Pain-Related Coping Behavior in ALS: The Interplay between Maladaptive Coping, the Patient’s Affective State and Pain
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Patient Characteristics
2.3. Patient-Reported Data and Assessment Instruments
2.4. Statistics
3. Results
3.1. Demographic and Clinical Data
3.2. Pain Characteristics, Treatment and Pain Relief
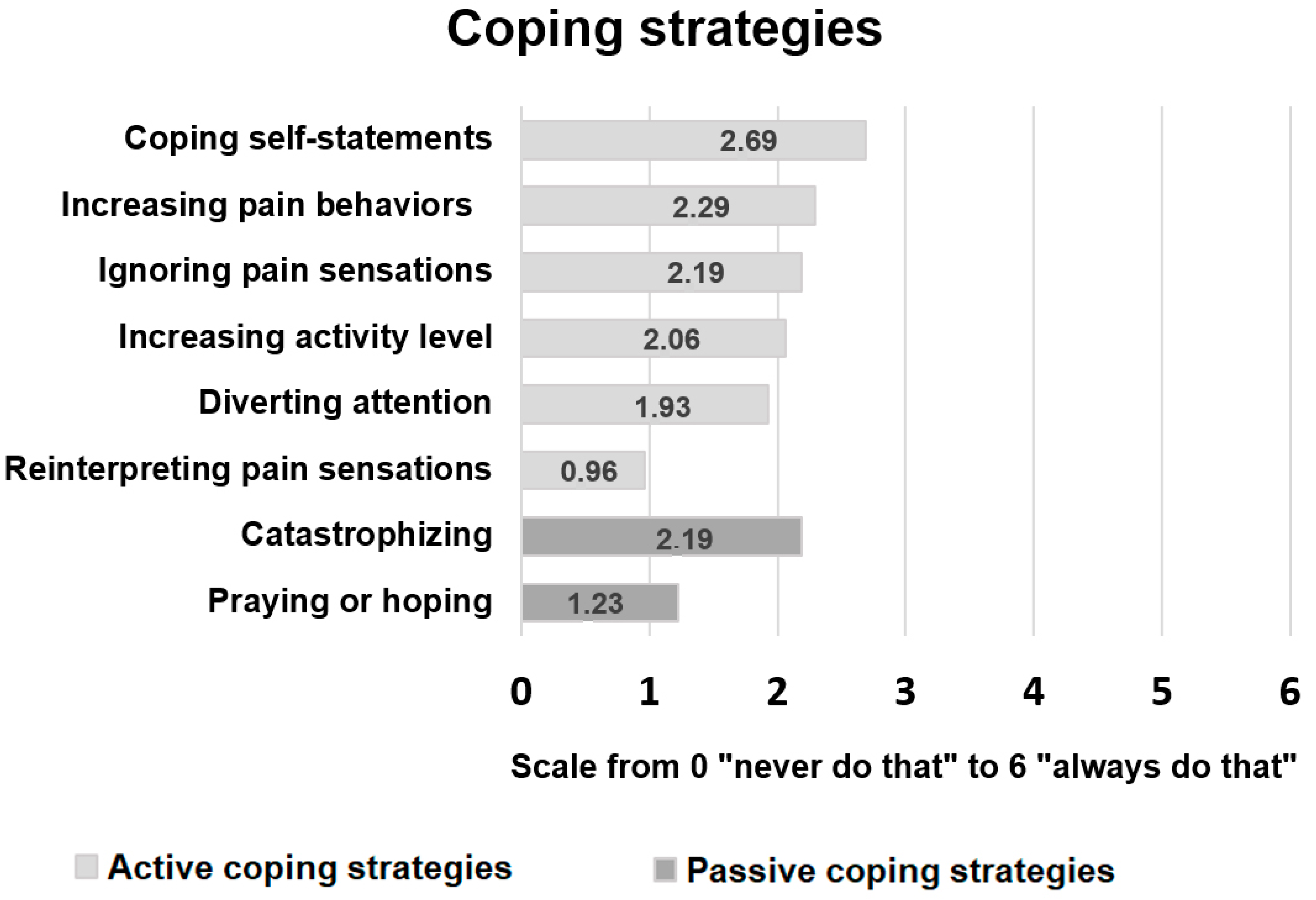
3.3. Pain-Related Coping Strategies
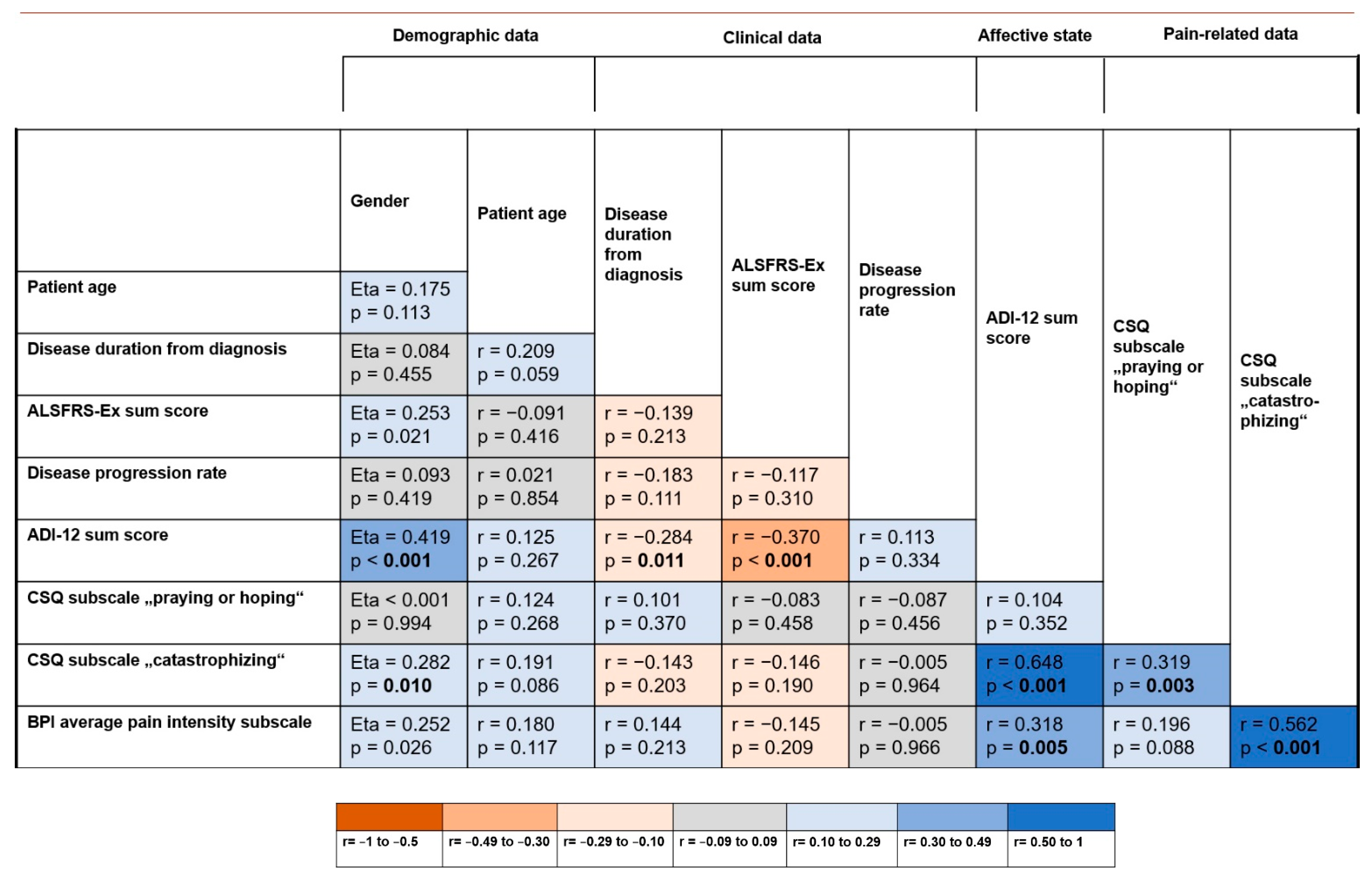
3.4. Relation between Demographic/Clinical Data, Pain-Related Features and the Affective State
3.5. Predictors of the Average Pain Intensity
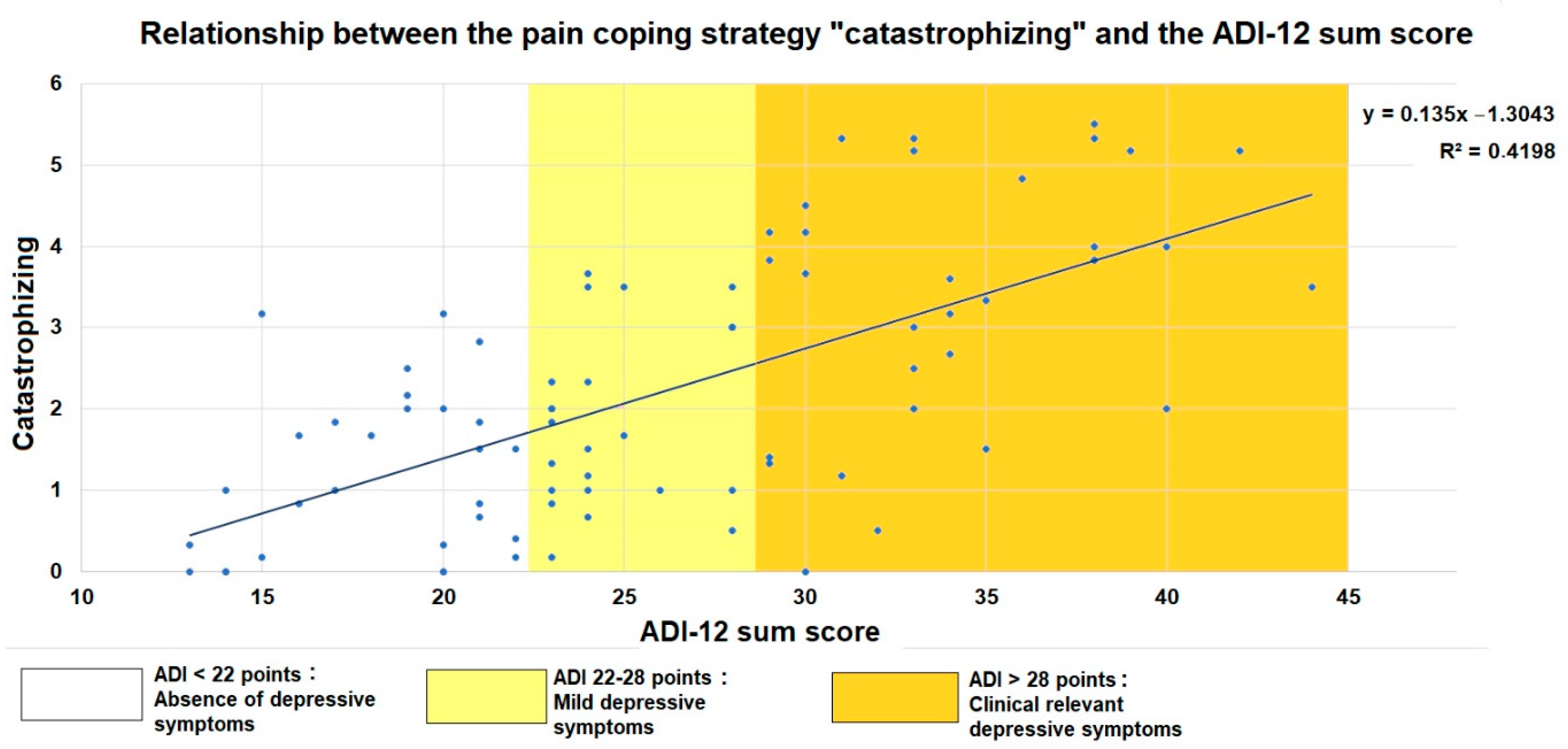
3.6. Catastrophizing in Relation to the Patients’ Functional Status and Emotional Aspects of Health-Related Quality of Life
3.7. Predictors of Pain-Related Catastrophizing
4. Discussion
4.1. Pain-Related Coping Behavior
4.2. Interplay between Pain, Maladaptive Coping and the Patients’ Affective State
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolf, J.; Safer, A.; Wöhrle, J.C.; Palm, F.; Nix, W.A.; Maschke, M.; Grau, A.J. Todesursachen bei amyotropher Lateralsklerose: Ergebnisse aus dem ALS-Register Rheinland-Pfalz. Nervenarzt 2017, 88, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Requardt, M.V.; Görlich, D.; Grehl, T.; Boentert, M. Clinical Determinants of Disease Progression in Amyotrophic Lateral Sclerosis-A Retrospective Cohort Study. J. Clin. Med. 2021, 10, 1623. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.M.; Hollinger, H.; McDermott, C.J. The evidence for symptomatic treatments in amyotrophic lateral sclerosis. Curr. Opin. Neurol. 2014, 27, 524–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiò, A.; Canosa, A.; Gallo, S.; Moglia, C.; Ilardi, A.; Cammarosano, S.; Papurello, D.; Calvo, A. Pain in amyotrophic lateral sclerosis: A population-based controlled study. Eur. J. Neurol. 2012, 19, 551–555. [Google Scholar] [CrossRef]
- Hanisch, F.; Skudlarek, A.; Berndt, J.; Kornhuber, M.E. Characteristics of pain in amyotrophic lateral sclerosis. Brain Behav. 2015, 5, e00296. [Google Scholar] [CrossRef]
- Ishida, N.; Hongo, S.; Kumano, A.; Hatta, H.; Zakoji, N.; Hirutani, M.; Yamamoto, Y.; Aono, H.; Tuigi, M.; Suzuki, R.; et al. Relationship between Pain and Functional Status in Patients with Amyotrophic Lateral Sclerosis: A Multicenter Cross-Sectional Study. J. Palliat. Med. 2018, 21, 588–591. [Google Scholar] [CrossRef]
- Moisset, X.; Cornut-Chauvinc, C.; Clavelou, P.; Pereira, B.; Dallel, R.; Guy, N. Is there pain with neuropathic characteristics in patients with amyotrophic lateral sclerosis? A cross-sectional study. Palliat. Med. 2016, 30, 486–494. [Google Scholar] [CrossRef]
- Edge, R.; Mills, R.; Tennant, A.; Diggle, P.J.; Young, C.A. Do pain, anxiety and depression influence quality of life for people with amyotrophic lateral sclerosis/motor neuron disease? A national study reconciling previous conflicting literature. J. Neurol. 2020, 267, 607–615. [Google Scholar] [CrossRef] [Green Version]
- Wallace, V.C.J.; Ellis, C.M.; Burman, R.; Knights, C.; Shaw, C.E.; Al-Chalabi, A. The evaluation of pain in amyotrophic lateral sclerosis: A case controlled observational study. Amyotroph. Lateral Scler. Front. Degener. 2014, 15, 520–527. [Google Scholar] [CrossRef]
- Ganzini, L.; Johnston, W.S.; Hoffman, W.F. Correlates of suffering in amyotrophic lateral sclerosis. Neurology 1999, 52, 1434–1440. [Google Scholar] [CrossRef]
- Hecht, M.; Hillemacher, T.; Gräsel, E.; Tigges, S.; Winterholler, M.; Heuss, D.; Hilz, M.-J.; Neundörfer, B. Subjective experience and coping in ALS. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2002, 3, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Rivera, I.; Ajroud-Driss, S.; Casey, P.; Heller, S.; Allen, J.; Siddique, T.; Sufit, R. Prevalence and characteristics of pain in early and late stages of ALS. Amyotroph. Lateral Scler. Front. Degener. 2013, 14, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Pizzimenti, A.; Aragona, M.; Onesti, E.; Inghilleri, M. Depression, pain and quality of life in patients with amyotrophic lateral sclerosis: A cross-sectional study. Funct. Neurol. 2013, 28, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Åkerblom, Y.; Jakobsson Larsson, B.; Zetterberg, L.; Åsenlöf, P. The multiple faces of pain in motor neuron disease: A qualitative study to inform pain assessment and pain management. Disabil. Rehabil. 2020, 42, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Pagnini, F.; Lunetta, C.; Banfi, P.; Rossi, G.; Fossati, F.; Marconi, A.; Castelnuovo, G.; Corbo, M.; Molinari, E. Pain in Amyotrophic Lateral Sclerosis: A psychological perspective. Neurol. Sci. 2012, 33, 1193–1196. [Google Scholar] [CrossRef]
- Meints, S.M.; Edwards, R.R. Evaluating psychosocial contributions to chronic pain outcomes. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2018, 87, 168–182. [Google Scholar] [CrossRef]
- Edwards, R.R.; Dworkin, R.H.; Sullivan, M.D.; Turk, D.C.; Wasan, A.D. The Role of Psychosocial Processes in the Development and Maintenance of Chronic Pain. J. Pain 2016, 17, T70–T92. [Google Scholar] [CrossRef] [Green Version]
- Snow-Turek, L.A.; Norris, M.P.; Tan, G. Active and passive coping strategies in chronic pain patients. Pain 1996, 64, 455–462. [Google Scholar] [CrossRef]
- Brown, G.K.; Nicassio, P.M. Development of a questionnaire for the assessment of active and passive coping strategies in chronic pain patients. Pain 1987, 31, 53–64. [Google Scholar] [CrossRef]
- Brown, G.K.; Nicassio, P.M.; Wallston, K.A. Pain coping strategies and depression in rheumatoid arthritis. J. Consult. Clin. Psychol. 1989, 57, 652–657. [Google Scholar] [CrossRef]
- Covic, T.; Adamson, B.; Hough, M. The impact of passive coping on rheumatoid arthritis pain. Rheumatology 2000, 39, 1027–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, M.J.; Thorn, B.; Haythornthwaite, J.A.; Keefe, F.; Martin, M.; Bradley, L.A.; Lefebvre, J.C. Theoretical perspectives on the relation between catastrophizing and pain. Clin. J. Pain 2001, 17, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.J.L.; Bishop, S.R.; Pivik, J. The Pain Catastrophizing Scale: Development and validation. Psychol. Assess. 1995, 7, 524–532. [Google Scholar] [CrossRef]
- Oh, J.; An, J.; Park, K. Coping in people with amyotrophic lateral sclerosis and motor neuron disease: Systematic review. J. Clin. Nurs. 2021, 30, 1838–1853. [Google Scholar] [CrossRef]
- Leandro, G.S.; Dourado Júnior, M.E.T.; Santana, G.C.; Dantas, L.S.X. Coping strategies among amyotrophic lateral sclerosis (ALS) patients: An integrative review. J. Neurol. 2021, 269, 693–702. [Google Scholar] [CrossRef]
- Lopes, L.C.G.; Galhardoni, R.; Silva, V.; Jorge, F.M.H.; Yeng, L.T.; Callegaro, D.; Chadi, G.; Teixeira, M.J.; Ciampi de Andrade, D. Beyond weakness: Characterization of pain, sensory profile and conditioned pain modulation in patients with motor neuron disease: A controlled study. Eur. J. Pain 2018, 22, 72–83. [Google Scholar] [CrossRef] [Green Version]
- Vogt, S.; Schlichte, I.; Schreiber, S.; Wigand, B.; Debska-Vielhaber, G.; Heitmann, J.; Meyer, T.; Dengler, R.; Petri, S.; Haghikia, A.; et al. A Multi-Center Cohort Study on Characteristics of Pain, Its Impact and Pharmacotherapeutic Management in Patients with ALS. J. Clin. Med. 2021, 10, 4552. [Google Scholar] [CrossRef]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef]
- Turner, M.R.; Wicks, P.; Brownstein, C.A.; Massagli, M.P.; Toronjo, M.; Talbot, K.; Al-Chalabi, A. Concordance between site of onset and limb dominance in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2011, 82, 853–854. [Google Scholar] [CrossRef] [Green Version]
- Roche, J.C.; Rojas-Garcia, R.; Scott, K.M.; Scotton, W.; Ellis, C.E.; Burman, R.; Wijesekera, L.; Turner, M.R.; Leigh, P.N.; Shaw, C.E.; et al. A proposed staging system for amyotrophic lateral sclerosis. Brain 2012, 135, 847–852. [Google Scholar] [CrossRef]
- Balendra, R.; Jones, A.; Jivraj, N.; Knights, C.; Ellis, C.M.; Burman, R.; Turner, M.R.; Leigh, P.N.; Shaw, C.E.; Al-Chalabi, A. Estimating clinical stage of amyotrophic lateral sclerosis from the ALS Functional Rating Scale. Amyotroph. Lateral Scler. Front. Degener. 2014, 15, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Wicks, P.; Massagli, M.P.; Wolf, C.; Heywood, J. Measuring function in advanced ALS: Validation of ALSFRS-EX extension items. Eur. J. Neurol. 2009, 16, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, S.; Vielhaber, S.; Körner, S.; Machts, J.; Heinze, H.-J.; Dengler, R.; Petri, S. Validation of the German version of the extended ALS functional rating scale as a patient-reported outcome measure. J. Neurol. 2013, 260, 2242–2255. [Google Scholar] [CrossRef] [PubMed]
- Radbruch, L.; Loick, G.; Kiencke, P.; Lindena, G.; Sabatowski, R.; Grond, S.; Lehmann, K.A.; Cleeland, C.S. Validation of the German Version of the Brief Pain Inventory. J. Pain Symptom Manag. 1999, 18, 180–187. [Google Scholar] [CrossRef]
- Rosenstiel, A.K.; Keefe, F.J. The use of coping strategies in chronic low back pain patients: Relationship to patient characteristics and current adjustment. Pain 1983, 17, 33–44. [Google Scholar] [CrossRef]
- Verra, M.L.; Angst, F.; Lehmann, S.; Aeschlimann, A. Translation, cross-cultural adaptation, reliability, and validity of the German version of the Coping Strategies Questionnaire (CSQ-D). J. Pain 2006, 7, 327–336. [Google Scholar] [CrossRef]
- Kübler, A.; Winter, S.; Kaiser, J.; Birbaumer, N.; Hautzinger, M. Das ALS-Depressionsinventar (ADI). Z. Für Klin. Psychol. Und Psychother. 2005, 34, 19–26. [Google Scholar] [CrossRef]
- Jenkinson, C.; Fitzpatrick, R.; Brennan, C.; Bromberg, M.; Swash, M. Development and validation of a short measure of health status for individuals with amyotrophic lateral sclerosis/motor neurone disease: The ALSAQ-40. J. Neurol. 1999, 246 (Suppl. S3), III16–III21. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Taylor and Francis: Hoboken, NJ, USA, 1988; ISBN 978-0-8058-0283-2. [Google Scholar]
- Ellis, C.M.; Simmons, A.; Jones, D.K.; Bland, J.; Dawson, J.M.; Horsfield, M.A.; Williams SCLeigh, P.N. Diffusion tensor MRI assesses corticospinal tract damage in ALS. Neurology 1999, 53, 1051–1058. [Google Scholar] [CrossRef]
- Hassett, A.L.; Cone, J.D.; Patella, S.J.; Sigal, L.H. The role of catastrophizing in the pain and depression of women with fibromyalgia syndrome. Arthritis Rheum. 2000, 43, 2493–2500. [Google Scholar] [CrossRef]
- Toth, C.; Brady, S.; Hatfield, M. The importance of catastrophizing for successful pharmacological treatment of peripheral neuropathic pain. J. Pain Res. 2014, 7, 327–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulla, S.; Vielhaber, S.; Kollewe, K.; Machts, J.; Heinze, H.-J.; Dengler, R.; Petri, S. The impact of physical impairment on emotional well-being in ALS. Amyotroph. Lateral Scler. Front. Degener. 2014, 15, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Prell, T.; Steinbach, R.; Witte, O.W.; Grosskreutz, J. Poor emotional well-being is associated with rapid progression in amyotrophic lateral sclerosis. Eneurologicalsci 2019, 16, 100198. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.M.; Kronfli, T.; Buenaver, L.F.; Smith, M.T.; Berna, C.; Haythornthwaite, J.A.; Edwards, R.R. Situational versus dispositional measurement of catastrophizing: Associations with pain responses in multiple samples. J. Pain 2010, 11, 443–453. [Google Scholar] [CrossRef] [Green Version]
- Litt, M.D.; Shafer, D.M.; Ibanez, C.R.; Kreutzer, D.L.; Tawfik-Yonkers, Z. Momentary pain and coping in temporomandibular disorder pain: Exploring mechanisms of cognitive behavioral treatment for chronic pain. Pain 2009, 145, 160–168. [Google Scholar] [CrossRef]
- Pinto, P.R.; McIntyre, T.; Almeida, A.; Araújo-Soares, V. The mediating role of pain catastrophizing in the relationship between presurgical anxiety and acute postsurgical pain after hysterectomy. Pain 2012, 153, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Keefe, F.J.; Brown, G.K.; Wallston, K.A.; Caldwell, D.S. Coping with rheumatoid arthritis pain: Catastrophizing as a maladaptive strategy. Pain 1989, 37, 51–56. [Google Scholar] [CrossRef]
- Hirsh, A.T.; Bockow, T.B.; Jensen, M.P. Catastrophizing, pain, and pain interference in individuals with disabilities. Am. J. Phys. Med. Rehabil. 2011, 90, 713–722. [Google Scholar] [CrossRef] [Green Version]
- Wigand, B.; Schlichte, I.; Schreiber, S.; Heitmann, J.; Meyer, T.; Dengler, R.; Petri, S.; Haghikia, A.; Vielhaber, S.; Vogt, S. Characteristics of pain and the burden it causes in patients with amyotrophic lateral sclerosis—A longitudinal study. Amyotroph. Lateral Scler. Front. Degener. 2021, 1–8. [Google Scholar] [CrossRef]
- Tedman, B.M.; Young, C.A.; Williams, I.R. Assessment of depression in patients with motor neuron disease and other neurologically disabling illness. J. Neurol. Sci. 1997, 152, s75–s79. [Google Scholar] [CrossRef]
- Stephens, H.E.; Lehman, E.; Raheja, D.; Yang, C.; Walsh, S.; Simmons, Z. The role of mental health and self-efficacy in the pain experience of patients with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2016, 17, 206–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsh, A.T.; George, S.Z.; Riley, J.L.; Robinson, M.E. An evaluation of the measurement of pain catastrophizing by the coping strategies questionnaire. Eur. J. Pain 2007, 11, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Geisser, M.E.; Robinson, M.E.; Keefe, F.J.; Weiner, M.L. Catastrophizing, depression and the sensory, affective and evaluative aspects of chronic pain. Pain 1994, 59, 79–83. [Google Scholar] [CrossRef]

| Patients with Pain | Patients without Pain | p-Value | |
|---|---|---|---|
| Gender (female/male) | 24/60 (28.6/71.4%) | 20/46 (30.3/69.7%) | 0.817 |
| Patient age | 61.2 ± 11.8 | 66.6 ± 8.0 | 0.037 |
| Disease duration from diagnosis in months | 33.5 ± 38.5 | 37.7 ± 51.3 | 0.569 |
| Symptom onset | 0.135 | ||
| Bulbar | 14 (16.7%) | 19 (28.8%) | |
| Upper limb | 37 (44%) | 21 (31.8%) | |
| Lower limb | 31 (36.9%) | 22 (33.3%) | |
| Missing information | 2 (2.4%) | 4 (6.1%) | |
| King’s clinical staging | 0.065 | ||
| Stage 1: Symptom onset/functional involvement of first region | 0 | 0 | |
| Stage 2A: Diagnosis | 7 (8.3%) | 5 (7.6%) | |
| Stage 2B: Functional involvement of a second region | 8 (9.5%) | 16 (24.2%) | |
| Stage 3: Functional involvement of a third region | 39 (46.4%) | 23 (34.8%) | |
| Stage 4A: Need for gastrostomy | 4 (4.8%) | 7 (10.6%) | |
| Stage 4B: Need for respiratory support (NIV) | 26 (31%) | 15 (22.7%) | |
| ALSFRS-EX | |||
| Sum score | 36.6 ± 12.9 | 42.6 ± 10.8 | 0.003 |
| Bulbar subscore | 11.6 ± 4.8 | 11.23 ± 3.4 | 0.344 |
| Fine motor subscore | 7.7 ± 4.3 | 10.0 ± 4.9 | 0.003 |
| Gross motor subscore | 8.2 ± 5.0 | 10.2 ± 4.6 | 0.012 |
| Respiratory subscore | 9.1 ± 3.2 | 10.2 ± 2.8 | 0.038 |
| Disease progression rate * | 0.9 ± 1.9 | 0.75 ± 0.9 | 0.639 |
| ADI-12 | |||
| Sum score | 26.1 ± 7.6 | 22.2 ± 7.1 | 0.002 |
| Absence of depressive symptoms (<22 points) | 25 (29.8%) | 33 (50%) | 0.029 |
| Mild depressive symptoms (22–28 points) | 26 (31%) | 18 (27.3%) | |
| Clinically relevant depressive symptoms (>28 points) | 31 (36.9%) | 14 (21.2%) | |
| ALSAQ-40 subscale score “emotional functioning” | 41.8 ± 22.6 | 30.9 ± 20.2 | 0.003 |
| Patients with Pain (n = 84) | |
|---|---|
| Pain interference with daily functions | |
| Normal work | 5.4 ± 3.0 |
| Walking ability | 4.8 ± 2.8 |
| General activity | 4.5 ± 3.2 |
| Sleep | 4.0 ± 3.7 |
| Mood | 4.2 ± 3.1 |
| Enjoyment of life | 3.6 ± 3.7 |
| Relations with other people | 2.9 ± 3.1 |
| Pain-related pharmacotherapy * | n = 54 (64.3%) |
| Non-opioid analgesics | n = 45 (53.6%) |
| Opioid analgesics | n = 16 (19.0%) |
| Tricyclic antidepressants ** | n = 5 (6.0%) |
| Anticonvulsants | n = 10 (12.0%) |
| Unstandardized Coefficients | Standardized Coefficients | T | p-Value | VIF | ||
|---|---|---|---|---|---|---|
| Regression Coefficient B | Standard Error | Beta | ||||
| Gender | −0.521 | 0.465 | −0.119 | −1.120 | 0.266 | 1.279 |
| CSQ subscales | ||||||
| Diverting attention | 0.323 | 0.170 | 0.216 | 1.899 | 0.062 | 1.471 |
| Catastrophizing | 0.621 | 0.160 | 0.529 | 3.873 | <0.001 | 2.121 |
| Increasing pain behaviors | 0.086 | 0.196 | 0.054 | 0.439 | 0.662 | 1.699 |
| ADI-12 sum score | −0.019 | 0.033 | −0.078 | −0.593 | 0.555 | 1.973 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlichte, I.; Petri, S.; Dengler, R.; Meyer, T.; Haghikia, A.; Vielhaber, S.; Vogt, S. Pain-Related Coping Behavior in ALS: The Interplay between Maladaptive Coping, the Patient’s Affective State and Pain. J. Clin. Med. 2022, 11, 944. https://doi.org/10.3390/jcm11040944
Schlichte I, Petri S, Dengler R, Meyer T, Haghikia A, Vielhaber S, Vogt S. Pain-Related Coping Behavior in ALS: The Interplay between Maladaptive Coping, the Patient’s Affective State and Pain. Journal of Clinical Medicine. 2022; 11(4):944. https://doi.org/10.3390/jcm11040944
Chicago/Turabian StyleSchlichte, Ina, Susanne Petri, Reinhard Dengler, Thomas Meyer, Aiden Haghikia, Stefan Vielhaber, and Susanne Vogt. 2022. "Pain-Related Coping Behavior in ALS: The Interplay between Maladaptive Coping, the Patient’s Affective State and Pain" Journal of Clinical Medicine 11, no. 4: 944. https://doi.org/10.3390/jcm11040944
APA StyleSchlichte, I., Petri, S., Dengler, R., Meyer, T., Haghikia, A., Vielhaber, S., & Vogt, S. (2022). Pain-Related Coping Behavior in ALS: The Interplay between Maladaptive Coping, the Patient’s Affective State and Pain. Journal of Clinical Medicine, 11(4), 944. https://doi.org/10.3390/jcm11040944







