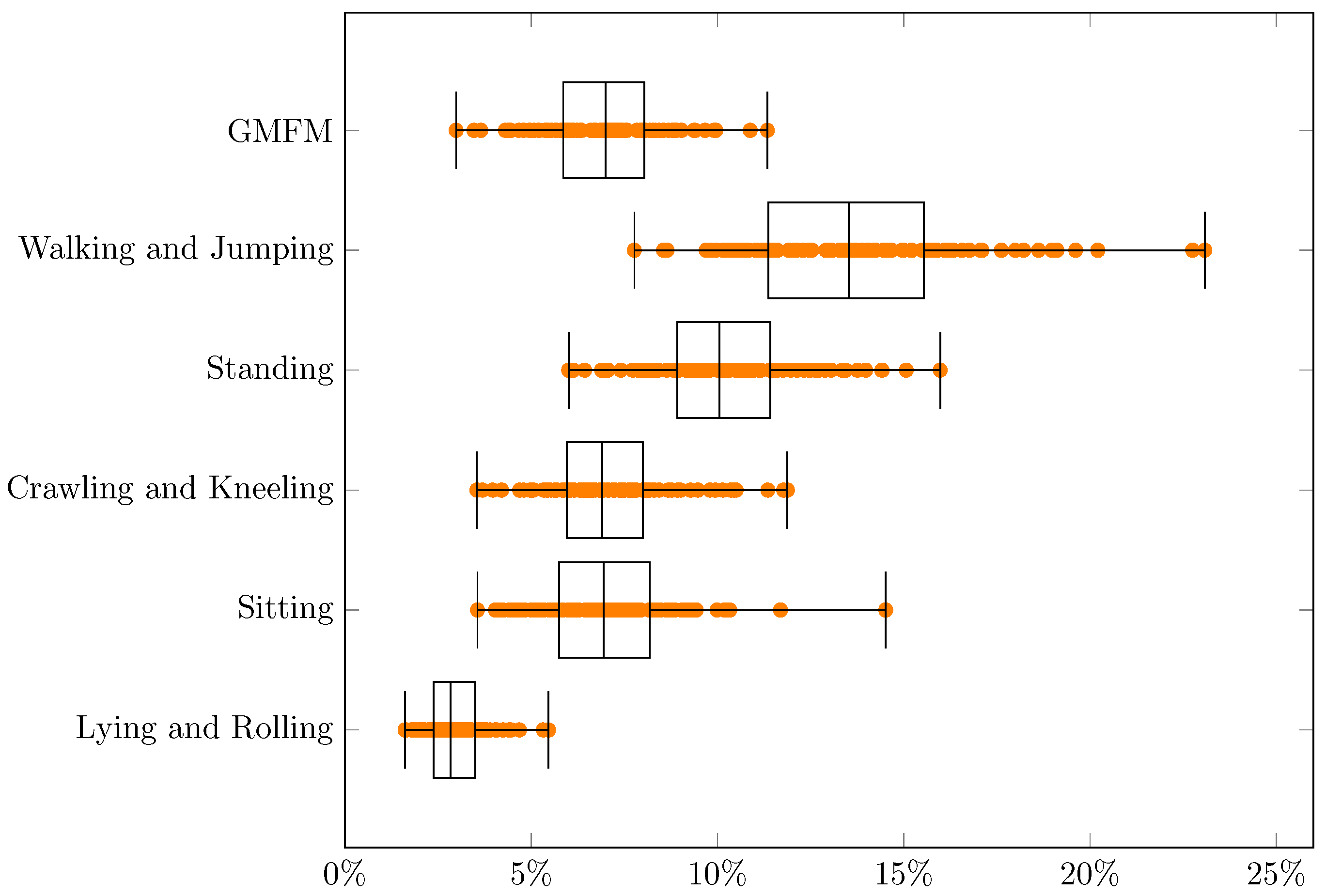
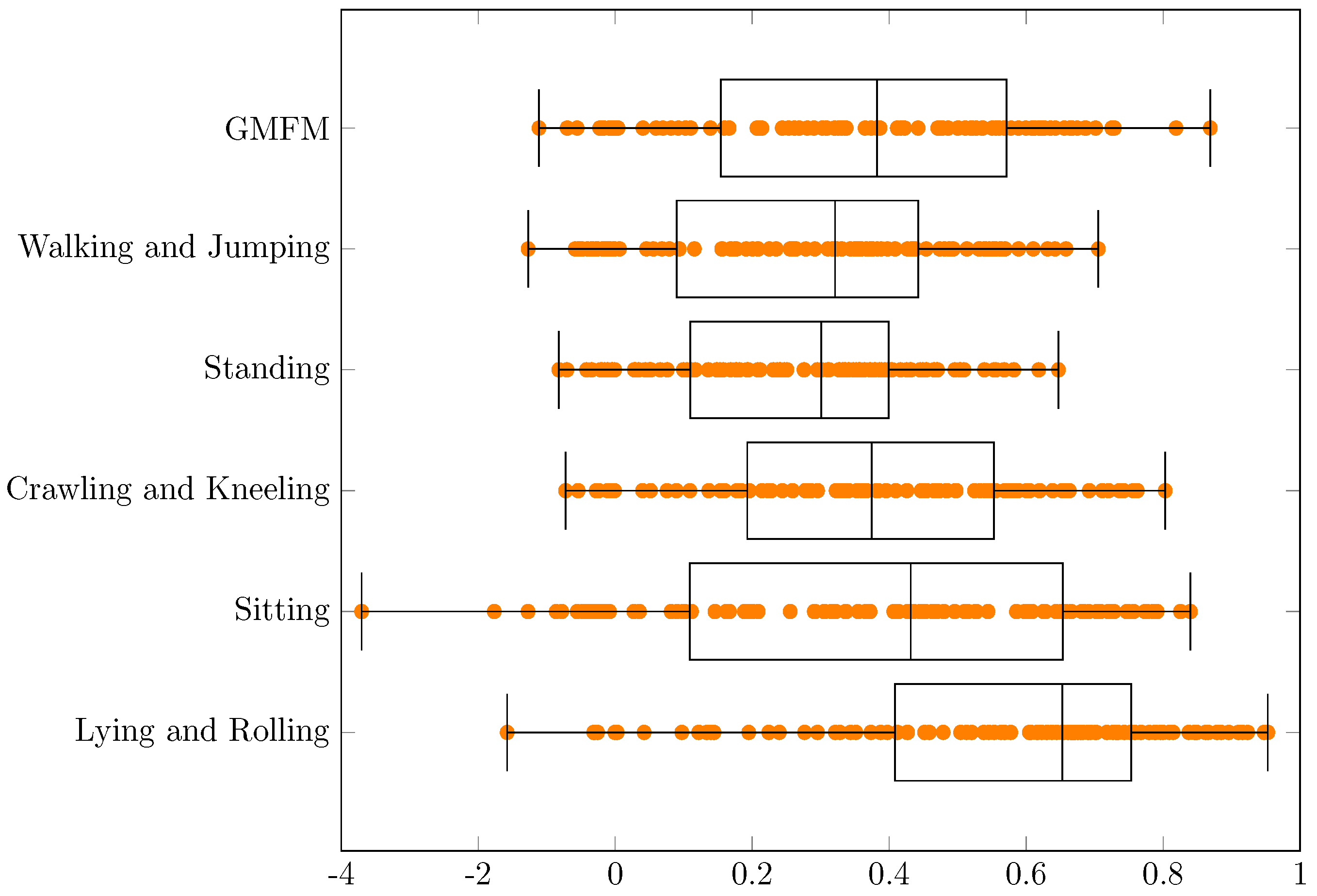
| Min | q1 | Median | q3 | Max | q3 − q1 | Range | |
|---|---|---|---|---|---|---|---|
| GMFM | 9.4 | 33.7 | 47.5 | 58.7 | 89.5 | 25.0 | 80.1 |
| Walking and Jumping | 43.7 | 79.1 | 101.1 | 120.1 | 234.2 | 41.0 | 190.5 |
| Standing | 33.3 | 63.2 | 79.3 | 93.7 | 155.4 | 30.5 | 122.2 |
| Crawling and Kneeling | 12.2 | 31.8 | 41.2 | 59.0 | 109.9 | 27.3 | 97.7 |
| Sitting | 16.1 | 40.9 | 59.3 | 71.9 | 144.5 | 30.9 | 128.4 |
| Lying and Rolling | 2.8 | 8.0 | 13.1 | 18.2 | 38.5 | 10.2 | 35.7 |
| Min | q1 | Median | q3 | Max | q3 − q1 | Range | |
|---|---|---|---|---|---|---|---|
| GMFM | 3.1 | 5.8 | 6.9 | 7.7 | 9.5 | 1.9 | 6.4 |
| Walking and Jumping | 6.6 | 8.9 | 10.1 | 11.0 | 15.3 | 2.1 | 8.7 |
| Standing | 5.8 | 7.9 | 8.9 | 9.7 | 12.5 | 1.7 | 6.7 |
| Crawling and Kneeling | 3.5 | 5.6 | 6.4 | 7.7 | 10.5 | 2.0 | 7.0 |
| Sitting | 4.0 | 6.4 | 7.7 | 8.5 | 12.0 | 2.1 | 8.0 |
| Lying and Rolling | 1.7 | 2.8 | 3.6 | 4.3 | 6.2 | 1.4 | 4.5 |
| Min | q1 | Median | q3 | Max | q3 − q1 | Range | |
|---|---|---|---|---|---|---|---|
| GMFM | 2.4 | 4.7 | 5.4 | 6.2 | 8.3 | 1.5 | 5.9 |
| Walking and Jumping | 5.4 | 7.7 | 8.6 | 9.4 | 13.3 | 1.7 | 7.9 |
| Standing | 4.4 | 6.7 | 7.5 | 8.1 | 11.0 | 1.5 | 6.6 |
| Crawling and Kneeling | 3.0 | 4.7 | 5.3 | 6.1 | 8.7 | 1.5 | 5.8 |
| Sitting | 3.4 | 4.9 | 5.8 | 6.7 | 9.7 | 1.8 | 6.4 |
| Lying and Rolling | 1.4 | 2.2 | 2.7 | 3.2 | 4.4 | 1.0 | 3.0 |
| Min | q1 | Median | q3 | Max | q3 − q1 | Range | |
|---|---|---|---|---|---|---|---|
| GMFM | 3.0% | 5.9% | 7.0% | 8.0% | 11.3% | 2.2% | 8.4% |
| Walking and Jumping | 7.8% | 11.4% | 13.5% | 15.5% | 23.1% | 4.2% | 15.3% |
| Standing | 6.0% | 8.9% | 10.1% | 11.4% | 16.0% | 2.5% | 10.0% |
| Crawling and Kneeling | 3.5% | 6.0% | 6.9% | 8.0% | 11.9% | 2.0% | 8.3% |
| Sitting | 3.6% | 5.7% | 6.9% | 8.2% | 14.5% | 2.4% | 11.0% |
| Lying and Rolling | 1.6% | 2.4% | 2.8% | 3.5% | 5.5% | 1.1% | 3.9% |
| Min | q1 | Median | q3 | Max | q3 − q1 | Range | |
|---|---|---|---|---|---|---|---|
| GMFM | −1.11 | 0.15 | 0.38 | 0.57 | 0.87 | 0.42 | 1.98 |
| Walking and Jumping | −1.27 | 0.09 | 0.32 | 0.44 | 0.71 | 0.35 | 1.98 |
| Standing | −0.82 | 0.11 | 0.30 | 0.40 | 0.65 | 0.29 | 1.47 |
| Crawling and Kneeling | −0.72 | 0.19 | 0.37 | 0.55 | 0.80 | 0.36 | 1.53 |
| Sitting | −3.70 | 0.11 | 0.43 | 0.65 | 0.84 | 0.54 | 4.54 |
| Lying and Rolling | −1.58 | 0.41 | 0.65 | 0.75 | 0.95 | 0.35 | 2.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bedla, M.; Pięta, P.; Kaczmarski, D.; Deniziak, S. Estimation of Gross Motor Functions in Children with Cerebral Palsy Using Zebris FDM-T Treadmill. J. Clin. Med. 2022, 11, 954. https://doi.org/10.3390/jcm11040954
Bedla M, Pięta P, Kaczmarski D, Deniziak S. Estimation of Gross Motor Functions in Children with Cerebral Palsy Using Zebris FDM-T Treadmill. Journal of Clinical Medicine. 2022; 11(4):954. https://doi.org/10.3390/jcm11040954
Chicago/Turabian StyleBedla, Mariusz, Paweł Pięta, Daniel Kaczmarski, and Stanisław Deniziak. 2022. "Estimation of Gross Motor Functions in Children with Cerebral Palsy Using Zebris FDM-T Treadmill" Journal of Clinical Medicine 11, no. 4: 954. https://doi.org/10.3390/jcm11040954
APA StyleBedla, M., Pięta, P., Kaczmarski, D., & Deniziak, S. (2022). Estimation of Gross Motor Functions in Children with Cerebral Palsy Using Zebris FDM-T Treadmill. Journal of Clinical Medicine, 11(4), 954. https://doi.org/10.3390/jcm11040954






