Prediction of Acute Kidney Injury by Cystatin C and [TIMP-2]*[IGFBP7] after Thoracic Aortic Surgery with Moderate Hypothermic Circulatory Arrest
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients (Inclusion and Exclusion Criteria)
2.2. Surgical Procedure
2.3. Biomarker Measurements
2.4. Definition of Endpoint and Outcomes
- AKI 1: Increase of serum creatinine by ≥0.3 mg/dL (≥26.4 µmol/L) or increase to ≥150–200% from baseline or urine output <0.5 mL/kg/h for >6 h;
- AKI 2: increase of serum creatinine to >200–300% from baseline and/or urine output <0.5 mL/kg/h for >12 h;
- AKI 3: increase of serum creatinine to >300% from baseline or serum creatinine ≥4.0 mg/dL (≥354 µmol/L) after a rise of at least 44 µmol/L or treatment with renal replacement therapy and/or urine output <0.3 mL/kg/h for >24 h or anuria for 12 h.
2.5. Sample Size Calculation
2.6. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Perioperative Course of Biomarkers
3.3. Prediction of AKI with Biomarkers
4. Discussion
5. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mori, Y.; Sato, N.; Kobayashi, Y.; Ochiai, R. Acute kidney injury during aortic arch surgery under deep hypothermic circulatory arrest. J. Anesth. 2011, 25, 799–804. [Google Scholar] [CrossRef]
- Ghincea, C.V.; Reece, T.B.; Eldeiry, M.; Roda, G.F.; Bronsert, M.R.; Jarrett, M.J.; Pal, J.D.; Cleveland, J.C., Jr.; Fullerton, D.A.; Aftab, M. Predictors of Acute Kidney Injury Following Aortic Arch Surgery. J. Surg. Res. 2019, 242, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Arnaoutakis, G.J.; Vallabhajosyula, P.; Bavaria, J.E.; Sultan, I.; Siki, M.; Naidu, S.; Milewski, R.K.; Williams, M.L.; Hargrove, W.C., 3rd; Desai, N.D.; et al. The Impact of Deep Versus Moderate Hypothermia on Postoperative Kidney Function After Elective Aortic Hemiarch Repair. Ann. Thorac. Surg. 2016, 102, 1313–1321. [Google Scholar] [CrossRef]
- Fang, Z.; Wang, G.; Liu, Q.; Zhou, H.; Zhou, S.; Lei, G.; Zhang, C.; Yang, L.; Shi, S.; Li, J.; et al. Moderate and deep hypothermic circulatory arrest has a comparable effect on acute kidney injury after total arch replacement with frozen elephant trunk procedure in type A aortic dissection. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; Park, M.H.; Kim, H.J.; Lim, H.Y.; Shim, H.S.; Sohn, J.T.; Kim, C.S.; Lee, S.M. Potentially modifiable risk factors for acute kidney injury after surgery on the thoracic aorta: A propensity score matched case-control study. Medicine 2015, 94, e273. [Google Scholar] [CrossRef] [PubMed]
- Waikar, S.S.; Betensky, R.A.; Emerson, S.C.; Bonventre, J.V. Imperfect gold standards for kidney injury biomarker evaluation. J. Am. Soc. Nephrol. 2012, 23, 13–21. [Google Scholar] [CrossRef]
- Göcze, I.; Jauch, D.; Götz, M.; Kennedy, P.; Jung, B.; Zeman, F.; Gnewuch, C.; Graf, B.M.; Gnann, W.; Banas, B.; et al. Biomarker-guided Intervention to Prevent Acute Kidney Injury After Major Surgery: The Prospective Randomized BigpAK Study. Ann. Surg. 2018, 267, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Pozzoli, S.; Simonini, M.; Manunta, P. Predicting acute kidney injury: Current status and future challenges. J. Nephrol. 2018, 31, 209–223. [Google Scholar] [CrossRef]
- Kellum, J.A.; Chawla, L.S. Cell-cycle arrest and acute kidney injury: The light and the dark sides. Nephrol. Dial. Transplant. 2016, 31, 16–22. [Google Scholar] [CrossRef]
- Pilarczyk, K.; Edayadiyil-Dudasova, M.; Wendt, D.; Demircioglu, E.; Benedik, J.; Dohle, D.S.; Jakob, H.; Dusse, F. Urinary [TIMP-2]*[IGFBP7] for early prediction of acute kidney injury after coronary artery bypass surgery. Ann. Intensive Care. 2015, 5, 50. [Google Scholar] [CrossRef]
- Dusse, F.; Edayadiyil-Dudásova, M.; Thielmann, M.; Wendt, D.; Kahlert, P.; Demircioglu, E.; Jakob, H.; Schaefer, S.T.; Pilarczyk, K. Early prediction of acute kidney injury after transapical and transaortic aortic valve implantation with urinary G1 cell cycle arrest biomarkers. BMC Anesthesiol. 2016, 16, 76. [Google Scholar]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.M.; Moher, D.; Rennie, D.; de Vet, H.C.; Lijmer, J.G. The STARD statement for reporting studies of diagnostic accuracy: Explanation and elaboration. Clin. Chem. 2003, 49, 7–18. [Google Scholar] [CrossRef]
- Friedrich, C.; Freundt, M.; Salem, M.A.; Panholzer, B.; Huenges, K.; Puehler, T.; Cremer, J.; Haneya, A. Sex-Specific Outcome after Ascending Aortic Surgery in Moderate Hypothermic Circulatory Arrest. Thorac. Cardiovasc. Surg. 2019, 69, 314–321. [Google Scholar]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012, 2, 1–138. [Google Scholar]
- Luo, X.; Jiang, L.; Du, B.; Wen, Y.; Wang, M.; Xi, X. Beijing Acute Kidney Injury Trial (BAKIT) workgroup. A comparison of different diagnostic criteria of acute kidney injury in critically ill patients. Crit Care 2014, 18, R144. [Google Scholar] [CrossRef]
- Wiersema, R.; Jukarainen, S.; Eck, R.J.; Kaufmann, T.; Koeze, J.; Keus, F.; Pettilä, V.; van der Horst, I.C.C.; Vaara, S.T. Different applications of the KDIGO criteria for AKI lead to different incidences in critically ill patients: A post hoc analysis from the prospective observational SICS-II study. Crit Care. 2020, 24, 164. [Google Scholar] [CrossRef]
- Xu, S.; Liu, J.; Li, L.; Wu, Z.; Li, J.; Liu, Y.; Zhu, J.; Sun, L.; Guan, X.; Gong, M.; et al. Cardiopulmonary bypass time is an independent risk factor for acute kidney injury in emergent thoracic aortic surgery: A retrospective cohort study. J Cardiothorac Surg. 2019, 14, 90. [Google Scholar] [CrossRef]
- Che, M.; Wang, X.; Xie, B.; Huang, R.; Liu, S.; Yan, Y.; Zhu, M.; Lu, R.; Qian, J.; Zhang, W.; et al. Use of Both Serum Cystatin C and Creatinine as Diagnostic Criteria for Cardiac Surgery-Associated Acute Kidney Injury and Its Correlation with Long-Term Major Adverse Events. Kidney Blood Press. Res. 2019, 44, 415–425. [Google Scholar] [CrossRef]
- Yong, Z.; Pei, X.; Zhu, B.; Yuan, H.; Zhao, W. Predictive value of serum cystatin C for acute kidney injury in adults: A meta-analysis of prospective cohort trials. Sci. Rep. 2017, 7, 41012. [Google Scholar] [CrossRef]
- Shlipak, M.G.; Coca, S.G.; Wang, Z.; Devarajan, P.; Koyner, J.L.; Patel, U.D.; Thiessen-Philbrook, H.; Garg, A.X.; Parikh, C.R.; TRIBE-AKI Consortium. Presurgical serum cystatin C and risk of acute kidney injury after cardiac surgery. Am. J. Kidney Dis. 2011, 58, 366–373. [Google Scholar] [CrossRef]
- Ledoux, D.; Monchi, M.; Chapelle, J.P.; Damas, P. Cystatin C blood level as a risk factor for death after heart surgery. Eur. Heart J. 2007, 28, 1848–1853. [Google Scholar] [CrossRef][Green Version]
- Kashani, K.; Al-Khafaji, A.; Ardiles, T.; Artigas, A.; Bagshaw, S.M.; Bell, M.; Bihorac, A.; Birkhahn, R.; Cely, C.M.; Chawla, L.S.; et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit. Care 2013, 17, R25. [Google Scholar] [CrossRef]
- Hoste, E.A.; McCullough, P.A.; Kashani, K.; Chawla, L.S.; Joannidis, M.; Shaw, A.D.; Feldkamp, T.; Uettwiller-Geiger, D.L.; McCarthy, P.; Shi, J.; et al. Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrol. Dial. Transplant. 2014, 29, 2054–2061. [Google Scholar] [CrossRef]
- Jo, S.K.; Yang, J.; Hwang, S.M.; Lee, M.S.; Park, S.H. Role of biomarkers as predictors of acute kidney injury and mortality in decompensated cirrhosis. Sci. Rep. 2019, 9, 14508. [Google Scholar] [CrossRef]
- Grieshaber, P.; Möller, S.; Arneth, B.; Roth, P.; Niemann, B.; Renz, H.; Böning, A. Predicting Cardiac Surgery-Associated Acute Kidney Injury Using a Combination of Clinical Risk Scores and Urinary Biomarkers. Thorac. Cardiovasc. Surg. 2020, 68, 389–400. [Google Scholar] [CrossRef]
- Wetz, A.J.; Richardt, E.M.; Wand, S.; Kunze, N.; Schotola, H.; Quintel, M.; Bräuer, A.; Moerer, O. Quantification of urinary TIMP-2 and IGFBP-7: An adequate diagnostic test to predict acute kidney injury after cardiac surgery? Crit. Care. 2015, 19, 3. [Google Scholar] [CrossRef]
- Brienza, N.; Giglio, M.T.; Marucci, M.; Fiore, T. Does perioperative hemodynamic optimization protect renal function in surgical patients? A meta-analytic study. Crit. Care Med. 2009, 37, 2079–2090. [Google Scholar] [CrossRef]
- Ponce, D.; Zorzenon Cde, P.; dos Santos, N.Y.; Balbi, A.L. Early nephrology consultation can have an impact on outcome of acute kidney injury patients. Nephrol. Dial. Transplant. 2011, 26, 3202–3206. [Google Scholar] [CrossRef]
- Zarbock, A.; Schmidt, C.; Van Aken, H.; Wempe, C.; Martens, S.; Zahn, P.K.; Wolf, B.; Goebel, U.; Schwer, C.I.; Rosenberger, P.; et al. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: A randomized clinical trial. JAMA 2015, 313, 2133–2141. [Google Scholar] [CrossRef]
- Karvellas, C.J.; Farhat, M.R.; Sajjad, I.; Mogensen, S.S.; Leung, A.A.; Wald, R.; Bagshaw, S.M. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: A systematic review and meta-analysis. Crit. Care 2011, 15, R72. [Google Scholar] [CrossRef]
- Liu, Y.; Davari-Farid, S.; Arora, P.; Porhomayon, J.; Nader, N.D. Early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury after cardiac surgery: A systematic review and meta-analysis. J. Cardiothorac. Vasc. Anesth. 2014, 28, 557–563. [Google Scholar] [CrossRef] [PubMed]
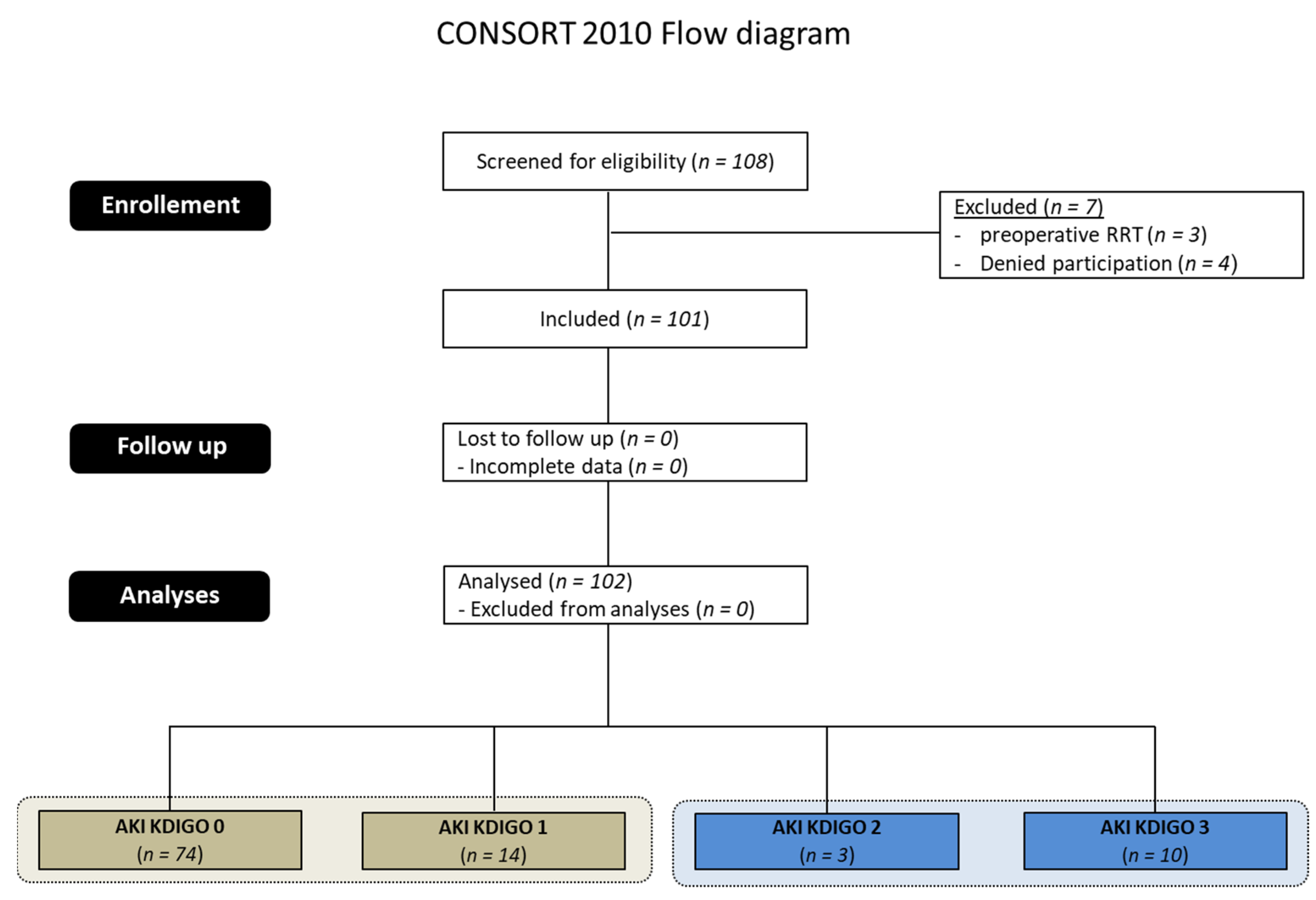
| AKI KDIGO Stage | Number | Classification Criterion for AKI Stage | |
|---|---|---|---|
| Increase of Serum Creatinine | Decrease of Diuresis or RRT | ||
| 1 | 14 (13.9%) | 8 | 6 |
| 2 | 3 (3.0%) | 2 | 1 |
| 3 | 10 (9.9%) | 1 | 9 |
| All (1–3) | 27 (26.7%) | 11 | 16 |
| AKI 0–1 (n = 88) | AKI 2–3 (n = 13) | p-Value | |
|---|---|---|---|
| Age (years) | 68.51 ± 11.46 | 72.72 ± 6.19 | 0.044 |
| Female Sex (n,%) | 60 (68.2) | 8 (61.5) | n.s. |
| Weight (kg) | 82.51 ± 16.02 | 8.67 ± 17.25 | n.s. |
| Height (cm) | 174.20 ± 9.01 | 171.73 ± 9.13 | n.s. |
| BMI(kg/m2) | 27.09 ± 4.28 | 27.20 ± 4.82 | n.s. |
| ES I log | 19.89 ± 14.66 | 29.68 ± 19.98 | 0.026 |
| ES II (%) | 5.73 ± 5.25 | 16.29 ± 11.49 | 0.003 |
| STS (%) | 3.38 ± 3.34 | 5.44 ± 4.64 | n.s. |
| EF (%) | 57.89 ± 13.71 | 53.69 ± 13.49 | n.s. |
| Acute MI (n,%) | 5 (56.8) | 2 (15.4) | n.s. |
| NYHA ≥ III (n,%) | 10 (11.4) | 6 (46.2) | 0.036 |
| COPD (n,%) | 8 (9.1) | 1(7.7) | n.s. |
| aHTN (n,%) | 63 (71.6) | 14 | n.s. |
| HLP (n, %) | 41(46.6) | 9 (69.2) | n.s. |
| pHTN (n,%) | 2 (2.2) | 1 (7.7) | n.s. |
| Afib (n,%) | 11(12.5) | 6 (46.2) | 0.017 |
| PAD (n,%) | 11 (12.5) | 3 (23.1) | n.s. |
| CAD (n,%) | 44 (50.0) | 9 (69.2) | n.s. |
| Redo (n,%) | 11 (12.5) | 3 (23.1) | n.s. |
| s/p Stroke (n,%) | 10 (11.4) | 1 (7.7) | n.s. |
| Endocarditis (n,%) | 2 (2.3) | 0 (0) | n.s |
| Smoking (n,%) | 18 (20.5) | 3 (23.1) | n.s. |
| CKD (n,%) | 0 (0) | 5 (38.5) | 0.001 |
| Urgent/Emergency Surgery (n,%) | 15 (17.0) | 5 (38.5) | 0.02 |
| Duration Surgery (min) | 297.77 ± 93.29 | 397.20 ± 139.04 | 0.017 |
| Duration CPB (min) | 170.57 ± 51.05 | 230.21 ± 68.29 | 0.007 |
| Cross Clamp Time (min) | 177.22 ± 42.93 | 151.50 ± 62.38 | 0.066 |
| CA Duration (min) | 24.70 ± 31.97 | 33.14 ± 31.77 | n.s. |
| RPBC intraop. (n, %) | 1.61 ± 2.74 | 4.27 ± 3.71 | 0.001 |
| Dosage of epinephrine (mg/h) | 0.21 ± 0.43 | 0.60 ± 0.51 | 0.011 |
| Diuresis intraop. (mL) | 989.82 ± 788.89 | 557.14 ± 605.06 | 0.008 |
| HF intraop (mL) | 2274.02 ± 2331.49 | 4388.88 ± 2168.20 | 0.013 |
| Aneurysm size (mm) | 52.51 ± 7.71 | 44.73 ± 9.79 | 0.004 |
| Marfan (n,%) | 2 (2.3) | 0 (0) | n.s. |
| Supra-coronary Asc.-Replacement (n, %) | 83 (94.3) | 12 (92.3) | n.s. |
| Hemiarch replacement (n,%) | 30 (34.1) | 4 (30.8) | n.s. |
| Total arch replacement (n, %) | 5 (5.7) | 2 (15.4) | n.s. |
| Aortic root surgery (n,%) | 14 (15.9) | 4 (30.8) | n.s. |
| David-Procedure (n,%) | 9 (10.2) | 1 (7.7) | n.s. |
| Elephant trunk (n,%) | 1 (1.1) | 2 (15.4) | n.s. |
| CABG (n, %) | 37 (42.0) | 7 (53.8) | n.s. |
| AVR (n,%) | 44 (50.0) | 7 (53.8) | n.s. |
| MVS (n,%) | 3 (3.4) | 3 (23.1) | 0.038 |
| TVS (n,%) | 0 (0) | 2 (15.4) | 0.02 |
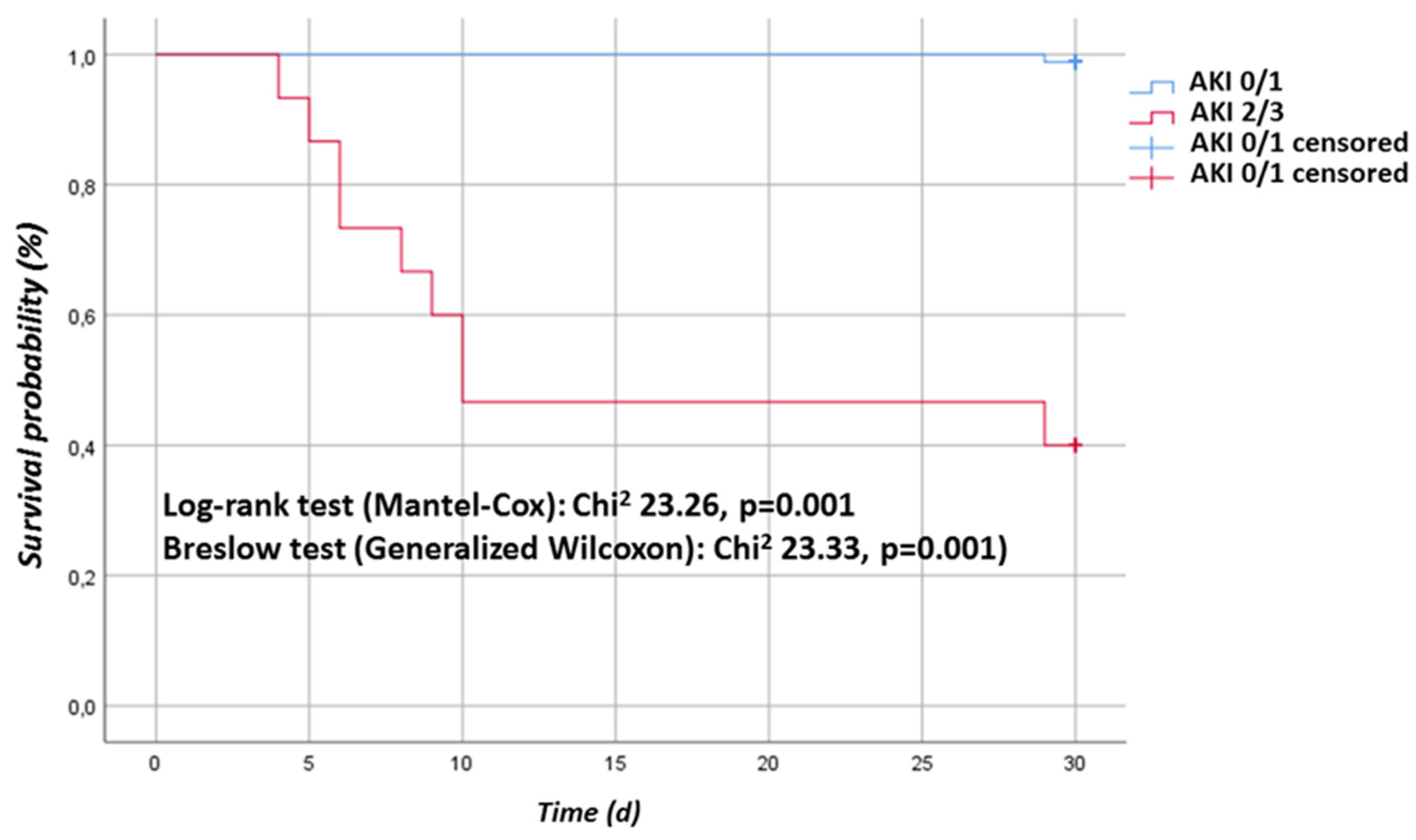
| Outcome | AKI 0–1 (n = 88) | AKI 2–3 (n = 13) | p-Value |
|---|---|---|---|
| Reintubation (n,%) | 7 (8.0) | 7 (53.8) | 0.001 |
| PDT (n,%) | 3 (3.4) | 6 (61.2) | 0.001 |
| Neurological complication (n,%) | 6 (6.8) | 6 (61.2) | 0.001 |
| CPR (n,%) | 0 (0) | 3 (21.4) | <0.001 |
| Myocardial infarction (n,%) | 0 (0) | 2 (14.3) | 0.002 |
| Pneumonia (n,%) | 4 (4.5) | 7 (53.8) | <0.001 |
| Sepsis (n,%) | 2 (2.3) | 7 (53.8) | <0.001 |
| Re-exploration for bleeding (n,%) | 3 (3.4) | 9 (64.3) | <0.001 |
| Infections (n %) | 2 (2.) | 3 (21.4) | 0.012 |
| DSWI (n,%) | 0 (0) | 1 (7.1) | 0.048 |
| 7 d mortality (n,%) | 0 (0) | 4 (30.8) | <0.001 |
| 30 d mortality (n,%) | 1 (1.1) | 9 (69.2) | <0.001 |
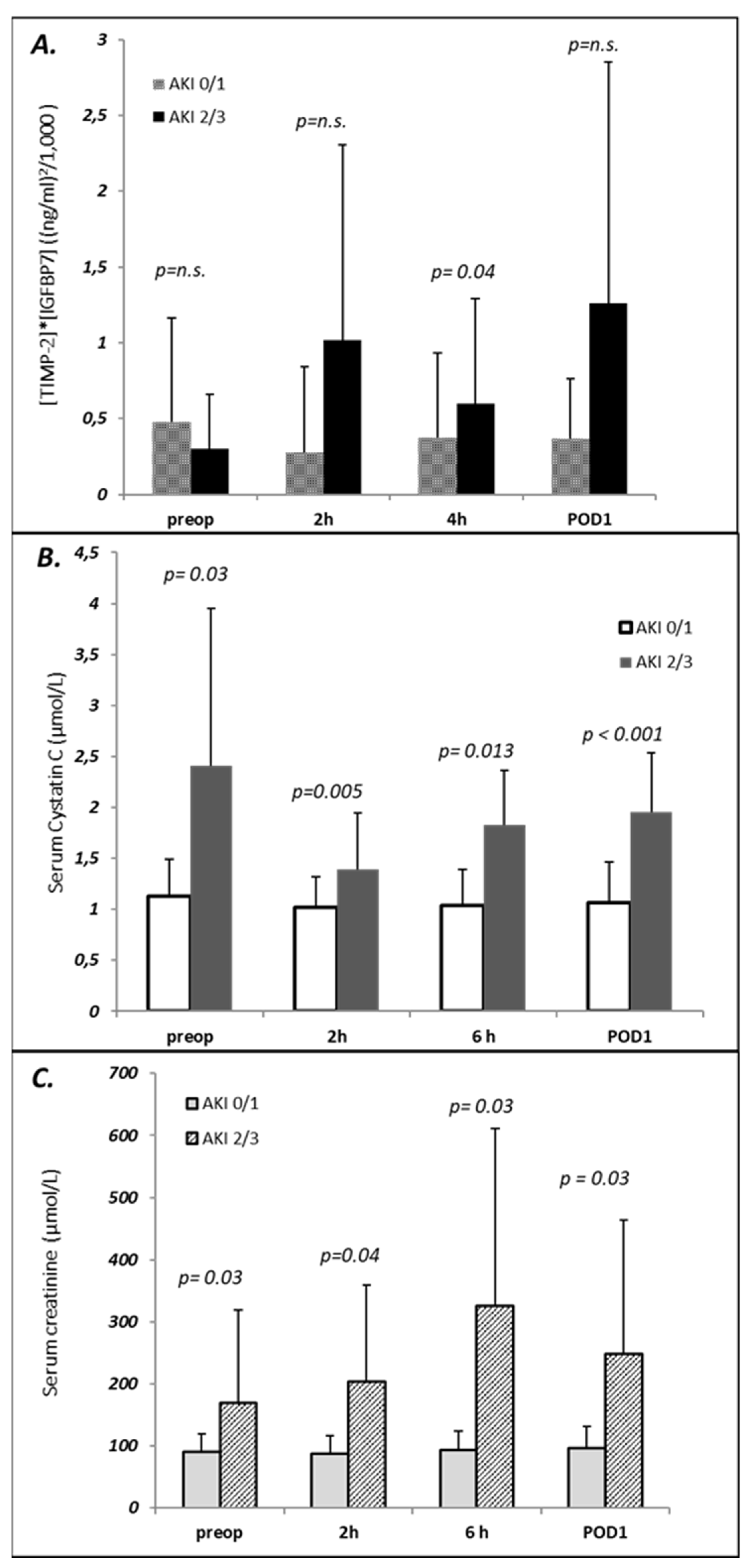
| Variable | AUC (95% CI) | SE | p-Value | Cut-Off | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| Prediction of AKI any stage | ||||||
| Cystatin C preop. | ||||||
| preoperative | 0.822 (0.710–0.935) | 0.057 | 0.000 | 1.19 | 0.79 | 0.75 |
| 2 h postoperative | 0.713 (0.594–0.869) | 0.070 | 0.0028 | 1.22 | 0.88 | 0.56 |
| 6 h postoperative | 0.872 (0.779–0.964) | 0.047 | <0.001 | 1.09 | 0.94 | 0.73 |
| POD 1 | 0.956 (0.841–0.970) | 0.033 | <0.001 | 1.09 | 1.00 | 0.71 |
| Serum-Creatinine | ||||||
| preoperative | 0.679 (0.530–0.828) | 0.076 | 0.022 | 123.5 | 0.47 | 0.96 |
| 2 h postoperative | 0.822 (0.729–0.915) | 0.047 | <0.001 | 80.5 | 0.45 | 0.95 |
| 6 h postoperative | 0.894 (0.783–1.000) | 0.0562 | <0.001 | 121.5 | 76.92 | 90.38 |
| POD 1 | 0.871 (0.779–0.962) | 0.046 | <0.001 | 96.5 | 0.95 | 0.67 |
| [TIMP-2]*[IGFBP7] | ||||||
| preoperative | 0.381 (0.212–0.549) | 0.086 | 0.165 | 0.205 | 0.5 | 0.442 |
| 2 h postoperative | 0.534 (0.336–0.733) | 0.101 | 0.734 | 0.19 | 0.417 | 0.692 |
| 4 h postoperative | 0.692 (0.537–0.848) | 0.079 | 0.015 | 0.205 | 0.583 | 0.712 |
| POD 1 | 0.635 (0.445–0.824) | 0.097 | 0.164 | 0.385 | 0.583 | 0.75 |
| Prediction of AKI stage 2–3 | ||||||
| Cystatin C preop. | ||||||
| preoperative | 0.930 (0.857–1.000) | 0.037 | 0.000 | 1.53 | 0.8 | 0.95 |
| 2 h postoperative | 0.731 (0.529–0.934) | 0.103 | 0.0438 | 1.27 | 0.87 | 0.57 |
| 6 h postoperative | 0.907 (0.806–1.000) | 0.0516 | 0.0005 | 1.42 | 0.89 | 0.86 |
| POD 1 | 0.909 (0.830–0.989) | 0.0406 | 0.004 | 1.33 | 1.00 | 0.78 |
| Serum-Creatinine | ||||||
| preoperative | 0.813 (0.643–0.982) | 0.087 | 0.002 | 130.0 | 0.7 | 0.97 |
| 2 h postoperative | 0.919 (0.857–0.983) | 0.032 | <0.001 | 98.5 | 1.00 | 0.76 |
| 6 h postoperative | 0.975 (0.925–1.000) | 0.026 | <0.001 | 120.5 | 1.00 | 0.83 |
| POD 1 | 0.878 (0.714–1.000) | 0.084 | <0.001 | 159.5 | 0.98 | 0.80 |
| [TIMP-2]*[IGFBP7] | ||||||
| preoperative | 0.409 (0.270–0.548) | 0.071 | 0.198 | 0.17 | 0.75 | 0.397 |
| 2 h postoperative | 0.740 (0.429–1.052) | 0.159 | 0.131 | 0.265 | 0.75 | 0.794 |
| 4 h postoperative | 0.724 (0.535–0.913) | 0.096 | 0.020 | 0.215 | 1.00 | 0.556 |
| POD 1 | 0.544 (0.233–0.854) | 0.159 | 0.783 | 0.165 | 0.75 | 0.413 |
| Prediction of RRT | ||||||
| Cystatin C | ||||||
| preoperative | 0.944 (0.853–1.036) | 0.047 | 0.000 | 1.53 | 0.86 | 0.92 |
| 2 h postoperative | 0.745 (0.537–0.953) | 0.106 | 0.033 | 1.46 | 0.57 | 0.95 |
| 6 h postoperative | 0.891 (0.774–1.000) | 0.059 | 0.001 | 1.42 | 0.84 | 0.87 |
| POD 1 | 0.934 (0.866–1.000) | 0.035 | <0.001 | 1.33 | 1.00 | 0.77 |
| Serum-Creatinine | ||||||
| preoperative | 0.861 (0.623–1.099) | 0.121 | 0.003 | 130.0 | 0.71 | 0.94 |
| 2 h postoperative | 0.882 (0.809–0.954) | 0.0367 | <0.001 | 104.0 | 1.00 | 0.76 |
| 6 h postoperative | 0.909 (0.807–1.000) | 0.0517 | 0.0175 | 121.5 | 1.00 | 0.81 |
| POD 1 | 0.788 (0.572–1.000) | 0.110 | 0.01 | 121.5 | 0.86 | 0.76 |
| [TIMP-2]*[IGFBP7] | ||||||
| preoperative | 0.378 (0.204–0.552) | 0.89 | 0.168 | 0.17 | 0.667 | 0.391 |
| 2 h postoperative | 0.667 (0.169–1.164) | 0.254 | 0.511 | 1.44 | 0.667 | 0.969 |
| 4 h postoperative | 0.643 (0.241–1.045) | 0.205 | 0.485 | 0.65 | 0.667 | 0.79 |
| POD 1 | 0.404 (0.078–0.885) | 0.246 | 0.695 | 1.28 | 0.478 | 0.984 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilarczyk, K.; Panholzer, B.; Huenges, K.; Salem, M.; Jacob, T.; Cremer, J.; Haneya, A. Prediction of Acute Kidney Injury by Cystatin C and [TIMP-2]*[IGFBP7] after Thoracic Aortic Surgery with Moderate Hypothermic Circulatory Arrest. J. Clin. Med. 2022, 11, 1024. https://doi.org/10.3390/jcm11041024
Pilarczyk K, Panholzer B, Huenges K, Salem M, Jacob T, Cremer J, Haneya A. Prediction of Acute Kidney Injury by Cystatin C and [TIMP-2]*[IGFBP7] after Thoracic Aortic Surgery with Moderate Hypothermic Circulatory Arrest. Journal of Clinical Medicine. 2022; 11(4):1024. https://doi.org/10.3390/jcm11041024
Chicago/Turabian StylePilarczyk, Kevin, Bernd Panholzer, Katharina Huenges, Mohamed Salem, Toni Jacob, Jochen Cremer, and Assad Haneya. 2022. "Prediction of Acute Kidney Injury by Cystatin C and [TIMP-2]*[IGFBP7] after Thoracic Aortic Surgery with Moderate Hypothermic Circulatory Arrest" Journal of Clinical Medicine 11, no. 4: 1024. https://doi.org/10.3390/jcm11041024
APA StylePilarczyk, K., Panholzer, B., Huenges, K., Salem, M., Jacob, T., Cremer, J., & Haneya, A. (2022). Prediction of Acute Kidney Injury by Cystatin C and [TIMP-2]*[IGFBP7] after Thoracic Aortic Surgery with Moderate Hypothermic Circulatory Arrest. Journal of Clinical Medicine, 11(4), 1024. https://doi.org/10.3390/jcm11041024






