Correlation between Coronary Artery Calcium- and Different Cardiovascular Risk Score-Based Methods for the Estimation of Vascular Age in Caucasian Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Populatioan
2.2. Coronary Computed Tomography Angiography
2.3. Calculation of Vascular Age Based on Coronary Artery Calcium Score
2.4. Calculation of Vascular Age Based on Framingham Risk Score (FRS)
2.5. Calculation of Vascular Age Based on Systematic Coronary Risk Evaluation (SCORE) Risk Score
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
- Eichler, K.; Puhan, M.A.; Steurer, J.; Bachmann, L.M. Prediction of first coronary events with the Framingham score: A systematic review. Am. Heart J. 2007, 153, 722–731.e8. [Google Scholar] [CrossRef] [PubMed]
- Versteylen, M.O.; Joosen, I.A.; Shaw, L.J.; Narula, J.; Hofstra, L. Comparison of Framingham, PROCAM, SCORE, and Diamond Forrester to predict coronary atherosclerosis and cardiovascular events. J. Nucl. Cardiol. Off. Publ. Am. Soc. Nucl. Cardiol. 2011, 18, 904–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karmali, K.N.; Lloyd-Jones, D.M. Adding a life-course perspective to cardiovascular-risk communication. Nat. Rev. Cardiol. 2013, 10, 111–115. [Google Scholar] [CrossRef]
- Hamczyk, M.R.; Nevado, R.M.; Barettino, A.; Fuster, V.; Andrés, V. Biological Versus Chronological Aging: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 919–930. [Google Scholar] [CrossRef]
- Villella, E.; Cho, J.S. Effect of aging on the vascular system plus monitoring and support. Surg. Clin. N. Am. 2015, 95, 37–51. [Google Scholar] [CrossRef]
- Gomez-Marcos, M.A.; Martinez-Salgado, C.; Gonzalez-Sarmiento, R.; Hernandez-Rivas, J.M.; Sanchez-Fernandez, P.L.; Recio-Rodriguez, J.I.; Rodriguez-Sanchez, E.; Garca-Ortiz, L. Association between different risk factors and vascular accelerated ageing (EVA study): Study protocol for a cross-sectional, descriptive observational study. BMJ Open 2016, 6, e011031. [Google Scholar] [CrossRef]
- Cuende, J.I.; Cuende, N.; Calaveras-Lagartos, J. How to calculate vascular age with the SCORE project scales: A new method of cardiovascular risk evaluation. Eur. Heart J. 2010, 31, 2351–2358. [Google Scholar] [CrossRef]
- D’Agostino, R.B.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham heart study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef] [Green Version]
- DeFilippis, A.P.; Young, R.; Carrubba, C.J.; McEvoy, J.W.; Budoff, M.J.; Blumenthal, R.S.; Kronmal, R.A.; McClelland, R.L.; Nasir, K.; Blaha, M.J. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann. Intern. Med. 2015, 162, 266–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wohlfahrt, P.; Bruthans, J.; Krajčoviechová, A.; Šulc, P.; Linhart, A.; Filipovský, J.; Mayer, O.J.; Widimský, J.J.; Blaha, M.; Abrahámová, J.; et al. Systematic COronary Risk Evaluation (SCORE) and 20-year risk of cardiovascular mortality and cancer. Eur. J. Intern. Med. 2020, 79, 63–69. [Google Scholar] [CrossRef]
- Stein, J.H.; Fraizer, M.C.; Aeschlimann, S.E.; Nelson-Worel, J.; McBride, P.E.; Douglas, P.S. Vascular age: Integrating carotid intima-media thickness measurements with global coronary risk assessment. Clin. Cardiol. 2004, 27, 388–392. [Google Scholar] [CrossRef]
- Mattace-Raso, F.U.S.; Van Der Cammen, T.J.M.; Hofman, A.; Van Popele, N.M.; Bos, M.L.; Schalekamp, M.A.D.H.; Asmar, R.; Reneman, R.S.; Hoeks, A.P.G.; Breteler, M.M.B.; et al. Arterial stiffness and risk of coronary heart disease and stroke: The Rotterdam Study. Circulation 2006, 113, 657–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasir, K.; Vasamreddy, C.; Blumenthal, R.S.; Rumberger, J.A. Comprehensive coronary risk determination in primary prevention: An imaging and clinical based definition combining computed tomographic coronary artery calcium score and national cholesterol education program risk score. Int. J. Cardiol. 2006, 110, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Greenland, P.; Smith, S.C.J.; Grundy, S.M. Improving coronary heart disease risk assessment in asymptomatic people: Role of traditional risk factors and noninvasive cardiovascular tests. Circulation 2001, 104, 1863–1867. [Google Scholar] [CrossRef] [Green Version]
- McClelland, R.L.; Nasir, K.; Budoff, M.; Blumenthal, R.S.; Kronmal, R.A. Arterial Age as a Function of Coronary Artery Calcium (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am. J. Cardiol. 2009, 103, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef] [Green Version]
- Conroy, R.M.; Pyörälä, K.; Fitzgerald, A.P.; Sans, S.; Menotti, A.; De Backer, G.; De Bacquer, D.; Ducimetière, P.; Jousilahti, P.; Keil, U.; et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur. Heart J. 2003, 24, 987–1003. [Google Scholar] [CrossRef]
- Lopez-Gonzalez, A.A.; Aguilo, A.; Frontera, M.; Bennasar-Veny, M.; Campos, I.; Vicente-Herrero, T.; Tomas-Salva, M.; De Pedro-Gomez, J.; Tauler, P. Effectiveness of the Heart Age tool for improving modifiable cardiovascular risk factors in a Southern European population: A randomized trial. Eur. J. Prev. Cardiol. 2015, 22, 389–396. [Google Scholar] [CrossRef]
- Gyöngyösi, H.; Kőrösi, B.; Batta, D.; Nemcsik-Bencze, Z.; László, A.; Tislér, A.; Cseprekál, O.; Torzsa, P.; Eörsi, D.; Nemcsik, J. Comparison of Different Cardiovascular Risk Score and Pulse Wave Velocity-Based Methods for Vascular Age Calculation. Heart Lung Circ. 2021, 30, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Khalil, Y.; Mukete, B.; Durkin, M.J.; Coccia, J.; Matsumura, M.E. A Comparison of Assessment of Coronary Calcium vs Carotid Intima Media Thickness for Determination of Vascular Age and Adjustment of the Framingham Risk Score. Prev. Cardiol. 2010, 13, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Velagaleti, R.; Vasan, R.S. Heart Failure in the 21st Century: Is it a Coronary Artery Disease Problem or Hypertension Problem? Cardiol Clin. 2008, 25, 487–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
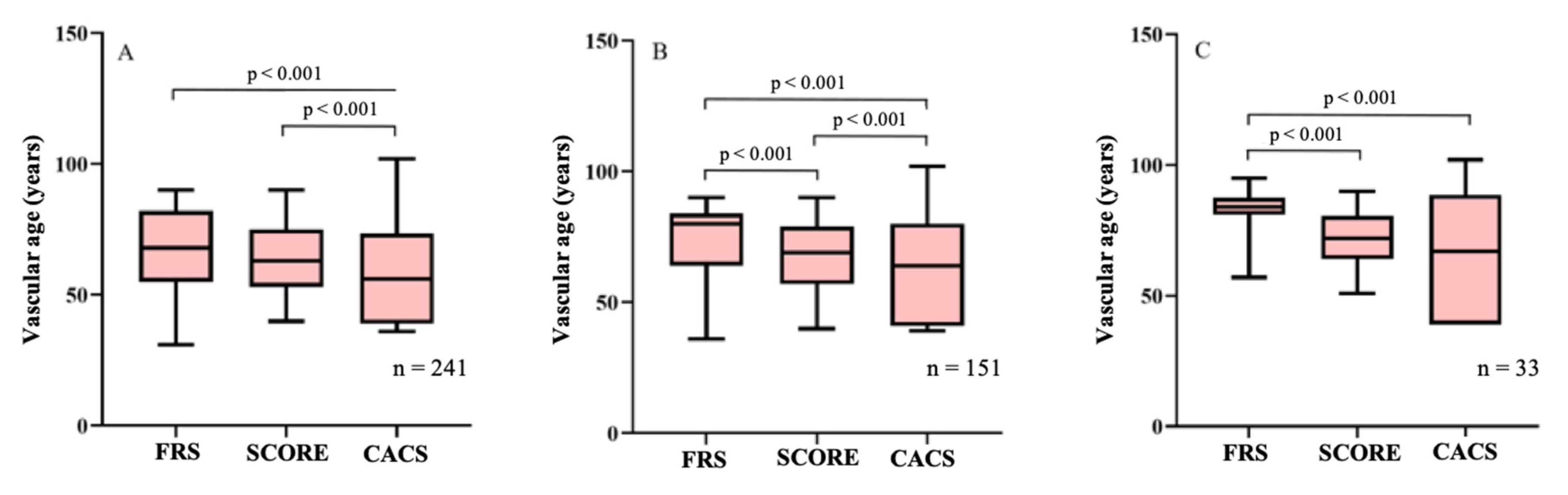

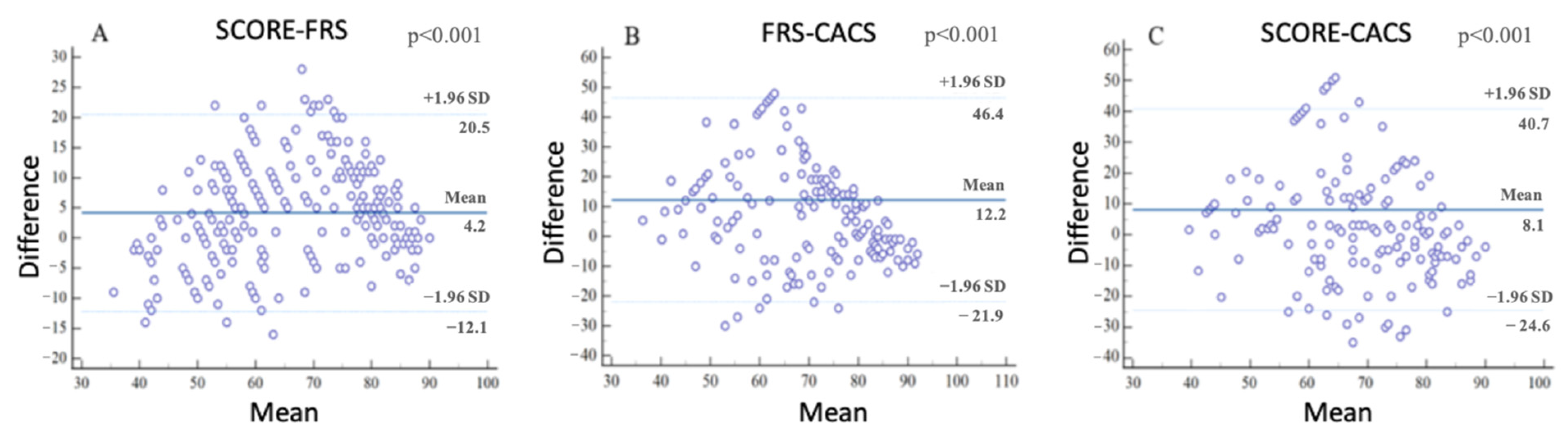
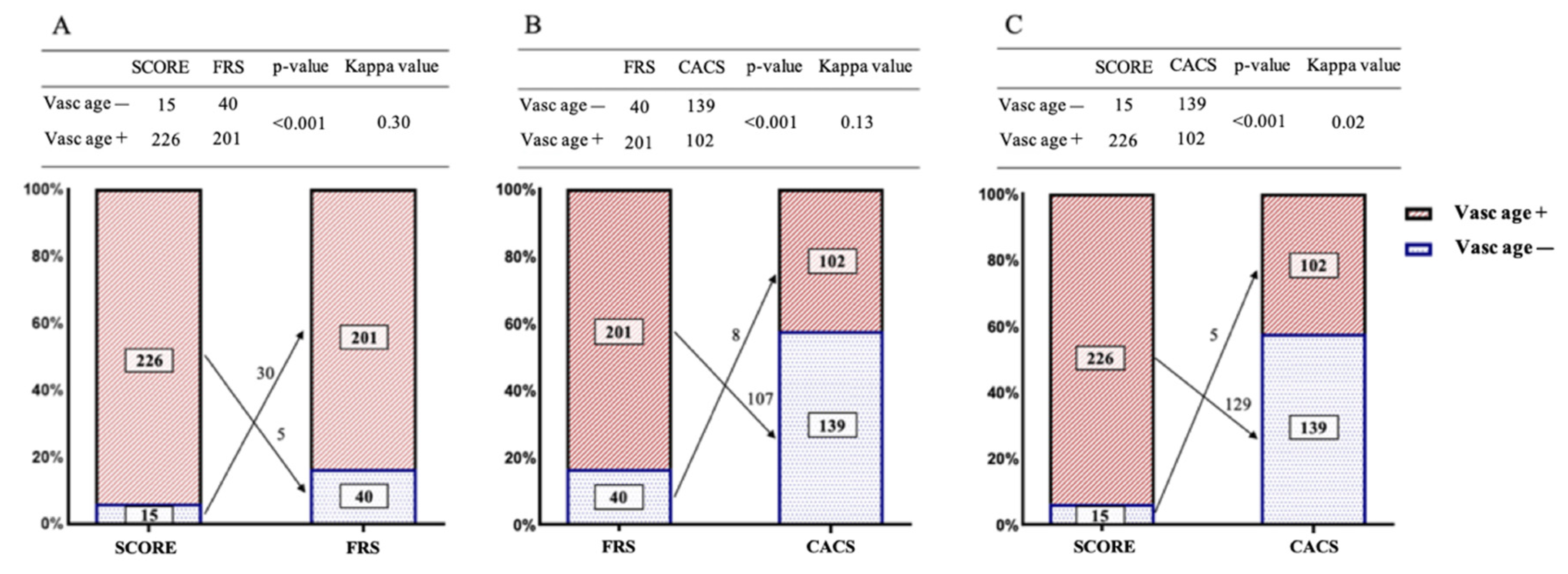
| All Subjects | CACS+ | FRS+ | SCORE+ | |
|---|---|---|---|---|
| Number, (%) | 241 | 102 (42.3) | 201 (83.4) | 226 (93.8) |
| Demographics | ||||
| Chronological age (years) | 56.2 [48.5–66.2] | 60.9 [50.2–68.1] | 57.3 [48.7–66.8] | 57.7 [48.5–66.4] |
| CACS vascular age (years) | 47.1 [39.1–72.3] | 75.7 [66.3–84.8] | 55.8 [39.1–75.9] * | 50.0 [39.1–72.5] * |
| FRS vascular age (years) | 68.0 [55.0–82.0] | 80.0 [60.0–84.0] | 76.0 [60.0–83.0] | 72.0 [57.0–82.0] |
| SCORE vascular age (years) | 63.0 [53.0–75.0] | 69.5 [57.0–78.3] | 65.0 [54.5–77.0] | 64.0 [54.0–76.0] |
| Female sex, n (%) | 117 (48.5) | 34 (33.3) | 87 (43.3) | 109 (48.2) * |
| BMI (kg/m2) | 27.2 [24.7–30.5] | 28.4 [25.4–31.7] | 27.6 [24.9–31.1] | 27.2 [24.7–30.5] |
| Cardiovascular risk factors | ||||
| Current smoker, n (%) | 38 (15.8) | 21 (20.6) | 36 (17.9) | 38 (16.8) |
| Hypertension, n (%) | 151 (62.7) | 75 (73.5) | 141 (70.1) | 139 (61.5) * |
| Diabetes mellitus, n (%) | 33 (13.7) | 18 (17.6) | 33 (16.4) | 32 (14.2) |
| Dyslipidemia, n (%) | 117 (48.5) | 63 (61.8) | 102 (50.7) | 109 (48.2) * |
| SBP (mmHg) | 146.5 ± 18.4 | 149.1 ± 17.8 | 149.8 ± 17.6 | 148.3 ± 17.6 |
| DBP (mmHg) | 88.9 ± 10.4 | 89.9 ± 10.5 | 90.1 ± 10.2 | 89.6 ± 10.1 |
| Laboratory parameters | ||||
| Glucose (mmol/L) | 5.4 [5.1–5.8] | 5.5 [5.2–6.1] | 5.4 [5.1–5.9] | 5.4 [5.1–5.8] |
| GFR (mL/min/1.732) | 78.5 [68.2–87.0] | 75.2 [67.6–87.0] | 78.5 [68.5–87.0] | 78.2 [68.2–87.6] |
| Total cholesterol (mmol/L) | 5.0 [4.2–5.9] | 5.0 [4.0–6.1] | 5.1 [4.2–6.1] | 5.1 [4.3–6.0] |
| LDL-cholesterol (mmol/L) | 3.3 [2.4–4.0] | 3.1 [2.3–4.2] | 3.4 [2.4–4.2] | 3.3 [2.4–4.1] |
| HDL-cholesterol (mmol/L) | 1.3 [1.1–1.7] | 1.3 [1.1–1.7] | 1.3 [1.1–1.6] | 1.3 [1.1–1.7] |
| Triglyceride (mmol/L) | 1.4 [1.0–2.3] | 1.5 [1.0–2.3] | 1.5 [1.1–2.4] | 1.5 [1.0–2.3] |
| Agatston score | 2.0 [0.0–99.0] | 154.5 [41.5–544.8] | 9.0 [0.0–154.5] * | 3.5 [0.0–103.3] * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vecsey-Nagy, M.; Szilveszter, B.; Kolossváry, M.; Boussoussou, M.; Vattay, B.; Merkely, B.; Maurovich-Horvat, P.; Radovits, T.; Nemcsik, J. Correlation between Coronary Artery Calcium- and Different Cardiovascular Risk Score-Based Methods for the Estimation of Vascular Age in Caucasian Patients. J. Clin. Med. 2022, 11, 1111. https://doi.org/10.3390/jcm11041111
Vecsey-Nagy M, Szilveszter B, Kolossváry M, Boussoussou M, Vattay B, Merkely B, Maurovich-Horvat P, Radovits T, Nemcsik J. Correlation between Coronary Artery Calcium- and Different Cardiovascular Risk Score-Based Methods for the Estimation of Vascular Age in Caucasian Patients. Journal of Clinical Medicine. 2022; 11(4):1111. https://doi.org/10.3390/jcm11041111
Chicago/Turabian StyleVecsey-Nagy, Milán, Bálint Szilveszter, Márton Kolossváry, Melinda Boussoussou, Borbála Vattay, Béla Merkely, Pál Maurovich-Horvat, Tamás Radovits, and János Nemcsik. 2022. "Correlation between Coronary Artery Calcium- and Different Cardiovascular Risk Score-Based Methods for the Estimation of Vascular Age in Caucasian Patients" Journal of Clinical Medicine 11, no. 4: 1111. https://doi.org/10.3390/jcm11041111
APA StyleVecsey-Nagy, M., Szilveszter, B., Kolossváry, M., Boussoussou, M., Vattay, B., Merkely, B., Maurovich-Horvat, P., Radovits, T., & Nemcsik, J. (2022). Correlation between Coronary Artery Calcium- and Different Cardiovascular Risk Score-Based Methods for the Estimation of Vascular Age in Caucasian Patients. Journal of Clinical Medicine, 11(4), 1111. https://doi.org/10.3390/jcm11041111






