Abdominal Aortic Aneurysm Morphology as an Essential Criterion for Stratifying the Risk of Aneurysm Rupture
Abstract
1. Introduction
2. Materials and Methods
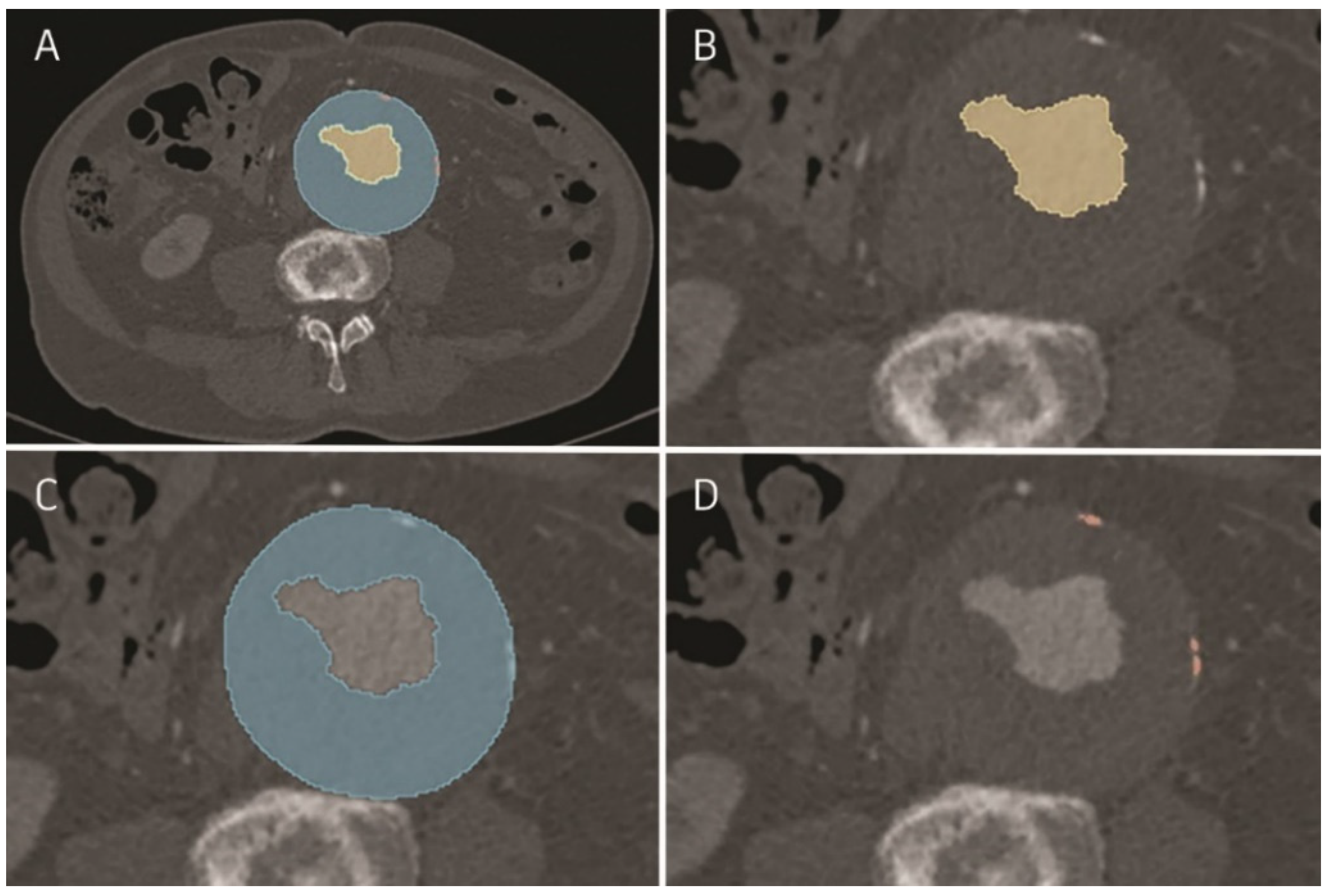
2.1. Image Analysis
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Altobelli, E.; Rapacchietta, L.; Profeta, V.F.; Fagnano, R. Risk factors for abdominal aortic aneurysm in population-based studies: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2018, 15, 2805. [Google Scholar] [CrossRef] [PubMed]
- Bown, M.J.; Sweeting, M.J.; Brown, L.C.; Powell, J.T.; Thompson, S.G. Surveillance intervals for small abdominal aortic aneurysms: A meta-analysis. JAMA-J. Am. Med. Assoc. 2013, 309, 806–813. [Google Scholar] [CrossRef][Green Version]
- De Rango, P.; Simonte, G.; Manzone, A.; Farchioni, L.; Cieri, E.; Verzini, F.; Parlani, G.; Isernia, G.; Lenti, M. Mortality Risk for Ruptured Abdominal Aortic Aneurysm in Women. Ann. Vasc. Surg. 2017, 39, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef] [PubMed]
- Sharp, M.A.; Collin, J. A myth exposed: Fast growth in diameter does not justify precocious abdominal aortic aneurysms repair. Eur. J. Vasc. Endovasc. Surg. 2003, 25, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.C.; Gardner, J.B.; Meissner, M.H.; Johansen, H.K. Rupture in small abdominal aortic aneurysms. J. Vasc. Surg. 1998, 28, 884–888. [Google Scholar] [CrossRef]
- Thubrikar, M.J.; Robicsek, F.; Labrosse, M.; Chervenkoff, V.; Fowler, B.L. Effect of thrombus on abdominal aortic aneurysm wall dilation and stress. J. Cardiovasc. Surg. 2003, 44, 67–77. [Google Scholar]
- Georgakarakos, E.; Ioannou, C.V.; Volanis, S.; Papaharilaou, Y.; Ekaterinaris, J.; Katsamouris, A.N. The influence of intraluminal thrombus on abdominal aortic aneurysm wall stress. Int. Angiol. 2009, 28, 325–333. [Google Scholar] [PubMed]
- Koole, D.; Zandvoort, H.J.A.; Schoneveld, A.; Vink, A.; Vos, J.A.; Van Den Hoogen, L.L.; De Vries, J.P.P.M.; Pasterkamp, G.; Moll, F.L.; Van Herwaarden, J.A. Intraluminal abdominal aortic aneurysm thrombus is associated with disruption of wall integrity. J. Vasc. Surg. 2013, 57, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Volokh, K.Y.; Aboudi, J. Aneurysm strength can decrease under calcification. J. Mech. Behav. Biomed. Mater. 2016, 57, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Siika, A.; Lindquist Liljeqvist, M.; Hultgren, R.; Gasser, T.C.; Roy, J. Aortic Lumen Area Is Increased in Ruptured Abdominal Aortic Aneurysms and Correlates to Biomechanical Rupture Risk. J. Endovasc. Ther. 2018, 25, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Moll, F.L.; Powell, J.T.; Fraedrich, G.; Verzini, F.; Haulon, S.; Waltham, M.; Van Herwaarden, J.A.; Holt, P.J.E.; Van Keulen, J.W.; Rantner, B.; et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur. J. Vasc. Endovasc. Surg. 2011, 41, S1–S58. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Iyer, V.; Jenkins, J.; Bradshaw, B.; Cronin, O.; Walker, P.J. Thrombus volume is similar in patients with ruptured and intact abdominal aortic aneurysms. J. Vasc. Surg. 2014, 59, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Wolanski, P.; Parr, A.; Buttner, P. Measurement and determinants of infrarenal aortic thrombus volume. Eur. Radiol. 2008, 18, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Fillinger, M.F.; Racusin, J.; Baker, R.K.; Cronenwett, J.L.; Teutelink, A.; Schermerhorn, M.L.; Zwolak, R.M.; Powell, R.J.; Walsh, D.B.; Rzucidlo, E.M. Anatomic characteristics of ruptured abdominal aortic aneurysm on conventional CT scans: Implications for rupture risk. J. Vasc. Surg. 2004, 39, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Hans, S.S.; Jareunpoon, O.; Balasubramaniam, M.; Zelenock, G.B. Size and location of thrombus in intact and ruptured abdominal aortic aneurysms. J. Vasc. Surg. 2005, 41, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.P.; Wong, S.A.; Moxon, J.V.; Gasser, T.C.; Golledge, J. Systematic review and meta-analysis of the association between intraluminal thrombus volume and abdominal aortic aneurysm rupture. J. Vasc. Surg. 2019, 70, 2065–2073.e10. [Google Scholar] [CrossRef] [PubMed]
- Buijs, R.V.C.; Willems, T.P.; Tio, R.A.; Boersma, H.H.; Tielliu, I.F.J.; Slart, R.H.J.A.; Zeebregts, C.J. Calcification as a risk factor for rupture of abdominal aortic aneurysm. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Schousboe, J.T.; Wilson, K.E.; Kiel, D.P. Detection of Abdominal Aortic Calcification With Lateral Spine Imaging Using DXA. J. Clin. Densitom. 2006, 9, 302–308. [Google Scholar] [CrossRef] [PubMed]
| Variable | Asymptomatic (n and % or ±SD) | Symptomatic (n and % or ±SD) | Ruptured (n and % or ±SD) | p Value |
|---|---|---|---|---|
| Age, years | 71.57 (±8.82) | 72.61 (±8.61) | 76.35 (±10.48) | 0.032 |
| Age > 80 | 12 (24.49%) | 10 (20.41%) | 22 (44.90%) | 0.018 |
| Male gender | 47 (95.92%) | 37 (75.51%) | 38 (77.55%) | 0.013 |
| HTN | 34 (69.39%) | 37 (75.51%) | 29 (59.18%) | >0.1 |
| CAD | 21 (42.86%) | 15 (30.61%) | 11 (22.45%) | >0.1 |
| CABG | 6 (12.24%) | 5 (10.20%) | 2 (4.08%) | >0.1 |
| Arrhytmia | 8 (16.33%) | 10 (20.41%) | 8 (16.33%) | >0.1 |
| HF | 13 (26.53%) | 3 (6.12%) | 6 (12.24%) | 0.015 |
| DM | 8 (16.33%) | 7 (14.29%) | 7 (14.29%) | >0.1 |
| CKD | 5 (10.20%) | 2 (4.08%) | 6 (12.24%) | >0.1 |
| Stroke | 6 (12.24%) | 6 (12.24%) | 4 (8.16%) | >0.1 |
| PAD | 9 (18.37%) | 10 (20.41%) | 5 (10.20%) | >0.1 |
| COPD | 10 (20.41%) | 5 (10.20%) | 7 (14.29%) | >0.1 |
| Obesity | 6 (12.24%) | 8 (16.33%) | 5 (10.20%) | >0.1 |
| aAAA | sAAA | rAAA | p-Value * | p-Value Post Hoc rAAA vs. aAAA | p-Value Post Hoc aAAA vs. sAAA | p-Value Post Hoc rAAA vs. sAAA | |
|---|---|---|---|---|---|---|---|
| Maximal aneurysm diameter [mm] | 82.52 (IQR 13.10) | 82.18 (IQR 14.00) | 83.65 (SD 14.00) | >0.1 | |||
| Total aneurysm volume [cm3] | 378.21 (IQR 147.16) | 344.07 (SD 144.66) | 381.59 (IQR 187.14) | >0.1 | |||
| AFL volume [cm3] | 116.83 (69.97) | 143.76 (101.39) | 184.44 (156.51) | 0.005 | 0.004 | >0.1 | >0.1 |
| AFL surface [mm2] | 16,436.85 (5201.20) * | 18,427.35 (8913.10) | 23,061.94 (12,065.20) | 0.005 | 0.004 | >0.1 | >0.1 |
| ILT volume [cm3] | 261.38 (144.49) | 200.30 (143.89) | 197.16 (160.23) | 0.009 | 0.013 | 0.051 | >0.1 |
| ILT surface [mm2] | 41,692.89 (11,309.40) | 41,143.71 (14,457.70) | 44,225.26 (18,255.84) * | >0.1 | |||
| Calcifications volume [cm3] | 3.01 (2.52) | 2.31 (2.12) | 1.54 (1.00) | <0.001 | <0.001 | >0.1 | 0.001 |
| Calcifications surface [mm2] | 2716.76 (2637.93) | 2412.74 (2306.52) | 1993.67 (859.57) | <0.001 | <0.001 | >0.1 | <0.001 |
| AFL/ILT volume ratio [cm3] | 0.63 (0.50) | 1.22 (1.03) | 1.35 (0.78) | <0.001 | <0.001 | 0.011 | >0.1 |
| AFL/ILT surface ratio [mm2] | 0.39 (0.10) | 0.45 (0.12) | 8.07 (0.12) | <0.001 | <0.001 | 0.033 | 0.016 |
| Calcifications/ ILT volume ratio [cm3] | 0.01 (0.01) | 0.02 (0.02) | 0.01 (0.01) | 0.004 | 0.037 | >0.1 | 0.005 |
| Variables | OR | 95% CI | p-Value * | Cut-Off Value | OR | 95% CI | p |
|---|---|---|---|---|---|---|---|
| AFL volume [cm3] | 1.010 | 1.004–1.016 | <0.001 | >181.56 | 7.170 | 2.428–21.174 | <0.001 |
| AFL surface [mm2] | 1.000 | 1.000–1.000 | 0.002 | >20,492.20 | 5.953 | 2.410–14.701 | <0.001 |
| ILT volume [cm3] | 0.997 | 0.993–1.000 | 0.040 | <172.16 | 4.786 | 1.957–11.704 | <0.001 |
| ILT surface [mm2] | 1.000 | 1.000–1.000 | >0.1 | * | * | * | * |
| Calcifications surface [mm2] | 1.000 | 1.000–1.000 | >0.1 | <782.12 | 8.366 | 3.294–21.249 | <0.001 |
| Calcifications volume [cm3] | 0.861 | 0.722–1.027 | >0.1 | <1.35 | 8.044 | 3.218–20.105 | <0.001 |
| AFL/ILT volume ratio [cm3] | 2.643 | 1.373–5.087 | 0.004 | >0.43 | 11.316 | 4.042–31.360 | <0.001 |
| AFL/ILT surface*100 ratio [mm2] | 1.187 | 1.099–1.281 | <0.001 | >0.40 | 13.895 | 4.676–41.285 | <0.001 |
| Calcifications/ILT volume*100 ratio [cm3] | 0.838 | 0.641–1.096 | >0.1 | * | * | * | * |
| Variables | OR | 95% CI | p-Value | Cut-Off Value | OR | 95% CI | p |
|---|---|---|---|---|---|---|---|
| AFL volume [cm3] | 1.004 | 1.000–1.009 | 0.049 | >189.26 | 2.591 | 1.077–6.232 | 0.034 |
| AFL surface [mm2] | 1.000 | 1.000–1.000 | 0.041 | >18,808.70 | 3.474 | 1.502–8.033 | 0.004 |
| ILT volume [cm3] | 1.000 | 0.997–1.003 | >0.1 | * | * | * | * |
| ILT surface [mm2] | 1.000 | 1.000–1.000 | >0.1 | * | * | * | * |
| Calcifications surface [mm2] | 1.000 | 1.000–1.000 | >0.1 | <1090.98 | 7.153 | 2.874–17.801 | <0.001 |
| Calcifications volume [cm3] | 0.915 | 0.777–1.077 | >0.1 | <1.57 | 6.250 | 2.353–16.598 | <0.001 |
| AFL/ILT volume*100 ratio [cm3] | 1.001 | 0.998–1.004 | >0.1 | * | * | * | * |
| AFL/ILT surface*100 ratio [mm2] | 1.045 | 1.004–1.087 | 0.03 | >0.45 | 2.991 | 1.310–6.826 | <0.001 |
| Calcifications /ILT volume*100 ratio [cm3] | 0.769 | 0.568–1.042 | >0.1 | <0.01 | 2.450 | 1.050–5.713 | 0.038 |
| Variables | OR | 95% CI | p-Value | Cut-Off Value | OR | 95% CI | p |
|---|---|---|---|---|---|---|---|
| AFL volume [cm3] | 1.005 | 1.000–1.011 | >0.1 | * | * | * | * |
| AFL surface [mm2] | 1.000 | 1.000–1.000 | >0.1 | * | * | * | * |
| ILT volume [cm3] | 0.996 | 0.993–1.000 | 0.027 | <170.12 | 3.450 | 1.413–8.426 | 0.007 |
| ILT surface [mm2] | 1.000 | 1.000–1.000 | >0.1 | * | * | * | * |
| Calcifications surface [mm2] | 1.000 | 1.000–1.000 | >0.1 | * | * | * | * |
| Calcifications volume [cm3] | 0.919 | 0.794–1.065 | >0.1 | * | * | * | * |
| AFL/ILT volume*100 ratio [cm3] | 1.006 | 1.001–1.011 | 0.022 | >0.47 | 3.552 | 1.544–8.170 | 0.003 |
| AFL/ILT surface*100 ratio [mm2] | 1.067 | 1.017–1.119 | 0.008 | >0.41 | 2.966 | 1.305–6.744 | 0.01 |
| Calcifications/ILT volume*100 ratio [cm3] | 1.060 | 0.873–1.287 | >0.1 | * | * | * | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niklas, N.; Gutowski, P.; Kazimierczak, A.; Rynio, P. Abdominal Aortic Aneurysm Morphology as an Essential Criterion for Stratifying the Risk of Aneurysm Rupture. J. Clin. Med. 2022, 11, 933. https://doi.org/10.3390/jcm11040933
Niklas N, Gutowski P, Kazimierczak A, Rynio P. Abdominal Aortic Aneurysm Morphology as an Essential Criterion for Stratifying the Risk of Aneurysm Rupture. Journal of Clinical Medicine. 2022; 11(4):933. https://doi.org/10.3390/jcm11040933
Chicago/Turabian StyleNiklas, Natalia, Piotr Gutowski, Arkadiusz Kazimierczak, and Paweł Rynio. 2022. "Abdominal Aortic Aneurysm Morphology as an Essential Criterion for Stratifying the Risk of Aneurysm Rupture" Journal of Clinical Medicine 11, no. 4: 933. https://doi.org/10.3390/jcm11040933
APA StyleNiklas, N., Gutowski, P., Kazimierczak, A., & Rynio, P. (2022). Abdominal Aortic Aneurysm Morphology as an Essential Criterion for Stratifying the Risk of Aneurysm Rupture. Journal of Clinical Medicine, 11(4), 933. https://doi.org/10.3390/jcm11040933






