Upper Body Physical Rehabilitation for Children with Ataxia through IMU-Based Exergame
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Issues
2.2. Participants
2.3. Procedure
2.4. Outcome Measure
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manto, M.-U. Clinical signs of cerebellar disorders. In The Cerebellum and Its Disorders; Cambridge University Press: Cambridge, UK, 2010; pp. 97–120. [Google Scholar] [CrossRef]
- Mariotti, C.; Fancellu, R.; Di Donato, S. An overview of the patient with ataxia. J. Neurol. 2005, 252, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Summa, S.; Schirinzi, T.; Favetta, M.; Romano, A.; Minosse, S.; Diodato, D.; Olivieri, G.; Martinelli, D.; Sancesario, A.; Zanni, G.; et al. A wearable video-oculography based evaluation of saccades and respective clinical correlates in patients with early onset ataxia. J. Neurosci. Methods 2020, 338, 108697. [Google Scholar] [CrossRef] [PubMed]
- Konczak, J.; Timmann, D. The effect of damage to the cerebellum on sensorimotor and cognitive function in children and adolescents. Neurosci. Biobehav. Rev. 2007, 31, 1101–1113. [Google Scholar] [CrossRef]
- Musselman, K.E.; Stoyanov, C.T.; Marasigan, R.; Jenkins, M.E.; Konczak, J.; Morton, S.M.; Bastian, A.J. Prevalence of ataxia in children: A systematic review. Neurology 2014, 82, 80–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, K.J.; Kantak, S.S.; Burtner, P.A. Motor learning in children: Feedback effects on skill acquisition. Phys. Ther. 2008, 88, 720–732. [Google Scholar] [CrossRef] [PubMed]
- Bodranghien, F.; Bastian, A.; Casali, C.; Hallett, M.; Louis, E.D.; Manto, M.; Mariën, P.; Nowak, D.A.; Schmahmann, J.D.; Serrao, M.; et al. Consensus paper: Revisiting the symptoms and signs of cerebellar syndrome. Cerebellum 2016, 15, 369–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanza, G.; Casabona, J.A.; Bellomo, M.; Cantone, M.; Fisicaro, F.; Bella, R.; Pennisi, G.; Bramanti, P.; Pennisi, M.; Bramanti, A. Update on intensive motor training in spinocerebellar ataxia: Time to move a step forward? J. Int. Med. Res. 2019, 48, 300060519854626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartley, H.; Cassidy, E.; Bunn, L.; Kumar, R.; Pizer, B.; Lane, S.; Carter, B. Exercise and physical therapy interventions for children with ataxia: A systematic review. Cerebellum 2019, 18, 951–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marquer, A.; Barbieri, G.; Pérennou, D. The assessment and treatment of postural disorders in cerebellar ataxia: A systematic review. Ann. Phys. Rehabil. Med. 2014, 57, 67–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacorte, E.; Bellomo, G.; Nuovo, S.; Corbo, M.; Vanacore, N.; Piscopo, P. The use of new mobile and gaming technologies for the assessment and rehabilitation of people with ataxia: A systematic review and meta-analysis. Cerebellum 2020, 20, 361–373. [Google Scholar] [CrossRef]
- Bates, C.; Baxter, P.; Bonney, H.; Bremner, F.; Bunn, L.; Carrillo Perez-Tome, M.; Chung, M.; Cipolotti, L.; de Silva, R.; Duberley, K. Management of the Ataxias towards Best Clinical Practice, 3rd ed.; Ataxia UK: London, UK, 2016. [Google Scholar]
- Sarva, H.; Shanker, V.L. Treatment options in degenerative cerebellar ataxia: A systematic review. Mov. Disord. Clin. Pract. 2014, 1, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Tartarisco, G.; Bruschetta, R.; Summa, S.; Ruta, L.; Favetta, M.; Busa, M.; Romano, A.; Castelli, E.; Marino, F.; Cerasa, A.; et al. Artificial intelligence for dysarthria assessment in children with ataxia: A hierarchical approach. IEEE Access 2021, 9, 166720–166735. [Google Scholar] [CrossRef]
- Gao, Z.; Lee, J.E.; Pope, Z.; Zhang, D. Effect of active videogames on underserved children’s classroom behaviors, effort, and fitness. Games Health J. 2016, 5, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Best, J.R. Exergaming in youth: Effects on physical and cognitive health. Z. Psychol. 2013, 221, 72–78. [Google Scholar] [CrossRef]
- Benzing, V.; Schmidt, M. Exergaming for children and adolescents: Strengths, weaknesses, opportunities and threats. J. Clin. Med. 2018, 7, 422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilg, W.; Schatton, C.; Schicks, J.; Giese, M.A.; Schöls, L.; Synofzik, M. Video game-based coordinative training improves ataxia in children with degenerative ataxia. Neurology 2012, 79, 2056–2060. [Google Scholar] [CrossRef] [PubMed]
- Schatton, C.; Synofzik, M.; Fleszar, Z.; Giese, M.A.; Schöls, L.; Ilg, W. Individualized exergame training improves postural control in advanced degenerative spinocerebellar ataxia: A rater-blinded, intra-individually controlled trial. Park. Relat. Disord. 2017, 39, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Ada, L.; Sherrington, C.; Canning, C.G.; Dean, C.M.; Scianni, A. Computerized tracking to train dexterity after cerebellar tumour: A single-case experimental study. Brain Inj. 2009, 23, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Milne, S.C.; Corben, L.A.; Roberts, M.; Szmulewicz, D.; Burns, J.; Grobler, A.C.; Williams, S.; Chua, J.; Liang, C.; Lamont, P.J.; et al. Rehabilitation for ataxia study: Protocol for a randomised controlled trial of an outpatient and supported home-based physiotherapy programme for people with hereditary cerebellar ataxia. BMJ Open 2020, 10, e040230. [Google Scholar] [CrossRef]
- Corben, L.A.; Tai, G.; Wilson, C.; Collins, V.; Churchyard, A.J.; Delatycki, M.B. A comparison of three measures of upper limb function in Friedreich ataxia. J. Neurol. 2010, 257, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, C.; Lessard, I.; Brais, B.; Côté, I.; Lavoie, C.; Synofzik, M.; Mathieu, J. Validity and reliability of outcome measures assessing dexterity, coordination, and upper limb strength in autosomal recessive spastic ataxia of charlevoix-saguenay. Arch. Phys. Med. Rehabil. 2018, 99, 1747–1754. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Weber, K.; Kashman, N.; Volland, G. Adult norms for the nine hole peg test of finger dexterity. Occup. Ther. J. Res. 1985, 5, 24–38. [Google Scholar] [CrossRef]
- Lynch, D.R.; Farmer, J.M.; Tsou, A.Y.; Perlman, S.; Subramony, S.H.; Gomez, C.M.; Ashizawa, T.; Wilmot, G.R.; Wilson, R.B.; Balcer, L.J. Measuring Friedreich ataxia: Complementary features of examination and performance measures. Neurology 2006, 66, 1711–1716. [Google Scholar] [CrossRef]
- Subramony, S.H. SARA—A new clinical scale for the assessment and rating of ataxia: Commentary. Nat. Clin. Pract. Neurol. 2007, 3, 136–137. [Google Scholar] [CrossRef]
- Yabe, I.; Matsushima, M.; Soma, H.; Basri, R.; Sasaki, H. Usefulness of the scale for assessment and rating of ataxia (SARA). J. Neurol. Sci. 2008, 266, 164–166. [Google Scholar] [CrossRef]
- Schmitz-Hübsch, T.; Du Montcel, S.T.; Baliko, L.; Berciano, J.; Boesch, S.; Depondt, C.; Giunti, P.; Globas, C.; Infante, J.; Kang, J.S.; et al. Scale for the assessment and rating of ataxia: Development of a new clinical scale. Neurology 2006, 66, 1717–1720. [Google Scholar] [CrossRef]
- Summa, S.; Schirinzi, T.; Bernava, G.M.; Romano, A.; Favetta, M.; Valente, E.M.; Bertini, E.; Castelli, E.; Petrarca, M.; Pioggia, G.; et al. Development of SaraHome: A novel, well-accepted, technology-based assessment tool for patients with ataxia. Comput. Methods Programs Biomed. 2020, 188, 105257. [Google Scholar] [CrossRef]
- Summa, S.; Tartarisco, G.; Favetta, M.; Buzachis, A.; Romano, A.; Bernava, G.M.; Vasco, G.; Pioggia, G.; Petrarca, M.; Castelli, E.; et al. Spatio-temporal parameters of ataxia gait dataset obtained with the Kinect. Data Br. 2020, 32, 106307. [Google Scholar] [CrossRef]
- Schirinzi, T.; Favetta, M.; Romano, A.; Sancesario, A.; Summa, S.; Minosse, S.; Zanni, G.; Castelli, E.; Bertini, E.; Petrarca, M.; et al. One-year outcome of coenzyme Q10 supplementation in ADCK3 ataxia (ARCA2). Cerebellum Ataxias 2019, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Summa, S.; Tartarisco, G.; Favetta, M.; Buzachis, A.; Romano, A.; Bernava, G.M.; Sancesario, A.; Vasco, G.; Pioggia, G.; Petrarca, M.; et al. Validation of low-cost system for gait assessment in children with ataxia. Comput. Methods Programs Biomed. 2020, 196, 105705. [Google Scholar] [CrossRef]
- Perez-Lloret, S.; van de Warrenburg, B.; Rossi, M.; Rodríguez-Blázquez, C.; Zesiewicz, T.; Saute, J.A.M.; Durr, A.; Nishizawa, M.; Martinez-Martin, P.; Stebbins, G.T.; et al. Assessment of ataxia rating scales and cerebellar functional tests: Critique and recommendations. Mov. Disord. 2021, 36, 283–297. [Google Scholar] [CrossRef]
- Fahey, M.C.; Corben, L.A.; Collins, V.; Churchyard, A.J.; Delatycki, M.B. The 25-foot walk velocity accurately measures real world ambulation in Friedreich ataxia. Neurology 2007, 68, 705–706. [Google Scholar] [CrossRef]
- Lynch, D.R.; Farmer, J.M.; Wilson, R.L.; Balcer, L.J. Performance measures in Friedreich ataxia: Potential utility as clinical outcome tools. Mov. Disord. 2005, 20, 777–782. [Google Scholar] [CrossRef]
- Armstrong, R.A. When to use the Bonferroni correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef]
- Schmitz-Hübsch, T.; Giunti, P.; Stephenson, D.A.; Globas, C.; Baliko, L.; Saccà, F.; Mariotti, C.; Rakowicz, M.; Szymanski, S.; Infante, J.; et al. SCA functional index. Neurology 2008, 71, 486–492. [Google Scholar] [CrossRef]
- Ayvat, E.; Onursal Kılınç, Ö.; Ayvat, F.; Savcun Demirci, C.; Aksu Yıldırım, S.; Kurşun, O.; Kılınç, M. The effects of exergame on postural control in individuals with ataxia: A rater-blinded, randomized controlled, cross-over study. Cerebellum 2021, 1–9. [Google Scholar] [CrossRef]
- Liang, Y.; Lau, P.W.C. Effects of active videogames on physical activity and related outcomes among healthy children: A systematic review. Games Health J. 2014, 3, 122–144. [Google Scholar] [CrossRef] [Green Version]
- Vaz, D.V.; Silva, P.L.; Mancini, M.C.; Carello, C.; Kinsella-Shaw, J. Towards an ecologically grounded functional practice in rehabilitation. Hum. Mov. Sci. 2017, 52, 117–132. [Google Scholar] [CrossRef]
- Chin A Paw, M.J.M.; Jacobs, W.M.; Vaessen, E.P.G.; Titze, S.; van Mechelen, W. The motivation of children to play an active video game. J. Sci. Med. Sport 2008, 11, 163–166. [Google Scholar] [CrossRef]
- Baranowski, T.; Maddison, R.; Maloney, A.; Medina, E.; Simons, M. Building a better mousetrap (exergame) to increase youth physical activity. Games Health J. 2014, 3, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Arpaia, P.; Cimmino, P.; De Matteis, E.; D’Addio, G. A low-cost force sensor-based posturographic plate for home care telerehabilitation exergaming. Measurement 2014, 51, 400–410. [Google Scholar] [CrossRef]
- Barry, G.; Galna, B.; Rochester, L. The role of exergaming in Parkinson’s disease rehabilitation: A systematic review of the evidence. J. Neuroeng. Rehabil. 2014, 11, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, S.; Kay, J. VRun: Running-in-place virtual reality exergame. In Proceedings of the 28th Australian Conference on Computer-Human Interaction, Launceston, Australia, 29 November–2 December 2016; pp. 562–566. [Google Scholar]
- de Souza, J.T. “Free to Fly”: Development and Evaluation of a Novel Exergame with a Low-Cost 3D Tracking Method for Post-Stroke Rehabilitation; Universidade Federal de Uberlândia: Uberlândia, Brazil, 2021. [Google Scholar]
- Sato, K.; Kuroki, K.; Saiki, S.; Nagatomi, R. Improving walking, muscle strength, and balance in the elderly with an exergame using Kinect: A randomized controlled trial. Games Health J. 2015, 4, 161–167. [Google Scholar] [CrossRef] [PubMed]
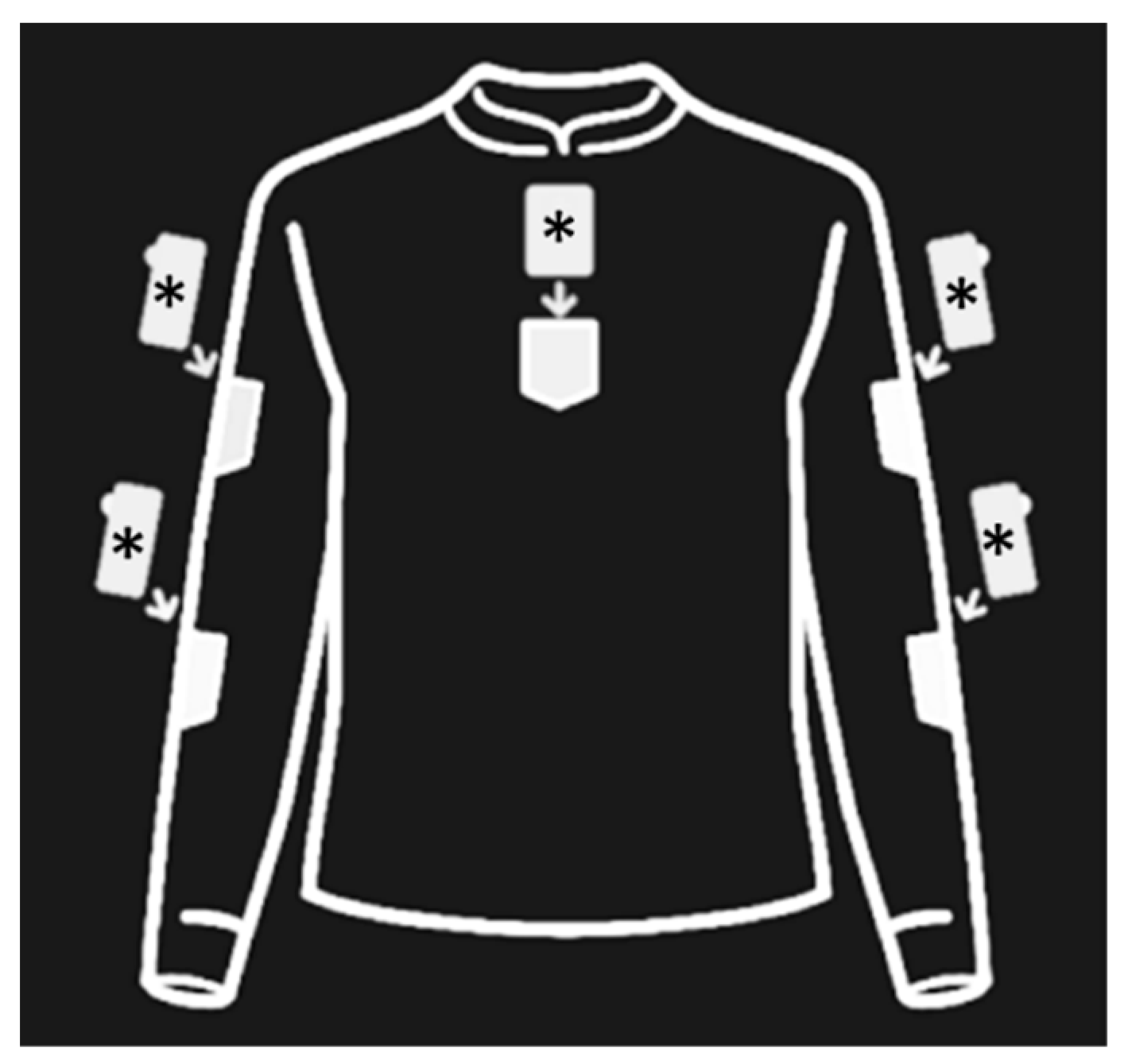

| Group | Pt. | Age at T0 (Years) | Sex | Diagnosis | SARA Scores | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gait | Stance | Sitting | Speech Disturbance | Finger Chase | Nose-Finger Test | Fast Alternating Hand Movements | Heel-Shin Slide | SARA Total Score | |||||
| IG | 1 | 14.9 | F | Non-genetic ataxia | 2 | 1 | 0 | 2 | 1 | 1 | 3 | 1 | 11 |
| 2 | 10.2 | M | Joubert’s ataxia | 3 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 8 | |
| 3 | 10 | F | ARCA2 | 3 | 0 | 0 | 1 | 1 | 1 | 3 | 1 | 10 | |
| 4 | 8.6 | M | ARCA2 | 1 | 0 | 0 | 0 | 0 | 1 | 1.5 | 0.5 | 4 | |
| 5 | 15.5 | F | ARCA2 | 1 | 0 | 0 | 2 | 1 | 1 | 3 | 1 | 9 | |
| 6 | 9.5 | F | Friedreich’s ataxia * | 2 | 2 | 1 | 0 | 1 | 1 | 3 | 2 | 11 | |
| 7 | 8.5 | M | Friedreich’s ataxia * | 2 | 2 | 0 | 1 | 1 | 1 | 1 | 2 | 10 | |
| 8 | 16.9 | F | Friedreich’s ataxia * | 2 | 2 | 0 | 2 | 0.5 | 1 | 0 | 1.5 | 9 | |
| 9 | 10.5 | F | ARSACS * | 2 | 1 | 0 | 1 | 1 | 1 | 2.5 | 2 | 10.5 | |
| CG | 10 | 17.2 | F | Non-genetic ataxia | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 8 |
| 11 | 15.5 | F | Joubert’s ataxia | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 5 | |
| 12 | 11.2 | M | Joubert’s ataxia | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 6 | |
| 13 | 10.4 | M | ARCA2 | 2 | 2 | 0 | 2 | 1 | 1 | 3 | 1.5 | 12.5 | |
| 14 | 7.7 | M | ARCA2 | 2 | 2 | 0 | 2 | 1 | 1.5 | 3 | 3.5 | 15 | |
| 15 | 9 | F | Friedreich’s ataxia * | 3 | 3 | 0 | 1 | 1 | 1.5 | 3 | 2.5 | 15 | |
| 16 | 12.6 | F | Friedreich’s ataxia * | 1 | 2 | 0 | 1 | 0.5 | 1 | 0.5 | 1.5 | 7.5 | |
| 17 | 15.4 | M | Friedreich’s ataxia * | 3 | 2 | 1 | 0 | 1 | 1 | 1 | 3 | 12 | |
| 18 | 5.1 | F | Ataxia telangiectasia * | 2 | 2 | 0 | 1 | 1 | 0 | 3 | 1 | 10 | |
| Exercises | Adherence Avg (SD) |
|---|---|
| Elbow flexion | 53.3 ± 0.4% |
| Shoulder 90° abduction | 64.1 ± 0.2% |
| Shoulder 180° abduction | 58.0 ± 0.3% |
| Shoulder 90° flexion | 64.4 ± 0.3% |
| Shoulder 180° flexion | 66.1 ± 0.3% |
| Ipsilateral target reaching | 62.8 ± 0.3% |
| Controlateral target reaching | 56.4 ± 0.3% |
| Trunk oscillation | 63.9 ± 0.3% |
| General adherence | 61.5 ± 3.9% |
| IG | p-Value IG T0 vs. T1 | CG | p-Value CG T0 vs. T1 | ∆ Scores | p-Value ∆ IG vs. ∆ CG | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T0 | T1 | ∆ IG | ∆ CG | |||||
| SARA Scores | Gait | 2 (0.7) | 2.1 (0.9) | 0.65 | 1.8 (0.8) | 2 (0.5) | 0.48 | −0.1 (0.8) | −0.2 (1) | 0.60 |
| Stance | 1 (0.9) | 1.1 (0.8) | 0.32 | 1.8 (0.7) | 1.6 (0.7) | 0.16 | −0.1 (0.3) | 0.2 (0.4) | 0.09 | |
| Sitting | 0.1 (0.3) | 0 (0) | 0.32 | 0.1 (0.3) | 0.3 (0.7) | 0.16 | 0.1 (0.3) | −0.2 (0.4) | 0.09 | |
| Speech disturbance | 1.1 (0.8) | 1.2 (0.8) | 1.00 | 1 (0.7) | 1.4 (1) | 0.18 | 0 (0.5) | −0.4 (1) | 0.30 | |
| Finger Chase | 0.8 (0.4) | 0.8 (0.4) | 0.32 | 0.9 (0.2) | 1.1 (0.8) | 0.59 | −0.1 (0.2) | −0.2 (0.8) | 0.90 | |
| Nose-finger test | 0.9 (0.3) | 1 (0) | 1.00 | 1 (0.4) | 1.2 (0.8) | 0.71 | 0 (0) | −0.2 (0.8) | 1.00 | |
| Fast alternating hand movements | 2 (1.1) | 1.9 (0.9) | 1.00 | 1.7 (1.3) | 1.8 (1.2) | 0.32 | 0.1 (0.8) | −0.1 (0.2) | 0.90 | |
| Heel-shin slide | 1.3 (0.6) | 1.4 (0.6) | 0.16 | 1.8 (1.1) | 1.7 (1) | 1.00 | −0.1 (0.2) | 0.1 (0.8) | 0.80 | |
| SARA Total score | 9.2 (2.2) | 9.6 (2.4) | 0.18 | 10.1 (3.7) | 11.1 (3.9) | 0.02 *↓ | −0.4 (0.9) | −0.9 (1) | 0.31 | |
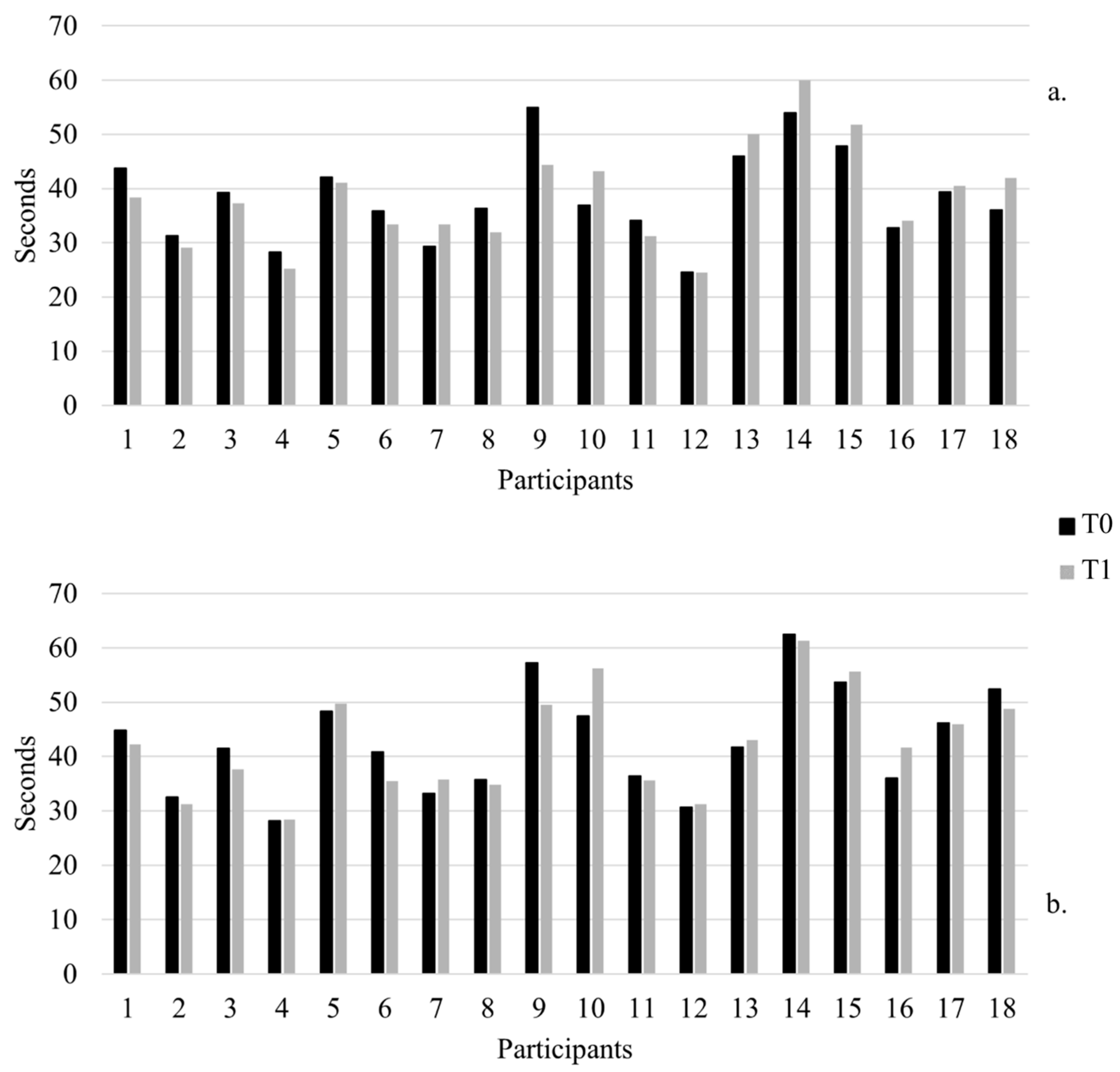
| T25FW | 5.3 (1) | 5.3 (0.5) | 0.86 | 6.3 (1.4) | 5.9 (1.2) | 0.31 | 0.1 (0.8) | 0.3 (0.7) | 0.31 | |
| 9HPT Dominant hand | 37.9 (8.4) | 34.9 (6) | 0.05 *↑ | 39.1 (8.9) | 41.9 (11) | 0.03 *↓ | 3 (3.9) | −2.6 (3.1) | 0.01 * | |
| 9HPT Non-dominant hand | 40.2 (9) | 38.3 (7.5) | 0.17 | 45.2 (10.1) | 46.6 (9.9) | 0.37 | 1.9 (3.3) | −1.6 (3.9) | 0.08 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, A.; Favetta, M.; Summa, S.; Schirinzi, T.; Bertini, E.S.; Castelli, E.; Vasco, G.; Petrarca, M. Upper Body Physical Rehabilitation for Children with Ataxia through IMU-Based Exergame. J. Clin. Med. 2022, 11, 1065. https://doi.org/10.3390/jcm11041065
Romano A, Favetta M, Summa S, Schirinzi T, Bertini ES, Castelli E, Vasco G, Petrarca M. Upper Body Physical Rehabilitation for Children with Ataxia through IMU-Based Exergame. Journal of Clinical Medicine. 2022; 11(4):1065. https://doi.org/10.3390/jcm11041065
Chicago/Turabian StyleRomano, Alberto, Martina Favetta, Susanna Summa, Tommaso Schirinzi, Enrico Silvio Bertini, Enrico Castelli, Gessica Vasco, and Maurizio Petrarca. 2022. "Upper Body Physical Rehabilitation for Children with Ataxia through IMU-Based Exergame" Journal of Clinical Medicine 11, no. 4: 1065. https://doi.org/10.3390/jcm11041065
APA StyleRomano, A., Favetta, M., Summa, S., Schirinzi, T., Bertini, E. S., Castelli, E., Vasco, G., & Petrarca, M. (2022). Upper Body Physical Rehabilitation for Children with Ataxia through IMU-Based Exergame. Journal of Clinical Medicine, 11(4), 1065. https://doi.org/10.3390/jcm11041065






