Pericardial Effusion on MRI in Autosomal Dominant Polycystic Kidney Disease
Abstract
1. Introduction
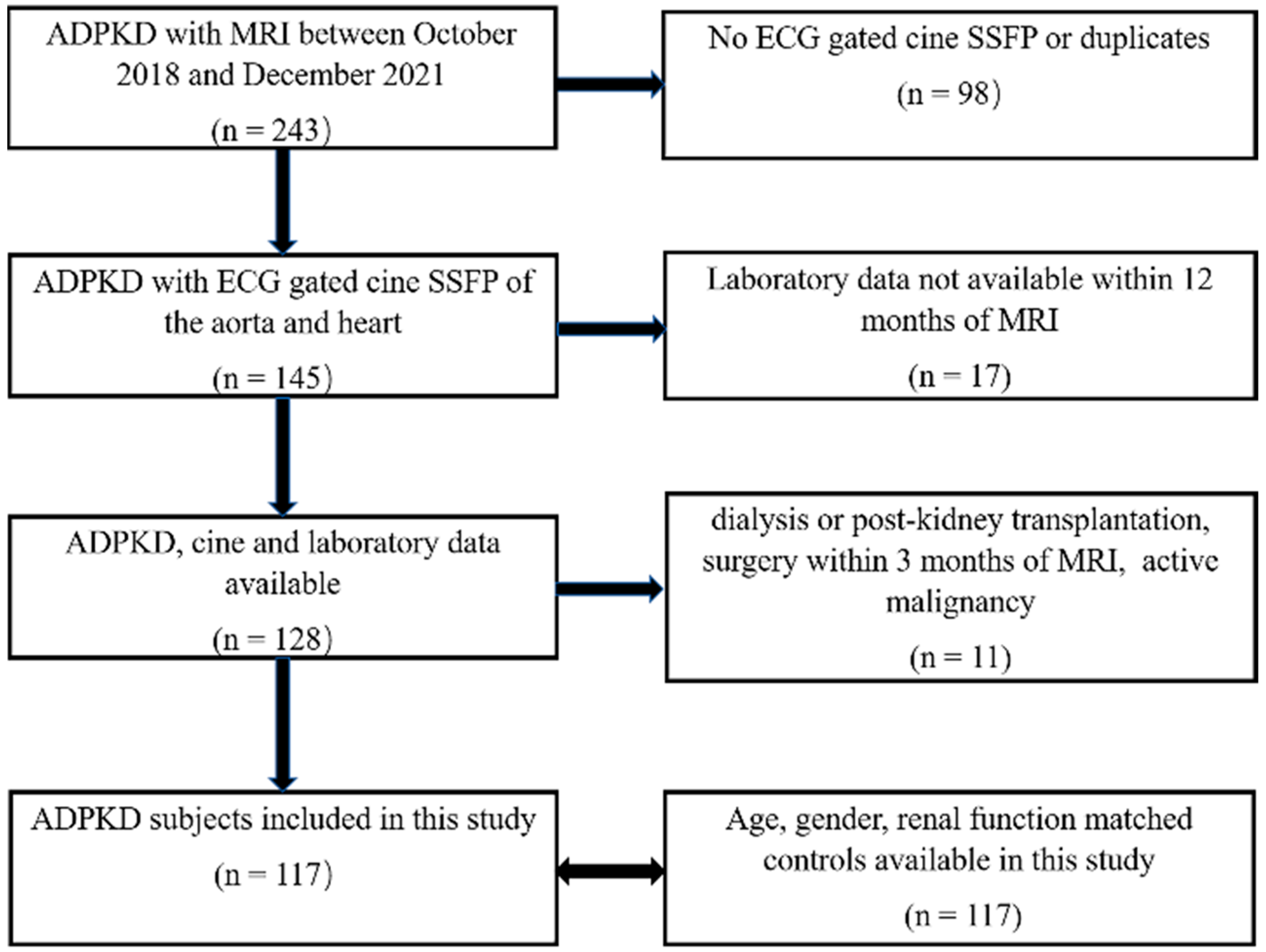
2. Materials and Methods
2.1. Study Design and Populations
2.2. Data Extraction
2.3. Image Acquisition
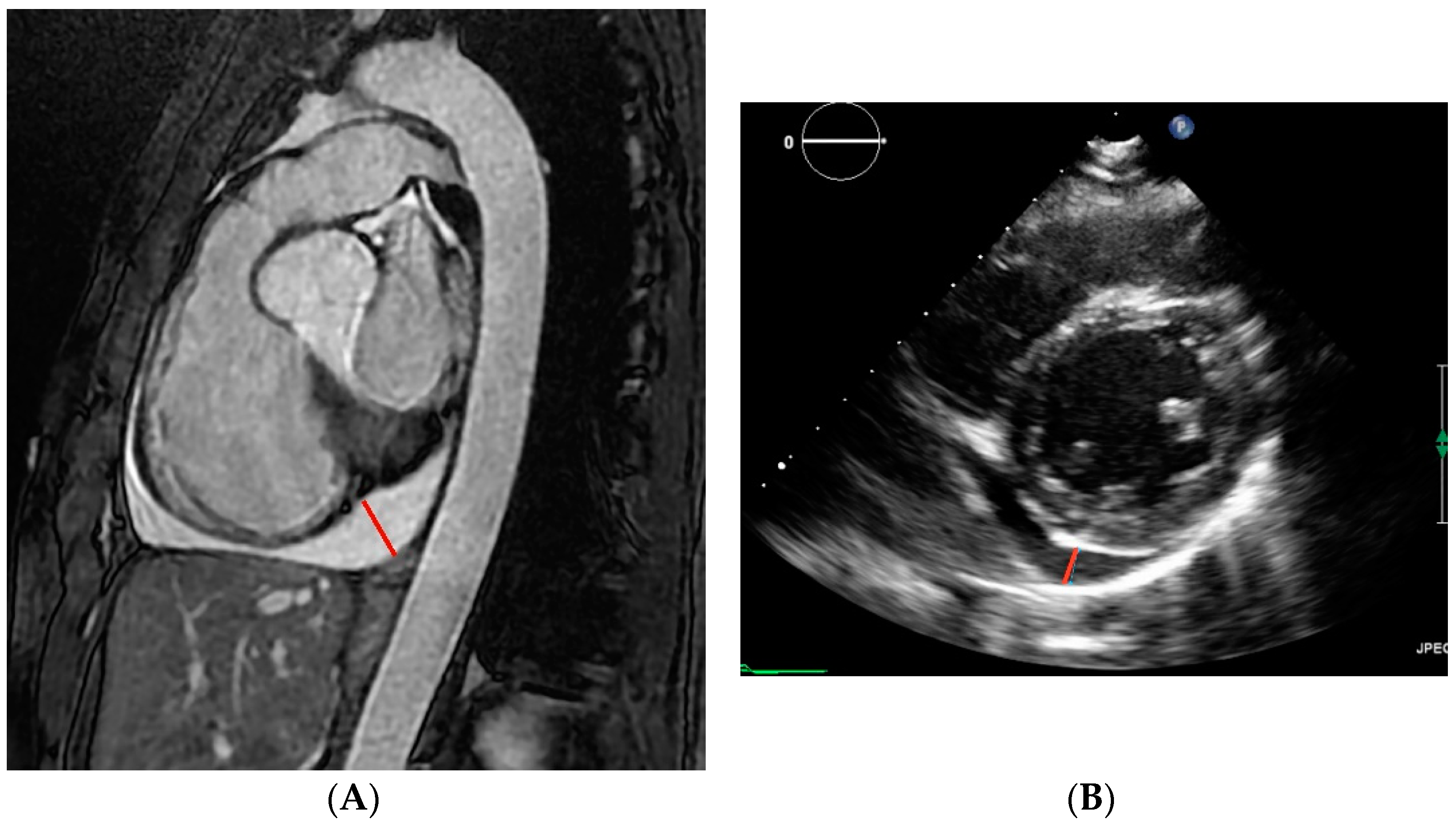
2.4. Image Analysis
2.5. Statistical Analysis
3. Results
3.1. Study Population and Characteristics
3.2. Interobserver and Intraobserver Variabilities
3.3. Prevalence of Pericardial Effusion
3.4. Correlation with Laboratory and Imaging Parameters
3.5. Comparing MRI and Echocardiography Measures of Pericardial Effusion
3.6. Genotype
3.7. Clinical Effects of Pericardial Effusion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cornec-Le Gall, E.; Alam, A.; Perrone, R.D. Autosomal dominant polycystic kidney disease. Lancet 2019, 393, 919–935. [Google Scholar] [CrossRef]
- Perumareddi, P.; Trelka, D.P. Autosomal Dominant Polycystic Kidney Disease. Prim. Care 2020, 47, 673–689. [Google Scholar] [CrossRef]
- Irazabal, M.V.; Rangel, L.J.; Bergstralh, E.J.; Osborn, S.L.; Harmon, A.J.; Sundsbak, J.L.; Bae, K.T.; Chapman, A.B.; Grantham, J.J.; Mrug, M.; et al. Imaging classification of autosomal dominant polycystic kidney disease: A simple model for selecting patients for clinical trials. J. Am. Soc. Nephrol. 2015, 26, 160–172. [Google Scholar] [CrossRef]
- Krishnappa, V.; Vinod, P.; Deverakonda, D.; Raina, R. Autosomal dominant polycystic kidney disease and the heart and brain. Cleve Clin. J. Med. 2017, 84, 471–481. [Google Scholar] [CrossRef]
- Perrone, R.D.; Malek, A.M.; Watnick, T. Vascular complications in autosomal dominant polycystic kidney disease. Nat. Rev. Nephrol. 2015, 11, 589–598. [Google Scholar] [CrossRef]
- Vlak, M.H.; Algra, A.; Brandenburg, R.; Rinkel, G.J. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011, 10, 626–636. [Google Scholar] [CrossRef]
- Chebib, F.T.; Hogan, M.C.; El-Zoghby, Z.M.; Irazabal, M.V.; Senum, S.R.; Heyer, C.M.; Madsen, C.D.; Cornec-Le Gall, E.; Behfar, A.; Harris, P.C.; et al. Autosomal Dominant Polycystic Kidney Patients May Be Predisposed to Various Cardiomyopathies. Kidney Int. Rep. 2017, 2, 913–923. [Google Scholar] [CrossRef]
- Lumiaho, A.; Ikäheimo, R.; Miettinen, R.; Niemitukia, L.; Laitinen, T.; Rantala, A.; Lampainen, E.; Laakso, M.; Hartikainen, J. Mitral valve prolapse and mitral regurgitation are common in patients with polycystic kidney disease type 1. Am. J. Kidney Dis. 2001, 38, 1208–1216. [Google Scholar] [CrossRef]
- Pei, Y.; Obaji, J.; Dupuis, A.; Paterson, A.D.; Magistroni, R.; Dicks, E.; Parfrey, P.; Cramer, B.; Coto, E.; Torra, R.; et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J. Am. Soc. Nephrol. 2009, 20, 205–212. [Google Scholar] [CrossRef]
- Pei, Y.; Hwang, Y.H.; Conklin, J.; Sundsbak, J.L.; Heyer, C.M.; Chan, W.; Wang, K.; He, N.; Rattansingh, A.; Atri, M.; et al. Imaging-based diagnosis of autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 2015, 26, 746–753. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef]
- Fernandez, G.A.P.; Ismail, M.Y. Autosomal Dominant Polycystic Kidney Disease and Pericardial Effusion. Oman Med. J. 2018, 33, 429–432. [Google Scholar] [CrossRef]
- Qian, Q.; Hartman, R.P.; King, B.F.; Torres, V.E. Increased occurrence of pericardial effusion in patients with autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 2007, 2, 1223–1227. [Google Scholar] [CrossRef]
- Çelik, A.; Bezgin, T.; Çağdaş, M. Autosomal dominant polycystic kidney disease with liver involvement and left ventricular noncompaction: An unusual coexistence. Anatol. J. Cardiol. 2021, 25, 5020. [Google Scholar] [CrossRef]
- Cosyns, B.; Plein, S.; Nihoyanopoulos, P.; Smiseth, O.; Achenbach, S.; Andrade, M.J.; Pepi, M.; Ristic, A.; Imazio, M.; Paelinck, B.; et al. European Association of Cardiovascular Imaging (EACVI) position paper: Multimodality imaging in pericardial disease. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 12–31. [Google Scholar] [CrossRef]
- Alter, P.; Figiel, J.H.; Rupp, T.P.; Bachmann, G.F.; Maisch, B.; Rominger, M.B. MR, CT, and PET imaging in pericardial disease. Heart Fail. Rev. 2013, 18, 289–306. [Google Scholar] [CrossRef]
- Thimmappa, N.D.; Blumenfeld, J.D.; Cerilles, M.A.; Dunning, A.; Donahue, S.L.; Bobb, W.O.; Zhang, H.L.; Prince, M.R. Cisterna chyli in autosomal dominant polycystic kidney disease. J. Magn. Reason. Imaging 2015, 41, 142–148. [Google Scholar] [CrossRef]
- Tangri, N.; Hougen, I.; Alam, A.; Perrone, R.; McFarlane, P.; Pei, Y. Total Kidney Volume as a Biomarker of Disease Progression in Autosomal Dominant Polycystic Kidney Disease. Can. J. Kidney Health Dis. 2017, 4, 1–6. [Google Scholar] [CrossRef]
- Neijenhuis, M.K.; Kievit, W.; Perrone, R.D.; Sloan, J.A.; Erwin, P.; Murad, M.H.; Gevers, T.J.G.; Hogan, M.C.; Drenth, J.P.H. The effect of disease severity markers on quality of life in autosomal dominant polycystic kidney disease: A systematic review, meta-analysis and meta-regression. BMC Nephrol. 2017, 18, 169. [Google Scholar] [CrossRef][Green Version]
- Bitarafan, F.; Garshasbi, M. Molecular genetic analysis of polycystic kidney disease 1 and polycystic kidney disease 2 mutations in pedigrees with autosomal dominant polycystic kidney disease. J. Res. Med. Sci. 2019, 24, 44. [Google Scholar] [CrossRef] [PubMed]

| Coronal SSFSE 1 | Axial SSFSE | Axial 3D LAVA 2 | Axial DWI 3 | Sagittal SSFP 4 Aorta and Heart | |
|---|---|---|---|---|---|
| Field of view | 40–48 | 30–42 | 30–42 | 30–42 | 30–42 |
| Matrix | 320 × 256 | 320 × 208 | 288 × 192 | 160 × 108 | 240 × 153 |
| Slice thickness | 5 mm | 5 mm | 3 mm | 5 mm | 7 mm |
| TR 5/TE 6/flip | 1200/91/130 | 1000/95/130 | 3.98/1.24/9 | 7500/90/90 | 58/1.2/39 |
| Characteristic | ADPKD Subjects n = 117 | Matched Controls n = 117 | p Value |
|---|---|---|---|
| Age | 47.3 ± 13 | 47.8 ± 13 | 0.74 |
| Male: female | 50: 67 | 50: 67 | 1 |
| White: Black: Asian: mixed: Native American: unknown | 76: 10: 8: 6: 1: 16 | 85: 11: 5: 6: 1: 9 | 0.55 |
| Body Mass Index (kg/m2) | 25 (23–29) | 26 (23–30) | 0.62 |
| Systolic Blood Pressure (mmHg) | 127 (120–136) | 125 (112–137) | 0.16 |
| Diuretic use | 24 (21%) | 26 (22%) | 0.75 |
| Rheumatological disease | 4 (3%) | 8 (7%) | 0.24 |
| Pericardial effusion (mm) mean ± SD | 2.9 ± 3.3 | 1.6 ± 1.5 | 0.001 |
| Pericardial effusion > 5 mm | 24 (21%) | 4 (3%) | 0.00006 |
| Estimated Glomerular Filtration Rate (mL/min/1.73 m2) | 67 (46–93) | 69 (52–94) | 0.44 |
| Blood Urea Nitrogen (mg/dL) | 19 (15–26) | 20 (15–27) | 0.99 |
| Albumin (g/dL) | 4.1 (4–4.3) | 4 (3.7–4.3) | 0.004 |
| Aspartate Transaminase (U/L) | 22 (18–26) | 23 (18–31) | 0.66 |
| Alanine Transaminase (U/L) | 21 (16–28) | 25 (16–36) | 0.59 |
| Right pleural effusion (mm) | 1.8 (0–3.2) | 0 (0–0) | <0.001 |
| Left pleural effusion (mm) | 0.6 (0–2.8) | 0 (0–0.67) | <0.001 |
| Pericardial Effusion: Characteristic | ≤5 mm n = 93 | >5 mm n = 24 | p Value |
|---|---|---|---|
| Age | 49 ± 13 | 42 ± 11 | 0.01 |
| Male: female | 43: 50 | 7: 17 | 0.32 |
| White:Black:Asian:mixed:Native American:unknown | 56:7:5:4:1:13 | 14:2:0:2:0:3 | - |
| Body Mass Index (kg/m2) | 25 (23–28) | 25 (23–30) | 0.69 |
| Systolic Blood Pressure (mmHg) | 129 ± 14 | 125 ± 25 | 0.32 |
| Estimated Glomerular Filtration Rate (mL/min/1.73 m2) | 65 (45–89) | 68 (46–93) | 0.72 |
| Right pleural effusion (mm) | 3.3 (2.1–4.6) | 0.7 (0–2.5) | <0.001 |
| Left pleural effusion (mm) | 2.4 (0.4–4) | 0 (0–2.6) | 0.004 |
| Tolvaptan use | 14 (15%) | 4 (17%) | 0.85 |
| Increased fluid intake (>3 L/day) | 35 (38%) | 8 (33%) | 0.70 |
| Thyroid stimulating hormone (mIU/mL) | 1.7 (1.2–2.3) | 1.6 (1.1–2.3) | 0.58 |
| Blood Urea Nitrogen (mg/dL) | 21 (17–30) | 19 (15–25) | 0.26 |
| Albumin (g/dL) | 4.2 (4.1–4.4) | 4.1 (3.9–4.3) | 0.18 |
| Aspartate Transaminase (U/L) | 21 (19–26) | 22 (18–26) | 0.43 |
| Alanine Transaminase (U/L) | 19 (16–30) | 21 (15–28) | 0.78 |
| Total Kidney Volume/height | 994 (660–1305) | 803 (519–1470) | 0.41 |
| Liver volume (mL) | 1895 (1523–2321) | 1792 (1517–2125) | 0.79 |
| Spleen volume (mL) | 246 (208–309) | 234 (187–285) | 0.24 |
| Cisterna chyli diameter (mm) | 4 (3–5) | 4 (3–5) | 0.84 |
| Genotype data available (%) | 59 (63%) | 16 (67%) | 0.09 |
| PKD1 1-only Mutation (%) | 29 of 59 (49%) | 11 of 16 (69%) | 0.16 |
| Truncating 2 | 20 of 29 (69%) | 4 of 11 (36%) | 0.07 |
| Nontruncating 2 | 6 of 29 (21%) | 5 of 11 (45%) | 0.07 |
| PKD2 3-only Mutation (%) | 13 of 59 (22%) | 1 of 16 (6%) | 0.15 |
| Truncating | 13 of 13 (100%) | 1 of 1 (100%) | 1 |
| Nontruncating | 0 of 13 | 0 of 1 | 1 |
| PKD1 & PKD2 Mutation (%) | 1 of 59 (2%) | 1 of 16 (6%) | 0.32 |
| No mutation detected (%) | 16 of 59 (27%) | 3 of 16 (19%) | 0.49 |
| Variables | Correlation Coefficient | p Value |
|---|---|---|
| Age | −0.28 | 0.003 |
| Gender | 0.24 | 0.010 |
| Body Mass Index | 0.01 | 0.892 |
| Systolic Blood Pressure | −0.15 | 0.118 |
| Right pleural effusion | 0.50 | 0.00000001 |
| Left pleural effusion | 0.37 | 0.00004 |
| Total Kidney Volume/height | 0.06 | 0.498 |
| Liver volume | 0.06 | 0.557 |
| Spleen volume | 0.13 | 0.164 |
| Cisterna chyli diameter | 0.03 | 0.787 |
| Serum Creatinine | 0.07 | 0.460 |
| Blood Urea Nitrogen | 0.03 | 0.748 |
| Estimated Glomerular Filtration Rate | −0.04 | 0.651 |
| Albumin | −0.003 | 0.970 |
| Aspartate Transaminase | −0.17 | 0.066 |
| Alanine Transaminase | −0.06 | 0.497 |
| Thyroid stimulating hormone | −0.06 | 0.54 |
| Tolvaptan use | −0.03 | 0.75 |
| Increased fluid intake | −0.14 | 0.12 |
| Coefficient | Standard Error | t Stat | p-Value | Lower 95% | Upper 95% | |
|---|---|---|---|---|---|---|
| Intercept | 2.24 | 1.25 | 1.79 | 0.08 | −0.23 | 4.72 |
| Pleural effusion | 0.60 | 0.11 | 5.47 | 0.0000003 | 0.38 | 0.81 |
| Age | −0.03 | 0.02 | −1.17 | 0.24 | −0.07 | 0.02 |
| Gender | 1.21 | 0.54 | 2.24 | 0.03 | 0.14 | 2.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Fujikura, K.; Dev, H.; Riyahi, S.; Blumenfeld, J.; Kim, J.; Rennert, H.; Prince, M.R. Pericardial Effusion on MRI in Autosomal Dominant Polycystic Kidney Disease. J. Clin. Med. 2022, 11, 1127. https://doi.org/10.3390/jcm11041127
Liu J, Fujikura K, Dev H, Riyahi S, Blumenfeld J, Kim J, Rennert H, Prince MR. Pericardial Effusion on MRI in Autosomal Dominant Polycystic Kidney Disease. Journal of Clinical Medicine. 2022; 11(4):1127. https://doi.org/10.3390/jcm11041127
Chicago/Turabian StyleLiu, Jin, Kana Fujikura, Hreedi Dev, Sadjad Riyahi, Jon Blumenfeld, Jiwon Kim, Hanna Rennert, and Martin R. Prince. 2022. "Pericardial Effusion on MRI in Autosomal Dominant Polycystic Kidney Disease" Journal of Clinical Medicine 11, no. 4: 1127. https://doi.org/10.3390/jcm11041127
APA StyleLiu, J., Fujikura, K., Dev, H., Riyahi, S., Blumenfeld, J., Kim, J., Rennert, H., & Prince, M. R. (2022). Pericardial Effusion on MRI in Autosomal Dominant Polycystic Kidney Disease. Journal of Clinical Medicine, 11(4), 1127. https://doi.org/10.3390/jcm11041127








