The Prevalence and the Impact of Frailty in Hepato-Biliary Pancreatic Cancers: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Quality and Risk of Bias Assessment
2.4. Data Extraction
2.5. Statistical Analysis
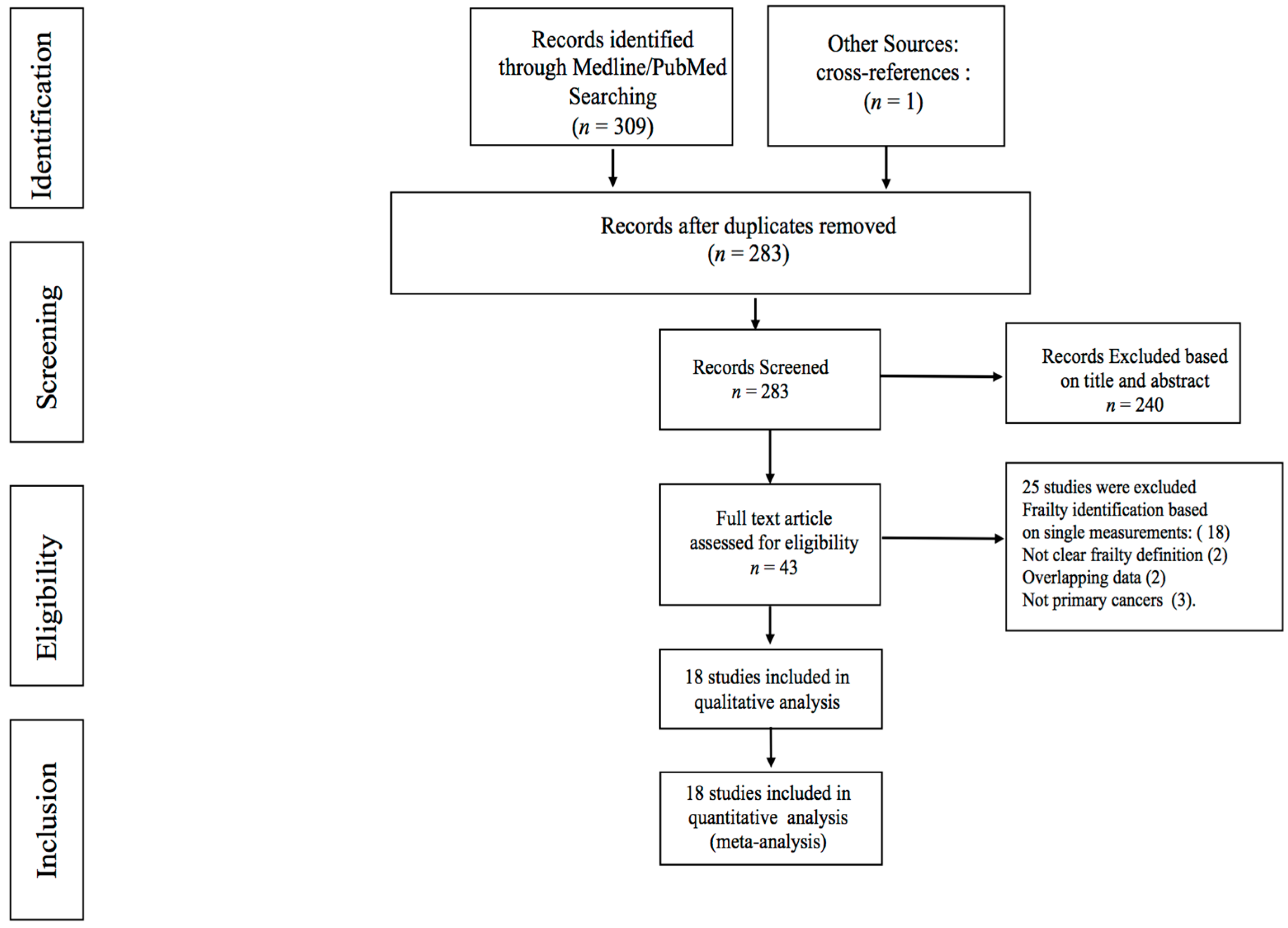
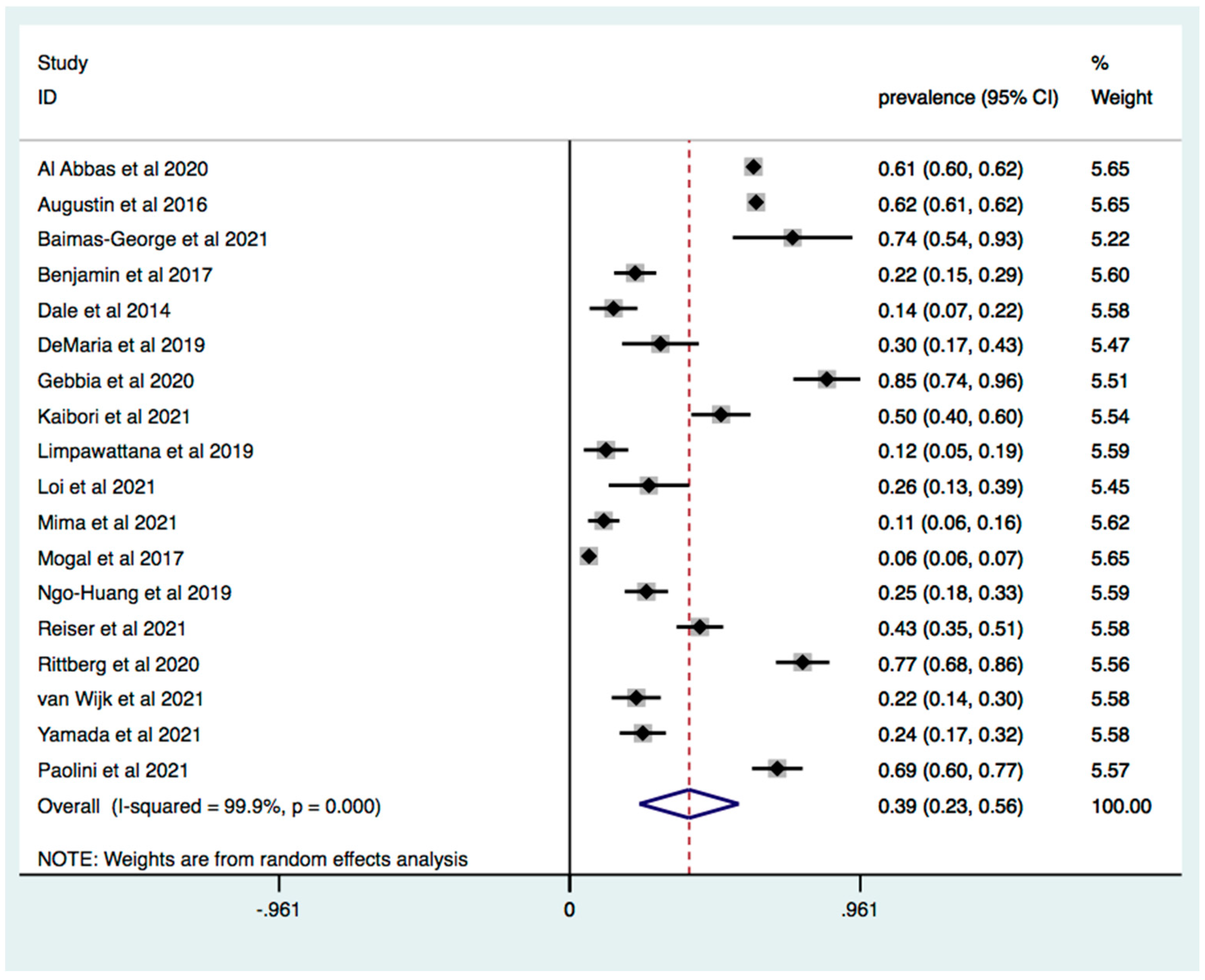
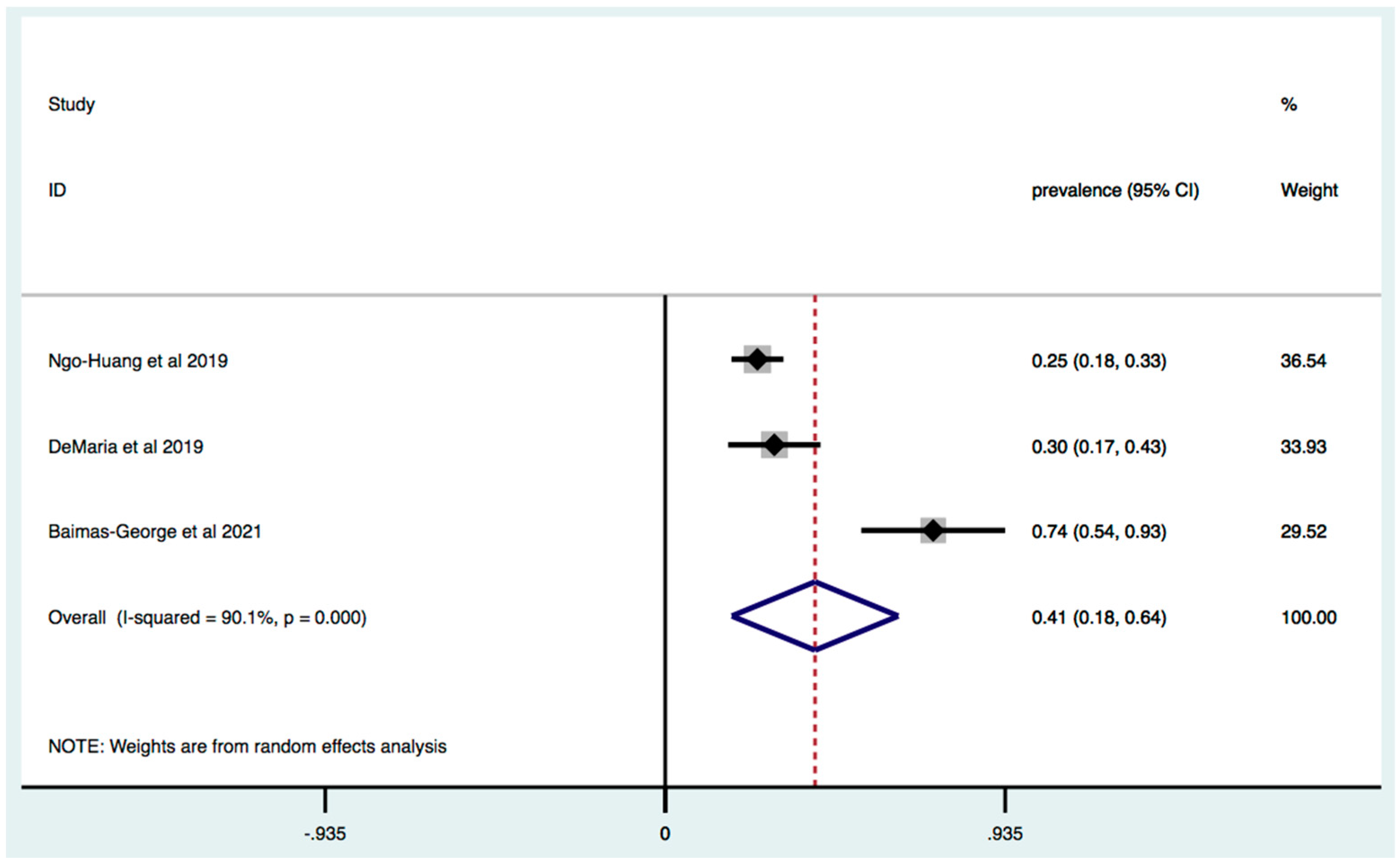
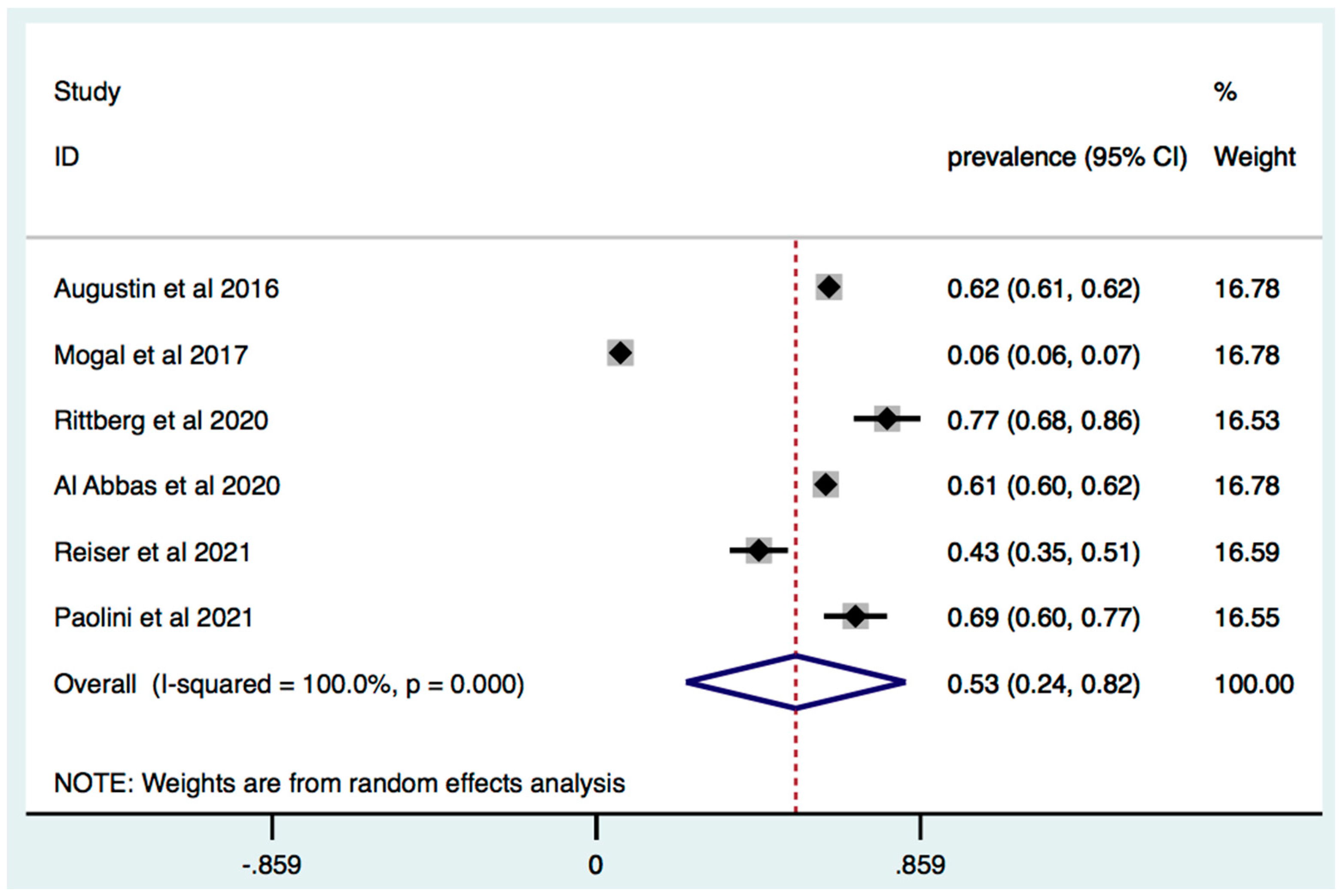
3. Results
3.1. Prevalence of Frailty in Patients with Pancreatic and Liver Cancer
3.2. Subgroup Analysis
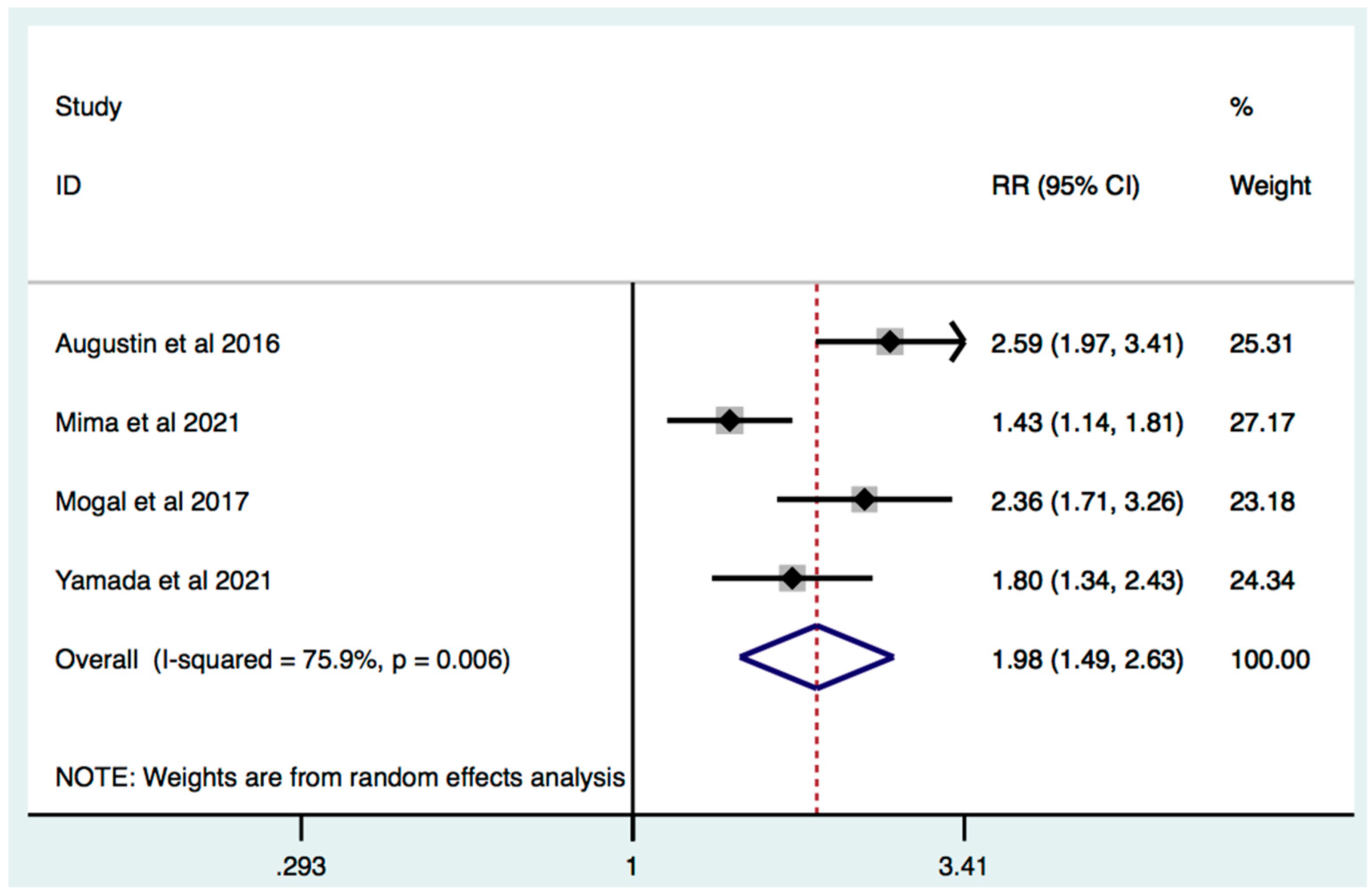
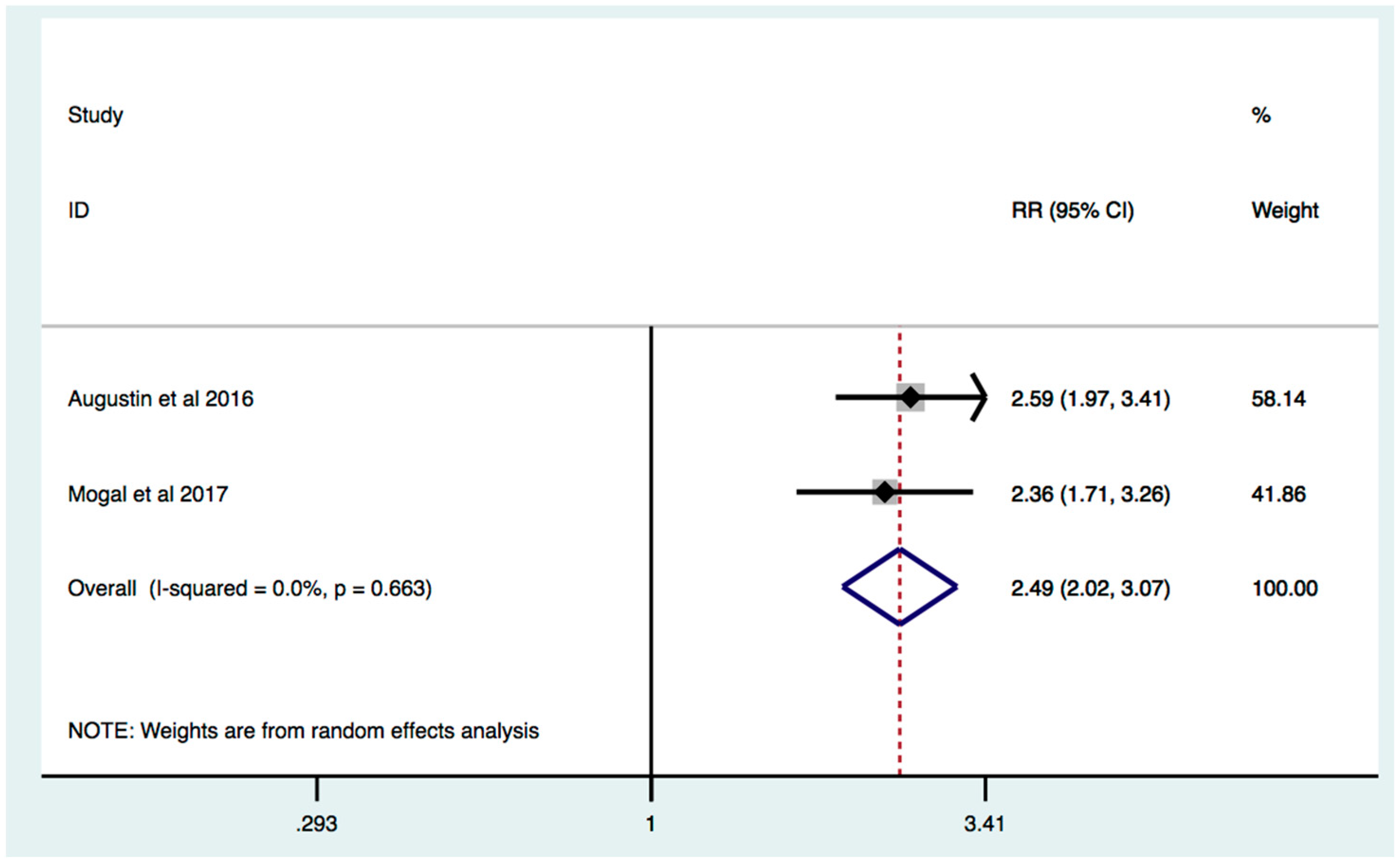
3.3. Frailty Is Associated with Increased Mortality in Patients with HPB Cancer
3.4. Study Quality
3.5. Publication Bias
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.W.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handforth, C.; Clegg, A.; Young, C.; Simpkins, S.; Seymour, M.T.; Selby, P.J.; Young, J. The prevalence and outcomes of frailty in older cancer patients: A systematic review. Ann. Oncol. 2014, 26, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Venuta, F.; Diso, D.; Onorati, I.; Anile, M.; Mantovani, S.; Rendina, E.A. Lung cancer in elderly patients. J. Thorac. Dis. 2016, 8 (Suppl. 11), S908–S914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Dale, W.; Hemmerich, J.; Kamm, A.; Posner, M.C.; Matthews, J.B.; Rothman, R.; Palakodeti, A.; Roggin, K.K. Geriatric assessment improves prediction of surgical outcomes in older adults undergoing pancreaticoduodenectomy: A prospective cohort study. Ann. Surg. 2014, 259, 960–965. [Google Scholar] [CrossRef]
- de la Fuente, S.G.; Bennett, K.M.; Pappas, T.N.; Scarborough, J.E. Pre- and intraoperative variables affecting early outcomes in elderly patients undergoing pancreaticoduodenectomy. HPB 2011, 13, 887–892. [Google Scholar] [CrossRef] [Green Version]
- Rostoft, S.; van Leeuwen, B. Frailty assessment tools and geriatric assessment in older patients with hepatobiliary and pancreatic malignancies. Eur. J. Surg. Oncol. 2021, 47, 514–518. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Open Med. 2009, 3, e123–e130. [Google Scholar] [PubMed]
- Lo, C.K.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef] [Green Version]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Thompson, S.G.; Sharp, S.J. Explaining heterogeneity in meta-analysis: A comparison of methods. Stat. Med. 1999, 18, 2693–2708. [Google Scholar] [CrossRef]
- Al Abbas, A.I.; Borrebach, J.D.; Pitt, H.A.; Bellon, J.; Hogg, M.E.; Zeh, H.J.; Zureikat, A.H. Development of a Novel Pancreatoduodenectomy-Specific Risk Calculator: An Analysis of 10,000 Patients. J. Gastrointest. Surg. 2020, 25, 1503–1511. [Google Scholar] [CrossRef]
- Augustin, T.; Burstein, M.D.; Schneider, E.B.; Morris-Stiff, G.; Wey, J.; Chalikonda, S.; Walsh, R.M. Frailty predicts risk of life-threatening complications and mortality after pancreatic resections. Surgery 2016, 160, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Baimas-George, M.; Watson, M.; Thompson, K.; Shastry, V.; Iannitti, D.; Martinie, J.B.; Baker, E.; Parala-Metz, A.; Vrochides, D. Prehabilitation for Hepatopancreatobiliary Surgical Patients: Interim Analysis Demonstrates a Protective Effect From Neoadjuvant Chemotherapy and Improvement in the Frailty Phenotype. Am. Surg. 2020, 87, 714–724. [Google Scholar] [CrossRef]
- DeMaria, S.D., Jr.; Khromava, M.; Schiano, T.D.; Lin, H.M.; Kim, S. Standardized measures of frailty predict hospital length of stay following orthotopic liver transplantation for hepatocellular carcinoma. Clin. Transplant. 2019, 33, e13746. [Google Scholar] [CrossRef]
- Gebbia, V.; Mare, M.; Cordio, S.; Valerio, M.R.; Piazza, D.; Bordonaro, R.; Firenze, A.; Giuffrida, D. Is G8 geriatric assessment tool useful in managing elderly patients with metastatic pancreatic carcinoma? J. Geriatr. Oncol. 2021, 12, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Kaibori, M.; Matsushima, H.; Ishizaki, M.; Kosaka, H.; Matsui, K.; Ogawa, A.; Yoshii, K.; Sekimoto, M. Perioperative Geriatric Assessment as A Predictor of Long-Term Hepatectomy Outcomes in Elderly Patients with Hepatocellular Carcinoma. Cancers 2021, 13, 842. [Google Scholar] [CrossRef]
- Limpawattana, P.; Wirasorn, K.; Sookprasert, A.; Sawanyawisuth, K.; Titapun, A.; Luvira, V.; Khuntikeo, N.; Chindaprasirt, J. Frailty Syndrome in Biliary Tract Cancer Patients: Prevalence and Associated Factors. Asian Pac. J. Cancer Prev. 2019, 20, 1497–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loi, M.; Comito, T.; Franzese, C.; Desideri, I.; Dominici, L.; Faro, L.L.; Clerici, E.; Franceschini, D.; Baldaccini, D.; Badalamenti, M.; et al. Charlson comorbidity index and G8 in older old adult (≥80 years) hepatocellular carcinoma patients treated with stereotactic body radiotherapy. J. Geriatr. Oncol. 2021, 12, 1100–1103. [Google Scholar] [CrossRef] [PubMed]
- Mima, K.; Hayashi, H.; Nakagawa, S.; Matsumoto, T.; Kinoshita, S.; Matsumura, K.; Kitamura, F.; Uemura, N.; Nakao, Y.; Itoyama, R.; et al. Frailty is associated with poor prognosis after resection for pancreatic cancer. Int. J. Clin. Oncol. 2021, 26, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Mogal, H.; Bs, S.A.V.; Dodson, R.; Hsu, F.-C.; Howerton, R.; Shen, P.; Clark, C.J. Modified Frailty Index Predicts Morbidity and Mortality After Pancreaticoduodenectomy. Ann. Surg. Oncol. 2017, 24, 1714–1721. [Google Scholar] [CrossRef] [PubMed]
- Ngo-Huang, A.; Holmes, H.M.; Bordes, J.K.A.D.; Parker, N.H.; Fogelman, D.; Petzel, M.Q.B.; Song, J.; Bruera, E.; Katz, M.H.G. Association between frailty syndrome and survival in patients with pancreatic adenocarcinoma. Cancer Med. 2019, 8, 2867–2876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieser, C.J.; Zenati, M.; Narayanan, S.; Bahary, N.; Lee, K.K.; Paniccia, A.; Bartlett, D.L.; Zureikat, A.H. Optimal Management of Resectable Pancreatic Head Cancer in the Elderly Patient: Does Neoadjuvant Therapy Offer a Survival Benefit? Ann. Surg. Oncol. 2021, 28, 6264–6272. [Google Scholar] [CrossRef] [PubMed]
- Rittberg, R.; Zhang, H.; Lambert, P.; Kudlovich, R.; Kim, C.A.; Dawe, D.E. Utility of the modified frailty index in predicting toxicity and cancer outcomes for older adults with advanced pancreatic cancer receiving first-line palliative chemotherapy. J. Geriatr. Oncol. 2020, 12, 112–117. [Google Scholar] [CrossRef]
- van Wijk, L.; van der Snee, L.; Buis, C.I.; Hentzen, J.E.K.R.; Haveman, M.E.; Klaase, J.M. A prospective cohort study evaluating screening and assessment of six modifiable risk factors in HPB cancer patients and compliance to recommended prehabilitation interventions. Perioper. Med. 2021, 10, 1–12. [Google Scholar] [CrossRef]
- Yamada, S.; Shimada, M.; Morine, Y.; Imura, S.; Ikemoto, T.; Saito, Y.; Miyazaki, K.; Tokunaga, T.; Nishi, M. Significance of frailty in prognosis after surgery in patients with pancreatic ductal adenocarcinoma. World J. Surg. Oncol. 2021, 19, 1–8. [Google Scholar] [CrossRef]
- Paolini, C.; Bencini, L.; Gabellini, L.; Urciuoli, I.; Pacciani, S.; Tribuzi, A.; Moraldi, L.; Calistri, M.; Coratti, A. Robotic versus open pancreaticoduodenectomy: Is there any difference for frail patients? Surg. Oncol. 2021, 37, 101515. [Google Scholar] [CrossRef]
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.; Doehner, W.; Evans, J.; et al. Frailty Consensus: A Call to Action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [Green Version]
- Corbi, G.; Cacciatore, F.; Komici, K.; Rengo, G.; Vitale, D.F.; Furgi, G.; Pagano, G.; Bencivenga, L.; Davinelli, S.; Ferrara, N. Inter-relationships between Gender, Frailty and 10-Year Survival in Older Italian Adults: An observational longitudinal study. Sci. Rep. 2019, 9, 18416. [Google Scholar] [CrossRef]
- Collard, R.M.; Boter, H.; Schoevers, R.A.; Voshaar, R.O. Prevalence of Frailty in Community-Dwelling Older Persons: A Systematic Review. J. Am. Geriatr. Soc. 2012, 60, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Marengoni, A.; Vetrano, D.L.; Manes-Gravina, E.; Bernabei, R.; Onder, G.; Palmer, K. The Relationship between COPD and Frailty: A Systematic Review and Meta-Analysis of Observational Studies. Chest 2018, 154, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Kitzman, D.; Whellan, D.J.; Duncan, P.W.; Mentz, R.J.; Pastva, A.M.; Nelson, M.B.; Upadhya, B.; Chen, H.; Reeves, G.R. Frailty Among Older Decompensated Heart Failure Patients: Prevalence, Association With Patient-Centered Outcomes, and Efficient Detection Methods. JACC Heart Fail. 2019, 7, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Laube, R.; Wang, H.; Park, L.; Heyman, J.K.; Vidot, H.; Majumdar, A.; Strasser, S.I.; Mccaughan, G.; Liu, K. Frailty in advanced liver disease. Liver Int. 2018, 38, 2117–2128. [Google Scholar] [CrossRef] [Green Version]
- Pericleous, M.; Khan, S.A. Epidemiology of HPB malignancy in the elderly. Eur. J. Surg. Oncol. 2021, 47, 503–513. [Google Scholar] [CrossRef]
- Park, J.; Chen, M.; Colombo, M.; Roberts, L.; Schwartz, M.; Chen, P.-J.; Kudo, M.; Johnson, P.; Wagner, S.; Orsini, L.S.; et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: The BRIDGE Study. Liver Int. 2015, 35, 2155–2166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosetti, C.; Bertuccio, P.; Negri, E.; La Vecchia, C.; Zeegers, M.; Boffetta, P. Pancreatic cancer: Overview of descriptive epidemiology. Mol. Carcinog. 2011, 51, 3–13. [Google Scholar] [CrossRef]
- Kojima, G. Frailty as a Predictor of Nursing Home Placement Among Community-Dwelling Older Adults: A Systematic Review and Meta-analysis. J. Geriatr. Phys. Ther. 2018, 41, 42–48. [Google Scholar] [CrossRef]
- Martínez-Velilla, N.; Herce, P.A.; Herrero, C.; Gutiérrez-Valencia, M.; de Asteasu, M.L.S.; Mateos, A.S.; Zubillaga, A.C.; Beroiz, B.I.; Jiménez, A.G.; Izquierdo, M. Heterogeneity of Different Tools for Detecting the Prevalence of Frailty in Nursing Homes: Feasibility and Meaning of Different Approaches. J. Am. Med. Dir. Assoc. 2017, 18, 898.e1–898.e8. [Google Scholar] [CrossRef]
- Woo, J.; Leung, J.; Morley, J.E. Comparison of Frailty Indicators Based on Clinical Phenotype and the Multiple Deficit Approach in Predicting Mortality and Physical Limitation. J. Am. Geriatr. Soc. 2012, 60, 1478–1486. [Google Scholar] [CrossRef]
- Eeles, E.M.P.; White, S.V.; O’Mahony, S.M.; Bayer, A.J.; Hubbard, R.E. The impact of frailty and delirium on mortality in older inpatients. Age Ageing 2012, 41, 412–416. [Google Scholar] [CrossRef] [Green Version]
- Balestroni, G.; Panzeri, A.; Omarini, P.; Cerutti, P.; Sacco, D.; Giordano, A.; Pistono, M.; Komici, K.; Ferrario, S.R. Psychophysical health of elderly inpatients in cardiac rehabilitation: A retrospective cohort study. Eur. J. Phys. Rehabilit. Med. 2020, 56, 197–205. [Google Scholar] [CrossRef]
- Calise, F.; Giuliani, A.; Sodano, L.; Crolla, E.; Bianco, P.; Rocca, A.; Ceriello, A. Segmentectomy: Is minimally invasive surgery going to change a liver dogma? Updates Surg. 2015, 67, 111–115. [Google Scholar] [CrossRef]
- Rovesti, G.; Valoriani, F.; Rimini, M.; Bardasi, C.; Ballarin, R.; Di Benedetto, F.; Menozzi, R.; Dominici, M.; Spallanzani, A. Clinical Implications of Malnutrition in the Management of Patients with Pancreatic Cancer: Introducing the Concept of the Nutritional Oncology Board. Nutrients 2021, 13, 3522. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, C.; de Heer, P.; Bjerre, E.D.; Suetta, C.; Hojman, P.; Pedersen, B.K.; Svendsen, L.B.; Christensen, J.F. Sarcopenia and Postoperative Complication Risk in Gastrointestinal Surgical Oncology: A meta-analysis. Ann. Surg. 2018, 268, 58–69. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; MacDonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer Cachexia in the Age of Obesity: Skeletal Muscle Depletion Is a Powerful Prognostic Factor, Independent of Body Mass Index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Meng, S.; Li, R.; Ye, J.; Zhao, L. Clinical significance of sarcopenia in the treatment of patients with primary hepatic malignancies, a systematic review and meta-analysis. Oncotarget 2017, 8, 102474–102485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EuroSurg Collaborative. Body mass index and complications following major gastrointestinal surgery: A prospective, international cohort study and meta-analysis. Color. Dis. 2018, 20, O215–O225. [Google Scholar] [CrossRef] [Green Version]
- Roderburg, C.; Wree, A.; Demir, M.; Schmelzle, M.; Tacke, F. The role of the innate immune system in the development and treatment of hepatocellular carcinoma. Hepatic Oncol. 2020, 7, HEP17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padoan, A.; Plebani, M.; Basso, D. Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity. Int. J. Mol. Sci. 2019, 20, 676. [Google Scholar] [CrossRef] [Green Version]
- Panzeri, A.; Komici, K.; Cerutti, P.; Sacco, D.; Pistono, M.; Ferrario, S.R. Gender differences and long term outcome of over 75 elderlies in cardiac rehabilitation: Highlighting the role of psychological and physical factors through a secondary analysis of a cohort study. Eur. J. Phys. Rehabil. Med. 2021, 57, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Souwer, E.T.; Moos, S.I.; van Rooden, C.J.; Bijlsma, A.Y.; Bastiaannet, E.; Steup, W.H.; Dekker, J.W.T.; Fiocco, M.; Bos, F.V.D.; Portielje, J.E. Physical performance has a strong association with poor surgical outcome in older patients with colorectal cancer. Eur. J. Surg. Oncol. 2020, 46, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.; Brunese, M.; Cappuccio, M.; Scacchi, A.; Martucci, G.; Buondonno, A.; Perrotta, F.; Quarto, G.; Avella, P.; Amato, B. Impact of Physical Activity on Disability Risk in Elderly Patients Hospitalized for Mild Acute Diverticulitis and Diverticular Bleeding Undergone Conservative Management. Medicina 2021, 57, 360. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.; Michaelson, J.S.; Hutter, M.M.; Lancaster, R.T.; Warshaw, A.L.; Henderson, W.G.; Khuri, S.F.; Tanabe, K.K. Morbidity and Mortality after Liver Resection: Results of the Patient Safety in Surgery Study. J. Am. Coll. Surg. 2007, 204, 1284–1292. [Google Scholar] [CrossRef]
- Kamiyama, T.; Nakanishi, K.; Yokoo, H.; Kamachi, H.; Tahara, M.; Yamashita, K.; Taniguchi, M.; Shimamura, T.; Matsushita, M.; Todo, S. Perioperative Management of Hepatic Resection Toward Zero Mortality and Morbidity: Analysis of 793 Consecutive Cases in a Single Institution. J. Am. Coll. Surg. 2010, 211, 443–449. [Google Scholar] [CrossRef]
- Ceccarelli, G.; Costa, G.; De Rosa, M.; Pisanelli, M.C.; Frezza, B.; De Prizio, M.; Bravi, I.; Scacchi, A.; Gallo, G.; Amato, B.; et al. Minimally Invasive Approach to Gastric GISTs: Analysis of a Multicenter Robotic and Laparoscopic Experience with Literature Review. Cancers 2021, 13, 4351. [Google Scholar] [CrossRef]
- Malot, C.; Durand-Bouteau, A.; Barizien, N.; Bizard, A.; Kennel, T.; Fischler, M.; Minnella, E.; Le Guen, M. Prehabilitation Program in Elderly Patients: A Prospective Cohort Study of Patients Followed Up Postoperatively for Up to 6 Months. J. Clin. Med. 2021, 10, 4500. [Google Scholar] [CrossRef]
- Ceccarelli, G.; Andolfi, E.; Biancafarina, A.; Rocca, A.; Amato, M.; Milone, M.; Scricciolo, M.; Frezza, B.; Miranda, E.; De Prizio, M.; et al. Robot-assisted surgery in elderly and very elderly population: Our experience in oncologic and general surgery with literature review. Aging Clin. Exp. Res. 2016, 29, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Giuliani, A.; Ceccarelli, G.; Rocca, A. The role of laparoscopic distal pancreatectomy in elderly patients. Minerva Chir. 2018, 73, 179–187. [Google Scholar] [CrossRef]
- Montroni, I.; Rostoft, S.; Spinelli, A.; Van Leeuwen, B.L.; Ercolani, G.; Saur, N.M.; Jaklitsch, M.T.; Somasundar, P.S.; Carino, N.D.L.; Ghignone, F.; et al. GOSAFE—Geriatric Oncology Surgical Assessment and Functional rEcovery after Surgery: Early analysis on 977 patients. J. Geriatr. Oncol. 2019, 11, 244–255. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Cecilia, D.; Cipriani, F.; Vishal, S.; Ratti, F.; Tranchart, H.; Barkhatov, L.; Tomassini, F.; Montalti, R.; Halls, M.; Troisi, R.I.; et al. Laparoscopic Versus Open Liver Resection for Colorectal Metastases in Elderly and Octogenarian Patients: A Multicenter Propensity Score Based Analysis of Short- and Long-term Outcomes. Ann. Surg. 2017, 265, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, D.; Marvaso, A.; Ceraso, S.; Cinelli, N.A.; Rocca, A.; Vitale, M.; Rossi, M.; Genovese, E.A.; Amato, B.; Cinelli, M. Minimal invasive surgery in treatment of liver metastases from colorectal carcinomas: Case studies and survival rates. BMC Surg. 2013, 13, S45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocca, A.; Scacchi, A.; Cappuccio, M.; Avella, P.; Bugiantella, W.; De Rosa, M.; Costa, G.; Polistena, A.; Codacci-Pisanelli, M.; Amato, B.; et al. Robotic surgery for colorectal liver metastases resection: A systematic review. Int. J. Med. Robot. Comput. Assist. Surg. 2021, 17, e2330. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.; Cipriani, F.; Belli, G.; Berti, S.; Boggi, U.; Bottino, V.; Cillo, U.; Cescon, M.; Cimino, M.; Corcione, F.; et al. The Italian Consensus on minimally invasive simultaneous resections for synchronous liver metastasis and primary colorectal cancer: A Delphi methodology. Updat. Surg. 2021, 73, 1247–1265. [Google Scholar] [CrossRef] [PubMed]
- Sena, G.; Picciariello, A.; Marino, F.; Goglia, M.; Rocca, A.; Meniconi, R.L.; Gallo, G. One-Stage Total Laparoscopic Treatment for Colorectal Cancer with Synchronous Metastasis. Is It Safe and Feasible? Front. Surg. 2021, 8, 752135. [Google Scholar] [CrossRef]
| First Author and Year | Study Design | Total Population | Mean Age | Male (%) | Frail | Non-Frail | Frailty Tool | Cancer Type | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| Al Abbas et al., 2020 | Retrospective Cohort Study | 9867 | 64.5 | 53.2 | 5996 | 3871 | Modified Frailty Index | Pancreatic Cancers (Adenocarcinoma 50%) | Surgery, Chemotherapy, Radiotherapy |
| Augustin et al., 2016 | Retrospective Cohort Study | 13,020 | N/R | 48.3 | 8024 | 4996 | Modified Frailty Index | Pancreatic Cancer, not specified | Surgery, Chemotherapy, Radiation |
| Baimas-George et al., 2021 | Prospective Cohort Study | 19 | 62 | 47 | 14 | 5 | Fried Phenotype Model | Pancreatic Adenocarcinoma (90%); Colangiocarcinoma (10%) | Chemotherapy, Surgery |
| Benjamin et al., 2017 | Prospective Cohort Study | 134 | 65.4 | 52 | 29 | 105 | SPPB | Pancreatic Ductal Adenocarcinoma | Surgery |
| Dale et al., 2014 | Prospective Cohort Study | 76 | 67.3 | 56.3 | 11 | 65 | VES-13, Fried and SPPB | Pancreatic Endocrine, Exocrine, Biliary | Surgery |
| DeMaria et al., 2019 | Prospective Cohort Study | 50 | 64 | 68 | 15 | 35 | Fried Phenotype Model | Hepatocellular Carcinoma | Surgery, liver transplantation |
| Gebbia et al., 2020 | Prospective Cohort Study | 40 | 74.7 | 65 | 34 | 6 | G8 | Advanced/metastatic Pancreatic Carcinoma | Chemotherapy |
| Kaibori et al., 2021 | Retrospective Cohort Study | 100 | 79 | N/R | 50 | 50 | G8, VES-13 | Hepatocellular Carcinoma | Surgery |
| Limpawattana et al., 2019 | Retrospective Cohort Study | 75 | N/R | 77.3 | 9 | 66 | Frail Scale | Biliary Cancer | Chemotherapy |
| Loi et al., 2021 | Retrospective Cohort Study | 42 | 85.3 | N/R | 11 | 31 | G8 | Hepatocellular Carcinoma | SBRT |
| Mima et al., 2021 | Retrospective Cohort Study | 142 | 56 | 16 | 126 | Clinical Frailty Scale | Pancreatic Cancer: Adenocarcinoma (98%) | Surgery | |
| Mogal et al., 2017 | Retrospective Cohort Study | 9986 | 64.1 | 51.2 | 637 | 9349 | Modified Frailty Index | Pancreatic Cancer, not specified | Surgery |
| Ngo-Huang et al., 2019 | Prospective Cohort Study | 142 | 65 | 65.5 | 36 | 106 | Fried Phenotype Model | Pancreatic Ductal Adenocarcinoma | Surgery, Chemotherapy, Radiation, Palliative |
| Reiser et al., 2021 | Retrospective Cohort Study | 158 | N/R | 37 | 68 | 90 | Modified Frailty Index | Pancreatic Ductal Adenocarcinoma | Surgery, Neoadjuvant therapy |
| Rittberg et al., 2020 | Retrospective Cohort Study | 87 | 73.7 | 54 | 67 | 20 | Modified Frailty Index | Advanced Pancreatic Cancer | Chemotherapy |
| van Wijk et al., 2021 | Prospective Cohort Study | 100 | 74 | 51 | 22 | 78 | Groningen frailty indicator | Hepatobiliary pancreatic cancers (Mixed population) | Scheduled for surgery |
| Yamada et al., 2021 | Retrospective Cohort Study | 120 | N/R | N/R | 29 | 91 | Clinical Frailty Scale | Pancreatic Ductal Adenocarcinoma | Surgery |
| Paolini et al., 2021 | Retrospective Cohort Study | 118 | 52.5 | 81 | 37 | Modified Frailty Index | Pancreatic, periampullary cancers, common bile duct cancers | Surgery (open or robotic) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komici, K.; Cappuccio, M.; Scacchi, A.; Vaschetti, R.; Delli Carpini, G.; Picerno, V.; Avella, P.; Brunese, M.C.; Rengo, G.; Guerra, G.; et al. The Prevalence and the Impact of Frailty in Hepato-Biliary Pancreatic Cancers: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1116. https://doi.org/10.3390/jcm11041116
Komici K, Cappuccio M, Scacchi A, Vaschetti R, Delli Carpini G, Picerno V, Avella P, Brunese MC, Rengo G, Guerra G, et al. The Prevalence and the Impact of Frailty in Hepato-Biliary Pancreatic Cancers: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(4):1116. https://doi.org/10.3390/jcm11041116
Chicago/Turabian StyleKomici, Klara, Micaela Cappuccio, Andrea Scacchi, Roberto Vaschetti, Giuseppe Delli Carpini, Vito Picerno, Pasquale Avella, Maria Chiara Brunese, Giuseppe Rengo, Germano Guerra, and et al. 2022. "The Prevalence and the Impact of Frailty in Hepato-Biliary Pancreatic Cancers: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 4: 1116. https://doi.org/10.3390/jcm11041116
APA StyleKomici, K., Cappuccio, M., Scacchi, A., Vaschetti, R., Delli Carpini, G., Picerno, V., Avella, P., Brunese, M. C., Rengo, G., Guerra, G., & Bencivenga, L. (2022). The Prevalence and the Impact of Frailty in Hepato-Biliary Pancreatic Cancers: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(4), 1116. https://doi.org/10.3390/jcm11041116









