rTMS Reduces Craving and Alcohol Use in Patients with Alcohol Use Disorder: Results of a Randomized, Sham-Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
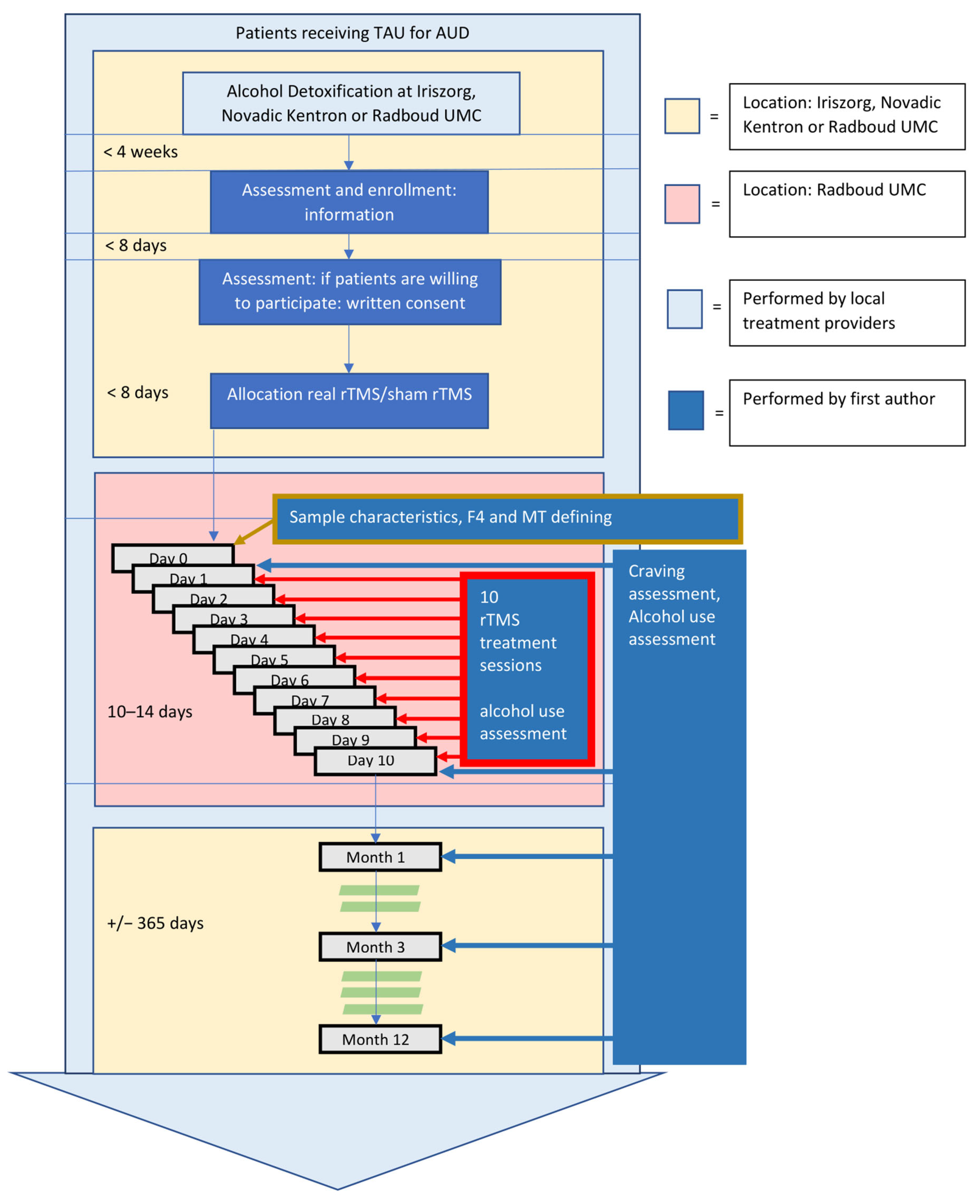
2.1. Study Design
2.2. Study Sample
2.3. Treatment
2.3.1. TAU
2.3.2. rTMS
2.3.3. Sham rTMS
2.4. Measurements
2.4.1. Sample and Treatment Characteristics
2.4.2. Outcome Measurements
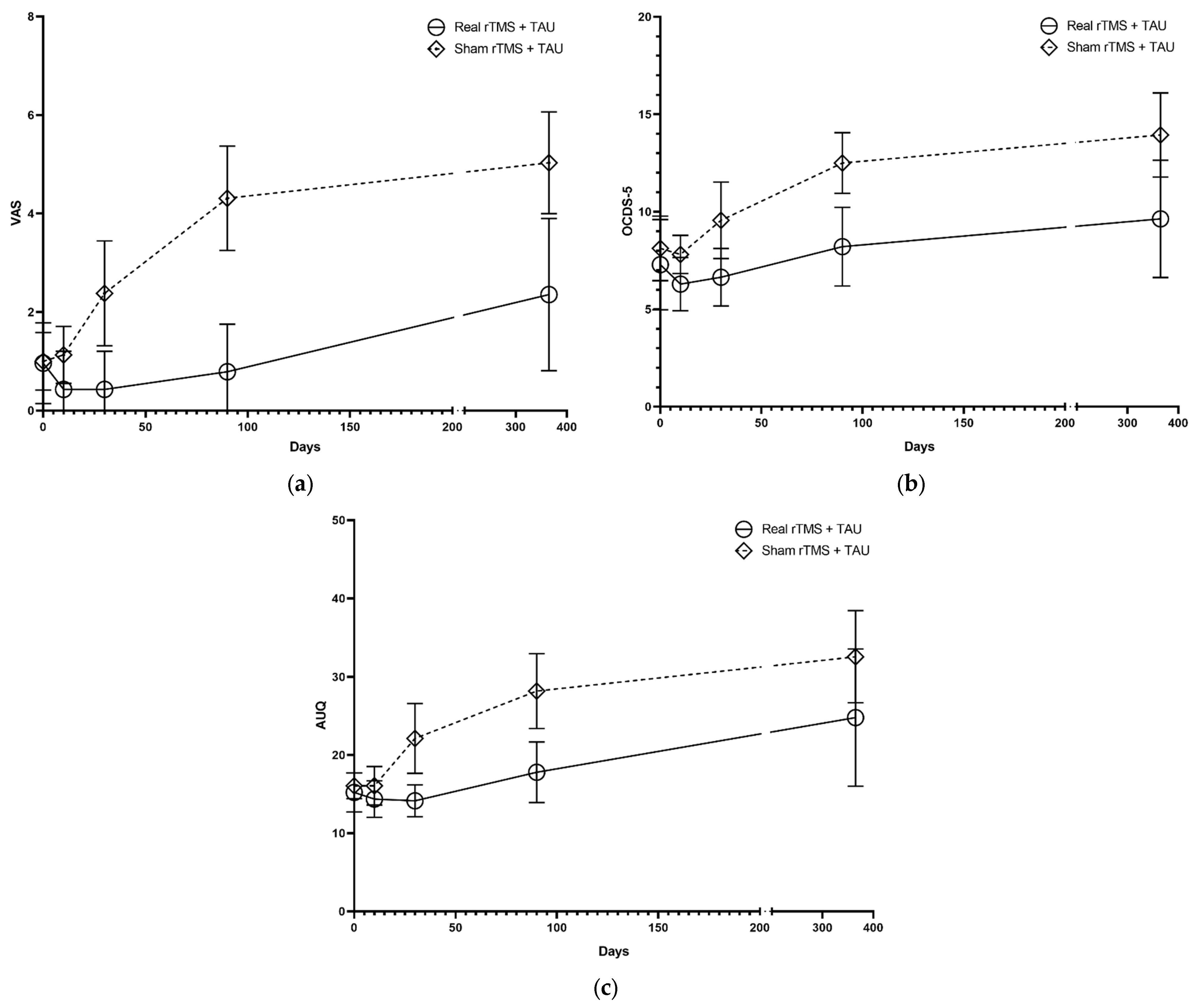
- Visual Analog Scale (VAS). The VAS is commonly used in studies to assess the severity of craving in patients with substance use disorder [31]. A total VAS score was defined by the mean of two VAS scores (on a 100 mm line, with anchor points of 0 (not at all/don’t agree at all) and 10 (desperately/totally agree)) on the question “How much do you want a drink at this moment?” and the statement “If I could drink, I would probably do it”.
- Alcohol Urge Questionnaire (AUQ). The AUQ is a validated instrument (Cronbach α = 0.918; test-retest reliability r = 0,82). It measures momentary alcohol craving in patients with AUD [32,33]. It contains eight items referring to statements such as the desire to drink, the expectation of the desired outcome from drinking, and the inability to avoid drinking if alcohol was available at that moment. Participants indicated their level of agreement on a seven-point scale (range 1–7, with anchor points: “Strongly disagree” and “Strongly agree”). A total score is calculated by summation of the item scores, with reversed scoring for two items. A higher score reflects a higher level of craving.
- Obsessive-Compulsive Drinking Scale—short version (OCDS-5). The OCDS-5 is a shortened version of the original OCDS [34] (Cronbach α = 0.814). The OCDS-5 is widely used in addiction treatment to measure mean craving over the past seven days [35]. It contains five items in a five-point (0–4) Likert-type scale format [36]. A total score is calculated by summation of the points attributed per item. Higher scores are indicative of higher craving levels.
- At baseline (day 1 of the rTMS treatment) and at the start of each rTMS treatment session (2nd–10th day), participants were asked about their alcohol use since the last treatment.
- At follow-up 1, 3, and 12 months after rTMS treatment, alcohol use was assessed using the TimeLine Follow-Back (TLFB) method over the previous period of 1 month. The TLFB is a validated instrument to systematically estimate alcohol use over a specified timeframe (Spearman’s ρ = 0.93) [37,38]. Participants indicated the number of days they had drunk alcohol and the quantity and type of beverage they had consumed, noted as the quantity of a standard drink (containing 10 mg alcohol).
2.5. Procedure
2.6. Analyses
2.6.1. Sample and Treatment Characteristics and Outcome Variables at Baseline
2.6.2. Craving
2.6.3. Alcohol Use
3. Results
3.1. Craving
3.2. Alcohol Outcome Measurements
3.3. Side Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Publishing. Diagnostic and Statistical Manual of Mental Disorder, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Stohs, M.E.; Schneekloth, T.D.; Geske, J.R.; Biernacka, J.M.; Karpyak, V.M. Alcohol Craving Predicts Relapse After Residential Addiction Treatment. Alcohol Alcohol. 2019, 54, 167–172. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Kendler, K.S.; Ohlsson, H.; Sundquist, J.; Sundquist, K. Alcohol Use Disorder and Mortality Across the Lifespan: A Longitudinal Cohort and Co-relative Analysis. JAMA Psychiatry 2016, 73, 575–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittchen, H.U.; Jacobi, F.; Rehm, J.; Gustavsson, A.; Svensson, M.; Jonsson, B.; Olesen, J.; Allgulander, C.; Alonso, J.; Faravelli, C.; et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 655–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, G.W.; Rehm, J. The effectiveness of psychosocial modalities in the treatment of alcohol problems in adults: A review of the evidence. Can. J. Psychiatry 2012, 57, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Lev-Ran, S.; Balchand, K.; Lefebvre, L.; Araki, K.; Le Foll, B. Pharmacotherapy of Alcohol Use Disorders and Concurrent Psychiatric Disorders: A Review. Can. J. Psychiatry 2012, 57, 342–349. [Google Scholar] [CrossRef] [Green Version]
- Goh, E.T.; Morgan, M.Y. Review article: Pharmacotherapy for alcohol dependence—the why, the what and the wherefore. Aliment. Pharmacol. Ther. 2017, 45, 865–882. [Google Scholar] [CrossRef] [Green Version]
- Agosti, V.; Nunes, E.V.; O’Shea, D. Do manualized psychosocial interventions help reduce relapse among alcohol-dependent adults treated with naltrexone or placebo? A meta-analysis. Am. J. Addict. 2012, 21, 501–507. [Google Scholar] [CrossRef]
- Luigjes, J.; Segrave, R.; de Joode, N.; Figee, M.; Denys, D. Efficacy of Invasive and Non-Iinvasive Brain Modulation Interventions for Addiction. Neuropsychol. Rev. 2019, 29, 116–138. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, E.V.; Pfefferbaum, A. Neurocircuitry in alcoholism: A substrate of disruption and repair. Psychopharmacology 2005, 180, 583–594. [Google Scholar] [CrossRef]
- Jansen, J.M.; Daams, J.G.; Koeter, M.W.; Veltman, D.J.; van den Brink, W.; Goudriaan, A.E. Effects of non-invasive neurostimulation on craving: A meta-analysis. Neurosci. Biobehav. Rev. 2013, 37, 2472–2480. [Google Scholar] [CrossRef]
- Enokibara, M.; Trevizol, A.; Shiozawa, P.; Cordeiro, Q. Establishing an effective TMS protocol for craving in substance addiction: Is it possible? Am. J. Addict. 2016, 25, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Coles, A.S.; Kozak, K.; George, T.P. A review of brain stimulation methods to treat substance use disorders. Am. J. Addict. 2018, 27, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zilverstand, A.; Gui, W.; Pan, X.; Zhou, X. Reducing craving and consumption in individuals with drug addiction, obesity or overeating through neuromodulation intervention: A systematic review and meta-analysis of its follow-up effects. Addiction 2021. [Google Scholar] [CrossRef] [PubMed]
- Maiti, R.; Mishra, B.R.; Hota, D. Effect of High-Frequency Transcranial Magnetic Stimulation on Craving in Substance Use Disorder: A Meta-Analysis. J. Neuropsychiatry Clin. Neurosci. 2017, 29, 160–171. [Google Scholar] [CrossRef]
- Ekhtiari, H.; Tavakoli, H.; Addolorato, G.; Baeken, C.; Bonci, A.; Campanella, S.; Castelo-Branco, L.; Challet-Bouju, G.; Clark, V.P.; Claus, E.; et al. Transcranial electrical and magnetic stimulation (tES and TMS) for addiction medicine: A consensus paper on the present state of the science and the road ahead. Neurosci. Biobehav. Rev. 2019, 104, 118–140. [Google Scholar] [CrossRef]
- Maatoug, R.; Bihan, K.; Duriez, P.; Podevin, P.; Silveira-Reis-Brito, L.; Benyamina, A.; Valero-Cabre, A.; Millet, B. Non-invasive and invasive brain stimulation in alcohol use disorders: A critical review of selected human evidence and methodological considerations to guide future research. Compr. Psychiatry 2021, 109, 152257. [Google Scholar] [CrossRef]
- Perini, I.; Kampe, R.; Arlestig, T.; Karlsson, H.; Lofberg, A.; Pietrzak, M.; Zangen, A.; Heilig, M. Repetitive transcranial magnetic stimulation targeting the insular cortex for reduction of heavy drinking in treatment-seeking alcohol-dependent subjects: A randomized controlled trial. Neuropsychopharmacology 2020, 45, 842–850. [Google Scholar] [CrossRef]
- Schluter, R.S.; van Holst, R.J.; Goudriaan, A.E. Effects of Ten Sessions of High Frequency Repetitive Transcranial Magnetic Stimulation (HF-rTMS) Add-on Treatment on Impulsivity in Alcohol Use Disorder. Front. Neurosci. 2019, 13, 1257. [Google Scholar] [CrossRef]
- First, M.B.; Williams, J.B.W.; Karg, R.S.; Spitzer, R.L. SCID-5-S Gestructureerd Klinisch Interview Voor DSM-5 Syndroomstoornissen; Boom Uitgevers Amsterdam: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Magstim Rapid2. Available online: https://www.magstim.com/row-en/product/rapid-family/ (accessed on 1 May 2016).
- Herwig, U.; Satrapi, P.; Schonfeldt-Lecuona, C. Using the international 10-20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr. 2003, 16, 95–99. [Google Scholar] [CrossRef]
- Ma, T.; Sun, Y.; Ku, Y. Effects of Non-invasive Brain Stimulation on Stimulant Craving in Users of Cocaine, Amphetamine, or Methamphetamine: A Systematic Review and Meta-Analysis. Front. Neurosci. 2019, 13, 1095. [Google Scholar] [CrossRef]
- Rossi, S.; Antal, A.; Bestmann, S.; Bikson, M.; Brewer, C.; Brockmoller, J.; Carpenter, L.L.; Cincotta, M.; Chen, R.; Daskalakis, J.D.; et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin. Neurophysiol. 2021, 132, 269–306. [Google Scholar] [CrossRef] [PubMed]
- Hoogendam, J.M.; Ramakers, G.M.; Di Lazzaro, V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010, 3, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Razza, L.B.; Moffa, A.H.; Moreno, M.L.; Carvalho, A.F.; Padberg, F.; Fregni, F.; Brunoni, A.R. A systematic review and meta-analysis on placebo response to repetitive transcranial magnetic stimulation for depression trials. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 81, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Lisanby, S.H.; Gutman, D.; Luber, B.; Schroeder, C.; Sackeim, H.A. Sham TMS: Intracerebral measurement of the induced electrical field and the induction of motor-evoked potentials. Biol. Psychiatry 2001, 49, 460–463. [Google Scholar] [CrossRef] [Green Version]
- Schmand, B.; Bakker, D.; Saan, R.; Louman, J. The Dutch Reading Test for Adults: A measure of premorbid intelligence level. Tijdschr. Gerontol. Geriatr. 1991, 22, 15–19. [Google Scholar]
- Sheehan, D.; Lecrubier, Y.; Sheehan, K.; Amorim, P.; Janavs, J.; Weiler, E. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59, 20–33. [Google Scholar]
- Beurmanjer, H.; Luykx, J.J.; De Wilde, B.; van Rompaey, K.; Buwalda, V.J.A.; De Jong, C.A.J.; Dijkstra, B.A.G.; Schellekens, A.F.A. Tapering with Pharmaceutical GHB or Benzodiazepines for Detoxification in GHB-Dependent Patients: A Matched-Subject Observational Study of Treatment-as-Usual in Belgium and The Netherlands. CNS Drugs 2020, 34, 651–659. [Google Scholar] [CrossRef]
- Bohn, M.J.; Krahn, D.D.; Staehler, B.A. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol. Clin. Exp. Res. 1995, 19, 600–606. [Google Scholar] [CrossRef]
- MacKillop, J. Factor structure of the alcohol urge questionnaire under neutral conditions and during a cue-elicited urge state. Alcohol. Clin. Exp. Res. 2006, 30, 1315–1321. [Google Scholar] [CrossRef]
- Anton, R.F.; Moak, D.H.; Latham, P. The Obsessive Compulsive Drinking Scale: A self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol. Clin. Exp. Res. 1995, 19, 92–99. [Google Scholar] [CrossRef]
- Schippers, G.M.; Broekman, T.G.; Buchholz, A.; Koeter, M.W.; van den Brink, W. Measurements in the Addictions for Triage and Evaluation (MATE): An instrument based on the World Health Organization family of international classifications. Addiction 2010, 105, 862–871. [Google Scholar] [CrossRef] [PubMed]
- De Wildt, W.A.; Lehert, P.; Schippers, G.M.; Nakovics, H.; Mann, K.; van den Brink, W. Investigating the structure of craving using structural equation modeling in analysis of the obsessive-compulsive drinking scale: A multinational study. Alcohol. Clin. Exp. Res. 2005, 29, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Sobell, L.C.; Brown, J.; Leo, G.I.; Sobell, M.B. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend. 1996, 42, 49–54. [Google Scholar] [CrossRef]
- Maisto, S.A.; Conigliaro, J.C.; Gordon, A.J.; McGinnis, K.A.; Justice, A.C. An experimental study of the agreement of self-administration and telephone administration of the Timeline Followback interview. J. Stud. Alcohol Drugs 2008, 69, 468–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehm, J. How should prevalence of alcohol use disorders be assessed globally? Int. J. Methods Psychiatr. Res. 2016, 25, 79–85. [Google Scholar] [CrossRef] [PubMed]
- IBM. IBM SPSS Statistics for Windows; Version 27.0; IBM Corp: Armonk, NY, USA, 2020. [Google Scholar]
- Antonelli, M.; Fattore, L.; Sestito, L.; Di Giuda, D.; Diana, M.; Addolorato, G. Transcranial Magnetic Stimulation: A review about its efficacy in the treatment of alcohol, tobacco and cocaine addiction. Addict. Behav. 2021, 114, 106760. [Google Scholar] [CrossRef] [PubMed]
- Herremans, S.C.; Baeken, C.; Vanderbruggen, N.; Vanderhasselt, M.A.; Zeeuws, D.; Santermans, L.; De Raedt, R. No influence of one right-sided prefrontal HF-rTMS session on alcohol craving in recently detoxified alcohol-dependent patients: Results of a naturalistic study. Drug Alcohol Depend. 2012, 120, 209–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNeill, A.; Monk, R.L.; Qureshi, A.W.; Makris, S.; Heim, D. Continuous Theta Burst Transcranial Magnetic Stimulation of the Right Dorsolateral Prefrontal Cortex Impairs Inhibitory Control and Increases Alcohol Consumption. Cogn. Affect. Behav. Neurosci. 2018, 18, 1198–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Felice, A.; Bellamoli, E.; Formaggio, E.; Manganotti, P.; Masiero, S.; Cuoghi, G.; Rimondo, C.; Genetti, B.; Sperotto, M.; Corso, F.; et al. Neurophysiological, psychological and behavioural correlates of rTMS treatment in alcohol dependence. Drug Alcohol Depend. 2016, 158, 147–153. [Google Scholar] [CrossRef]
- Raikwar, S.; Divinakumar, K.J.; Prakash, J.; Khan, S.A.; GuruPrakash, K.V.; Batham, S. A sham-controlled trial of repetitive transcranial magnetic stimulation over left dorsolateral prefrontal cortex and its effects on craving in patients with alcohol dependence. Ind. Psychiatry J. 2020, 29, 245–250. [Google Scholar] [CrossRef]
- Rapinesi, C.; Kotzalidis, G.D.; Serata, D.; Del Casale, A.; Bersani, F.S.; Solfanelli, A.; Scatena, P.; Raccah, R.N.; Brugnoli, R.; Digiacomantonio, V.; et al. Efficacy of add-on deep transcranial magnetic stimulation in comorbid alcohol dependence and dysthymic disorder: Three case reports. Prim. Care Companion CNS Disord. 2013, 15. [Google Scholar] [CrossRef] [PubMed]
- Dunner, D.L.; Aaronson, S.T.; Sackeim, H.A.; Janicak, P.G.; Carpenter, L.L.; Boyadjis, T.; Brock, D.G.; Bonneh-Barkay, D.; Cook, I.A.; Lanocha, K.; et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: Durability of benefit over a 1-year follow-up period. J. Clin. Psychiatry 2014, 75, 1394–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, A.; Pavlicova, M.; Avery, D.; Nahas, Z.; McDonald, W.M.; Wajdik, C.D.; Holtzheimer, P.E., III; George, M.S.; Sackeim, H.A.; Lisanby, S.H. Long-term efficacy of repeated daily prefrontal transcranial magnetic stimulation (TMS) in treatment-resistant depression. Depress Anxiety 2012, 29, 883–890. [Google Scholar] [CrossRef]
- Philip, N.S.; Dunner, D.L.; Dowd, S.M.; Aaronson, S.T.; Brock, D.G.; Carpenter, L.L.; Demitrack, M.A.; Hovav, S.; Janicak, P.G.; George, M.S. Can Medication Free, Treatment-Resistant, Depressed Patients Who Initially Respond to TMS Be Maintained Off Medications? A Prospective, 12-Month Multisite Randomized Pilot Study. Brain Stimul. 2016, 9, 251–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connor, J.P.; Feeney, G.F.; Young, R.M. A comparison of the Yale-Brown Obsessive Compulsive Scale for "heavy drinking" with a single item craving measure: Construct validity and clinical utility. Subst. Use Misuse 2005, 40, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.P.; Jack, A.; Feeney, G.F.; Young, R.M. Validity of the obsessive compulsive drinking scale in a heavy drinking population. Alcohol. Clin. Exp. Res. 2008, 32, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, E.; Koski-Jannes, A.; Salaspuro, M.; Ahtinen, H.; Alho, H. A randomized, multicentre, open-label, comparative trial of disulfiram, naltrexone and acamprosate in the treatment of alcohol dependence. Alcohol Alcohol. 2008, 43, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Rubio, G.; Jimenez-Arriero, M.A.; Ponce, G.; Palomo, T. Naltrexone versus acamprosate: One year follow-up of alcohol dependence treatment. Alcohol Alcohol. 2001, 36, 419–425. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, S.; Tulachan, P.; Ojha, S.P.; Chapagai, M.; Dhungana, S.; Pant, S.B. Comparison of Disulfiram and Naltrexone in Cases of Alcohol Dependence Syndrome. J. Nepal Health Res. Counc. 2020, 18, 75–81. [Google Scholar] [CrossRef]
- Van Amsterdam, J.; van den Brink, W. Reduced-risk drinking as a viable treatment goal in problematic alcohol use and alcohol dependence. J. Psychopharmacol. 2013, 27, 987–997. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Edwards, M.J.; Rounis, E.; Bhatia, K.P.; Rothwell, J.C. Theta burst stimulation of the human motor cortex. Neuron 2005, 45, 201–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopala-Sibley, D.C.; Chartier, G.B.; Bhanot, S.; Cole, J.; Chan, P.Y.; Berlim, M.T.; McGirr, A. Personality trait predictive utility and stability in transcranial magentic stimulation (rTMS) for Major Depression: Dissociation of neuroticism and sefl-criticism. Can. J. Psychiatry 2020, 65, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.; Mitjans, M.; Nitsche, M.A.; Kuo, M.F.; Ehrenreich, H. Excitation-inhibition dysbalance as predictor of autistic phenotypes. J. Psychiatr. Res. 2018, 104, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Ward, H.B.; Yip, A.; Siddiqui, R.; Morales, O.G.; Seiner, S.J.; Siddiqi, S.H. Borderline personality traits do not influence response to TMS. J. Affect. Disord. 2021, 281, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, S.A.; Khaleghi, A.; Mohammadi, M.R. Noninvasive brain stimulation in alcohol craving: A systematic review and meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 101, 109938. [Google Scholar] [CrossRef]
- Notzon, S.; Steinberg, C.; Zwanzger, P.; Junghofer, M. Modulating Emotion Perception: Opposing Effects of Inhibitory and Excitatory Prefrontal Cortex Stimulation. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 329–336. [Google Scholar] [CrossRef]
- Jansen, J.M.; van den Heuvel, O.A.; van der Werf, Y.D.; de Wit, S.J.; Veltman, D.J.; van den Brink, W.; Goudriaan, A.E. The Effect of High-Frequency Repetitive Transcranial Magnetic Stimulation on Emotion Processing, Reappraisal, and Craving in Alcohol Use Disorder Patients and Healthy Controls: A Functional Magnetic Resonance Imaging Study. Front. Psychiatry 2019, 10, 272. [Google Scholar] [CrossRef] [Green Version]
- Killgore, W.D.; Yurgelun-Todd, D.A. The right-hemisphere and valence hypotheses: Could they both be right (and sometimes left)? Soc. Cogn. Affect. Neurosci. 2007, 2, 240–250. [Google Scholar] [CrossRef]
- Aron, A.R.; Robbins, T.W.; Poldrack, R.A. Right inferior frontal cortex: Addressing the rebuttals. Front. Hum. Neurosci. 2014, 8, 905. [Google Scholar] [CrossRef] [Green Version]
- Momenan, R.; Steckler, L.E.; Saad, Z.S.; van Rafelghem, S.; Kerich, M.J.; Hommer, D.W. Effects of alcohol dependence on cortical thickness as determined by magnetic resonance imaging. Psychiatry Res. 2012, 204, 101–111. [Google Scholar] [CrossRef]
- Schluter, R.S.; Jansen, J.M.; van Holst, R.J.; van den Brink, W.; Goudriaan, A.E. Differential Effects of Left and Right Prefrontal High-Frequency Repetitive Transcranial Magnetic Stimulation on Resting-State Functional Magnetic Resonance Imaging in Healthy Individuals. Brain Connect. 2018, 8, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Luijten, M.; Schellekens, A.F.; Kuhn, S.; Machielse, M.W.; Sescousse, G. Disruption of Reward Processing in Addiction: An Image-Based Meta-analysis of Functional Magnetic Resonance Imaging Studies. JAMA Psychiatry 2017, 74, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.; Gallinat, J. Common biology of craving across legal and illegal drugs—a quantitative meta-analysis of cue-reactivity brain response. Eur. J. Neurosci. 2011, 33, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Harel, M.; Perini, I.; Kämpe, R.; Alyagon, U.; Shalev, H.; Besser, I.; Sommer, W.H.; Heilig, M.; Zangen, A. Repetitive transcranial magnetic stimulation in alcohol dependence: A randomized, double-blind, sham-controlled proof-of-concept trial targeting medial prefrontal and anterior cingulate cortex. Biol. Psychiatry 2021. [Google Scholar] [CrossRef]
- Mitra, S.; Mehta, U.M.; Binukumar, B.; Venkatasubramanian, G.; Thirthalli, J. Statistical power estimation in non-invasive brain stimulation studies and its clinical implications: An exploratory study of the meta-analyses. Asian J. Psychiatr. 2019, 44, 29–34. [Google Scholar] [CrossRef]
- Ioannidis, J.P. Why most discovered true associations are inflated. Epidemiology 2008, 19, 640–648. [Google Scholar] [CrossRef] [Green Version]
- Hunter, A.M.; Minzenberg, M.J.; Cook, I.A.; Krantz, D.E.; Levitt, J.G.; Rotstein, N.M.; Chawla, S.A.; Leuchter, A.F. Concomitant medication use and clinical outcome of repetitive Transcranial Magnetic Stimulation (rTMS) treatment of Major Depressive Disorder. Brain Behav. 2019, 9, e01275. [Google Scholar] [CrossRef] [Green Version]
- Pourzitaki, C.; Dardalas, I.; Poutoglidou, F.; Kouvelas, D.; Kimiskidis, V.K. The Combination of rTMS and Pharmacotherapy on In Vitro Models: A Mini-Review. CNS Neurol. Disord. Drug Targets 2020, 19, 220–226. [Google Scholar] [CrossRef]
- FDA. Repetitive Transcranial Magnetic Stimulation (rTMS) Systems—Class II Special Controls Guidance for Industry and FDA Staff. Available online: https://www.fda.gov/medical-devices/guidance-documents-medical-devices-and-radiation-emitting-products/repetitive-transcranial-magnetic-stimulation-rtms-systems-class-ii-special-controls-guidance (accessed on 31 January 2022).
- Neacsiu, A.D.; Luber, B.M.; Davis, S.W.; Bernhardt, E.; Strauman, T.J.; Lisanby, S.H. On the Concurrent Use of Self-System Therapy and Functional Magnetic Resonance Imaging-Guided Transcranial Magnetic Stimulation as Treatment for Depression. J. ECT 2018, 34, 266–273. [Google Scholar] [CrossRef]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef]
- Hoeft, F.; Wu, D.A.; Hernandez, A.; Glover, G.H.; Shimojo, S. Electronically switchable sham transcranial magnetic stimulation (TMS) system. PLoS ONE 2008, 3, e1923. [Google Scholar] [CrossRef] [PubMed]
- Duecker, F.; Sack, A.T. Rethinking the role of sham TMS. Front. Psychol. 2015, 6, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variables | rTMS + TAU (n = 16) | TAU (n = 18) | Total Sample (n = 34) |
|---|---|---|---|
| Demographics | |||
| Age, M (SD) | 49.3 (7.9) | 45.8 (9.7) | 47.4 (8.9) |
| Gender, %male | 100 | 89 | 94 |
| IQ score, M (SD) | 95.8 (14.5) | 97.6 (14.7) | 96.8 (14.4) |
| AUD, M (SD) | |||
| Age first ever alcohol use | 12.3 (3.5) | 13.3 (3.4) | 12.8 (3.4) |
| Years of problematic use | 16.4 (6.5) | 14.3 (7.4) | 15.7 (7.0) |
| Consumption alcohol (gr/day) | 132 (54) | 122 (58) | 127 (56) |
| Number previous treatments | 3.8 (0.3) | 4.6 (1.0) | 4.2 (1.3) |
| Other substance use disorders | |||
| Tobacco, % | 81 | 94 | 88 |
| Cannabis, % | 0 | 6 | 3 |
| Stimulants, % | 0 | 6 | 3 |
| Benzodiazepine, % | 0 | 6 | 3 |
| Psychiatric comorbidity | |||
| PTSD, % | 0 | 44 | 26 |
| Depression, % | 13 | 17 | 15 |
| OCD, % | 0 | 22 | 12 |
| Panic disorder, % | 0 | 0 | 0 |
| Measurements baseline | |||
| VAS, M (SD) | 0.9 (1.4) | 0.8 (1.2 | 0.9 (1.2) |
| OCDS, M (SD) | 7.3 (4.0) | 8.1 (3.1) | 7.7 (3.5) |
| AUQ, M (SD) | 15.9 (5.3) | 14.3 (5.9) | 15.1 (5.7) |
| Abstinent, % | 100 | 94 | 97 |
| Alcohol use (gr/day), M (SD) | 0 (0) | 0.13 (0.13) | 0.07 (0.03) |
| Heavy drinking, M (SD) | 0 (0) | 0 (0) | 0 (0) |
| Use of medication | |||
| Anticraving, % | 6 | 6 | 6 |
| Antidepressants, % | 19 | 44 | 32 |
| Antipsychotics, % | 19 | 44 | 32 |
| Benzodiazepines, % | 50 | 72 | 62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belgers, M.; Van Eijndhoven, P.; Markus, W.; Schene, A.H.; Schellekens, A. rTMS Reduces Craving and Alcohol Use in Patients with Alcohol Use Disorder: Results of a Randomized, Sham-Controlled Clinical Trial. J. Clin. Med. 2022, 11, 951. https://doi.org/10.3390/jcm11040951
Belgers M, Van Eijndhoven P, Markus W, Schene AH, Schellekens A. rTMS Reduces Craving and Alcohol Use in Patients with Alcohol Use Disorder: Results of a Randomized, Sham-Controlled Clinical Trial. Journal of Clinical Medicine. 2022; 11(4):951. https://doi.org/10.3390/jcm11040951
Chicago/Turabian StyleBelgers, Maarten, Philip Van Eijndhoven, Wiebren Markus, Aart H. Schene, and Arnt Schellekens. 2022. "rTMS Reduces Craving and Alcohol Use in Patients with Alcohol Use Disorder: Results of a Randomized, Sham-Controlled Clinical Trial" Journal of Clinical Medicine 11, no. 4: 951. https://doi.org/10.3390/jcm11040951
APA StyleBelgers, M., Van Eijndhoven, P., Markus, W., Schene, A. H., & Schellekens, A. (2022). rTMS Reduces Craving and Alcohol Use in Patients with Alcohol Use Disorder: Results of a Randomized, Sham-Controlled Clinical Trial. Journal of Clinical Medicine, 11(4), 951. https://doi.org/10.3390/jcm11040951






