A Novel Echocardiographic-Based Classification for the Prediction of Peri-Device Leakage following Left Atrial Appendage Occluder Implantation
Abstract
1. Introduction
2. Methods
2.1. LAAO Procedure
2.2. Transesophageal Echocardiography
2.3. Biomarker
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Echocardiographic Features Following LAAO
3.3. Predictors of Type C LAAO
3.4. Correlation of BNP Values with Morphological Features
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glikson, M.; Wolff, R.; Hindricks, G.; Mandrola, J.; Camm, A.J.; Lip, G.Y.H.; Fauchier, L.; Betts, T.R.; Lewalter, T.; Saw, J.; et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion—An update. EP Europace 2019, 22, 184. [Google Scholar] [CrossRef]
- Meier, B.; Blaauw, Y.; Khattab, A.A.; Lewalter, T.; Sievert, H.; Tondo, C.; Glikson, M.; Lip, G.Y.H.; Lopez-Minguez, J.; Roffi, M.; et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion. EP Europace 2014, 16, 1397–1416. [Google Scholar] [CrossRef] [PubMed]
- Vainrib, A.F.; Harb, S.C.; Jaber, W.; Benenstein, R.J.; Aizer, A.; Chinitz, L.A.; Saric, M. Left atrial appendage occlusion/exclusion: Procedural image guidance with transesophageal echocardiography. J. Am. Soc. Echocardiogr. 2018, 31, 454–474. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, M.A.; Chaker, Z.; Al Hajji, M.; Alqahtani, F.; Raybuck, B. Incidence, characteristics and management of residual peri-device leak after percutaneous left atrial appendage occlusion. J. Am. Coll. Cardiol. 2018, 72, B114–B115. [Google Scholar] [CrossRef]
- Ayhan, H.; Mohanty, S.; Gedikli, Ö.; Trivedi, C.; Canpolat, U.; Tapia, A.C.; Chen, Q.; Della Rocca, D.G.; Gianni, C.; Salwan, A.; et al. A simple method to detect leaks after left atrial appendage occlusion with Watchman. J. Cardiovasc. Electrophysiol. 2020, 31, 2338–2343. [Google Scholar] [CrossRef]
- Saw, J.; Tzikas, A.; Shakir, S.; Gafoor, S.; Omran, H.; Nielsen-Kudsk, J.E.; Kefer, J.; Aminian, A.; Berti, S.; Santoro, G.; et al. Incidence and clinical impact of device-associated thrombus and peri-device leak following left atrial appendage closure with the amplatzer cardiac plug. JACC Cardiovasc. Interv. 2017, 10, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, J.R.; Lewis, A.J.; Perez, E.J.; Sanchez, A.M.; Baez-Escudero, J.L.; Navia, J.L.; Asher, C.R.; Cubeddu, R.J. Management of peri-device leak following left atrial appendage closure: A systematic review. Catheter. Cardiovasc. Interv. 2021, 98, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Viles-Gonzalez, J.F.; Kar, S.; Douglas, P.; Dukkipati, S.; Feldman, T.; Horton, R.; Holmes, D.; Reddy, V.Y. The clinical impact of incomplete left atrial appendage closure with the Watchman Device in patients with atrial fibrillation: A PROTECT AF (Percutaneous Closure of the Left Atrial Appendage versus Warfarin Therapy for Prevention of Stroke in Patients with Atrial Fibrillation) substudy. J. Am. Coll. Cardiol. 2012, 59, 923–929. [Google Scholar]
- Albaghdadi, M.; Kadlec, A.; Adler, A.; Siddiqui, U.; Romanov, A.; Kuck, K.-H.; Sievert, H.; Leon, M.B.; Vahl, T. Peri-device leaks after percutaneous left atrial appendage closure: Clinical significance and unmet diagnostic needs. Struct. Heart 2020, 4, 475–481. [Google Scholar] [CrossRef]
- Gianni, C.; Di Biase, L.; Trivedi, C.; Mohanty, S.; Gökoğlan, Y.; Güneş, M.F.; Bai, R.; Al-Ahmad, A.; Burkhardt, J.D.; Horton, R.P.; et al. Clinical implications of leaks following left atrial appendage ligation with the LARIAT device. JACC Cardiovasc. Interv. 2016, 9, 1051–1057. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Sievert, H.; Halperin, J.; Doshi, S.K.; Buchbinder, M.; Neuzil, P.; Huber, K.; Whisenant, B.; Kar, S.; Swarup, V.; et al. Percutaneous left atrial appendage closure vs. warfarin for atrial fibrillation: A randomized clinical trial. JAMA 2014, 312, 1988–1998. [Google Scholar] [CrossRef]
- Saw, J.; Fahmy, P.; DeJong, P.; Lempereur, M.; Spencer, R.; Tsang, M.; Gin, K.; Jue, J.; Mayo, J.; McLaughlin, P.; et al. Cardiac CT angiography for device surveillance after endovascular left atrial appendage closure. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Möbius-Winkler, S.; Sandri, M.; Mangner, N.; Lurz, P.; Dähnert, I.; Schuler, G. The WATCHMAN left atrial appendage closure device for atrial fibrillation. J. Vis. Exp. 2012, e3671. [Google Scholar] [CrossRef] [PubMed]
- Ijuin, S.; Hamadanchi, A.; Haertel, F.; Baez, L.; Schulze, P.C.; Franz, M.; Moebius-Winkler, S. Improvement in left atrial strain among patients undergoing percutaneous left atrial appendage closure. J. Cardiovasc. Echogr. 2020, 30, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Goebel, B.; Wieg, S.; Hamadanchi, A.; Otto, S.; Jung, C.; Kretzschmar, D.; Figulla, H.R.; Schulze, P.C.; Poerner, T. Interventional left atrial appendage occlusion: Added value of 3D transesophageal echocardiography for device sizing. Int. J. Cardiovasc. Imaging 2016, 32, 1363–1370. [Google Scholar] [CrossRef]
- Boersma, L.V.; Schmidt, B.; Betts, T.; Sievert, H.; Tamburino, C.; Teiger, E.; Pokushalov, E.; Kische, S.; Schmitz, T.; Stein, K.M.; et al. Implant success and safety of left atrial appendage closure with the WATCHMAN device: Peri-procedural outcomes from the EWOLUTION registry. Eur. Heart J. 2016, 37, 2465–2474. [Google Scholar] [CrossRef]
- Boersma, L.V.; Schmidt, B.; Betts, T.; Sievert, H.; Tamburino, C.; Teiger, E.; Stein, K.M.; Bergmann, M.W. EWOLUTION: Design of a registry to evaluate real-world clinical outcomes in patients with AF and high stroke risk-treated with the WATCHMAN left atrial appendage closure technology. Catheter. Cardiovasc. Interv. 2016, 88, 460–465. [Google Scholar] [CrossRef]
- Holmes, D.R.; Kar, S.; Price, M.J.; Whisenant, B.; Sievert, H.; Doshi, S.K.; Huber, K.; Reddy, V.Y. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J. Am. Coll. Cardiol. 2014, 64, 1–12. [Google Scholar] [CrossRef]
- Yerasi, C.; Lazkani, M.; Kolluru, P.; Miryala, V.; Kim, J.; Moole, H.; Sawant, A.C.; Morris, M.; Pershad, A. An updated systematic review and meta-analysis of early outcomes after left atrial appendage occlusion. J. Interv. Cardiol. 2018, 31, 197–206. [Google Scholar] [CrossRef]
- Alkhouli, M.; Chaker, Z.; Clemetson, E.; Alqahtani, F.; Al Hajji, M.; Lobban, J.; Sengupta, P.P.; Raybuck, B. Incidence, characteristics and management of persistent peri-device flow after percutaneous left atrial appendage occlusion. Struct. Heart 2019, 3, 491–498. [Google Scholar] [CrossRef]
- Alkhouli, M.; Chaker, Z.; Alqahtani, F.; Raslan, S.; Raybuck, B. Outcomes of routine intracardiac echocardiography to guide left atrial appendage occlusion. JACC Clin. Electrophysiol. 2020, 6, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Lindner, S.; Behnes, M.; Wenke, A.; Sartorius, B.; Ansari, U.; Akin, M.; Mashayekhi, K.; Vogler, N.; Haubenreisser, H.; Schoenberg, S.O.; et al. Assessment of peri-device leaks after interventional left atrial appendage closure using standardized imaging by cardiac computed tomography angiography. Int. J. Cardiovasc. Imaging 2019, 35, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Neuzner, J.; Dietze, T.; Paliege, R.; Möller, M.; Saeed, G.; Gradaus, R. Left atrial appendage closure with the Amplatzer™ Cardiac Plug: Rationale for a higher degree of device oversizing at implantation. Cardiol. J. 2015, 22, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.J.; Wong, S.C.; Mosadegh, B. Leaks after left atrial appendage closure: Ignored or neglected? Cardiology 2021, 146, 384–391. [Google Scholar] [CrossRef]
- Pracon, R.; Bangalore, S.; Dzielinska, Z.; Konka, M.; Kepka, C.; Kruk, M.; Kaczmarska-Dyrda, E.; Petryka-Mazurkiewicz, J.; Bujak, S.; Solecki, M.; et al. Device thrombosis after percutaneous left atrial appendage occlusion is related to patient and procedural characteristics but not to duration of postimplantation dual antiplatelet therapy. Circ. Cardiovasc. Interv. 2018, 11, e005997. [Google Scholar] [CrossRef] [PubMed]
- Kleinecke, C.; Gloekler, S.; Meier, B. Utilization of percutaneous left atrial appendage closure in patients with atrial fibrillation: An update on patient outcomes. Expert Rev. Cardiovasc. Ther. 2020, 18, 517–530. [Google Scholar] [CrossRef]
- Aryana, A.; Singh, S.K.; Singh, S.M.; O’Neill, P.G.; Bowers, M.R.; Allen, S.L.; Lewandowski, S.L.; Vierra, E.C.; D’Avila, A. Association between incomplete surgical ligation of left atrial appendage and stroke and systemic embolization. Heart Rhythm 2015, 12, 1431–1437. [Google Scholar] [CrossRef]
- Kanderian, A.S.; Gillinov, A.M.; Pettersson, G.B.; Blackstone, E.; Klein, A.L. Success of surgical left atrial appendage closure: Assessment by transesophageal echocardiography. J. Am. Coll. Cardiol. 2008, 52, 924–929. [Google Scholar] [CrossRef]
- Nguyen, A.; Gallet, R.; Riant, E.; Deux, J.-F.; Boukantar, M.; Mouillet, G.; Dubois-Randé, J.-L.; Lellouche, N.; Teiger, E.; Lim, P.; et al. Peridevice leak after left atrial appendage closure: Incidence, risk factors, and clinical impact. Can. J. Cardiol. 2019, 35, 405–412. [Google Scholar] [CrossRef]
- Raphael, C.E.; Friedman, P.A.; Saw, J.; Pislaru, S.V.; Munger, T.M.; Holmes, D.R., Jr. Residual leaks following percutaneous left atrial appendage occlusion: Assessment and management implications. Eurointervention 2017, 13, 1218–1225. [Google Scholar] [CrossRef]
- Tower-Rader, A.; Wazni, O.; Jaber, W.A. Intradevice leak on late follow-up after Watchman implantation. CASE 2018, 2, 192–196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Granier, M.; Laugaudin, G.; Massin, F.; Cade, S.; Winum, P.F.; Freitag, C.; Pasquie, J.-L. Occurrence of incomplete endothelialization causing residual permeability after left atrial appendage closure. J. Invasive Cardiol. 2018, 30, 245–250. [Google Scholar] [PubMed]
- Majunke, N.; Sandri, M.; Adams, V.; Daehnert, I.; Mangner, N.; Schuler, G.; Moebius-Winkler, S. Atrial and brain natriuretic peptide secretion after percutaneous closure of the left atrial appendage with the watchman device. J. Invasive Cardiol. 2015, 27, 448–452. [Google Scholar] [PubMed]
- Di Biase, L.; Santangeli, P.; Anselmino, M.; Mohanty, P.; Salvetti, I.; Gili, S.; Horton, R.; Sanchez, J.E.; Bai, R.; Mohanty, S.; et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J. Am. Coll. Cardiol. 2012, 60, 531–538. [Google Scholar] [CrossRef]
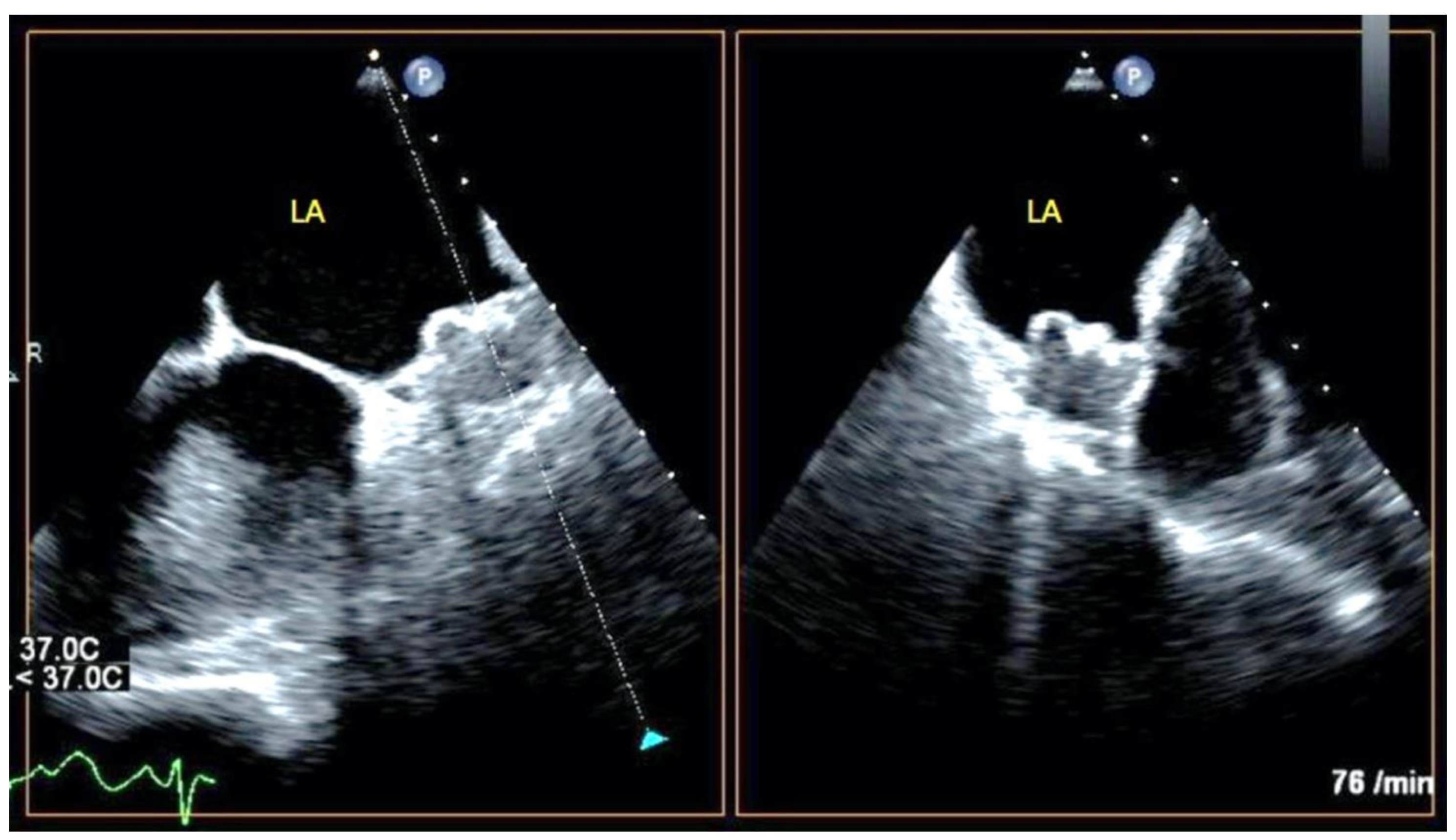

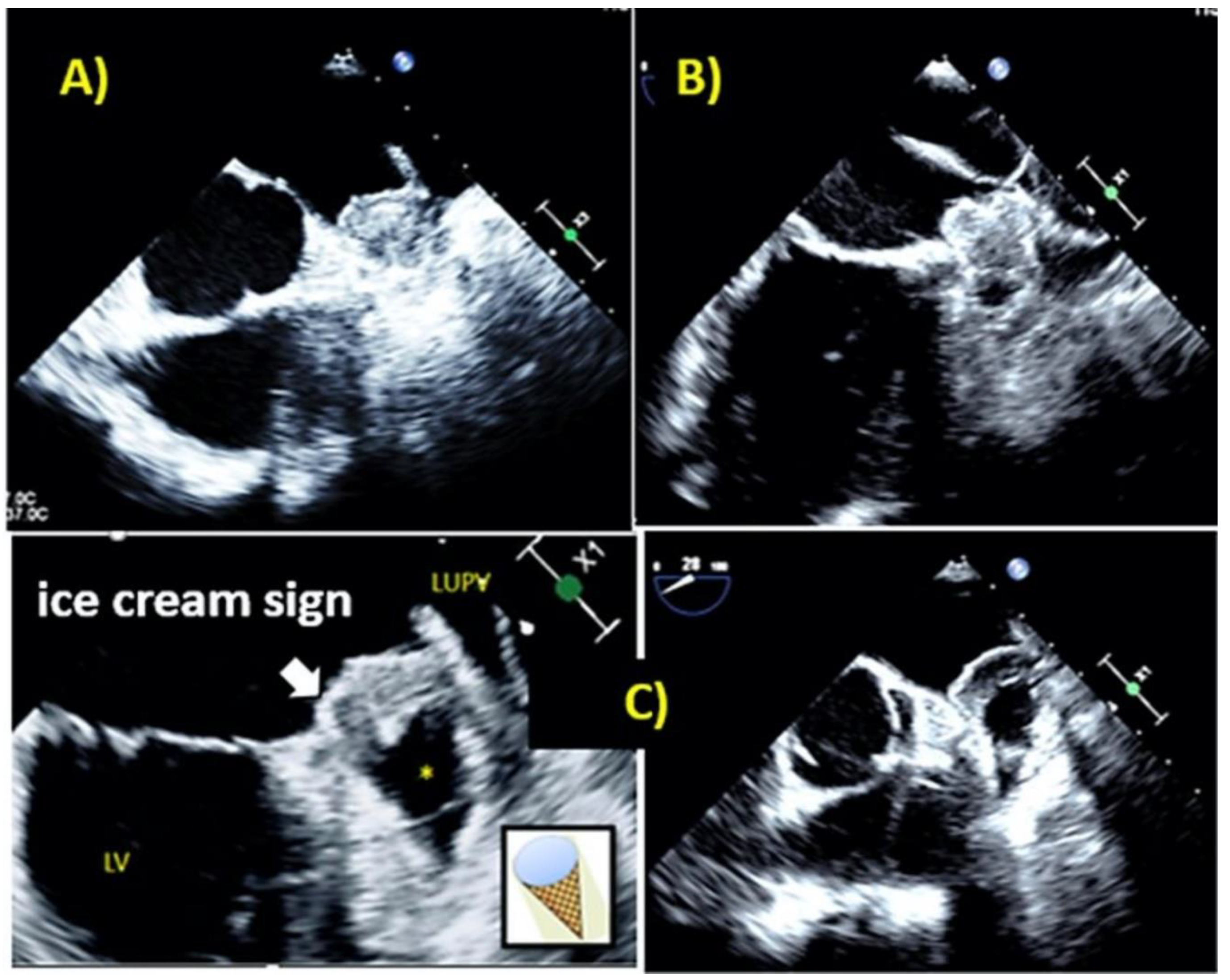
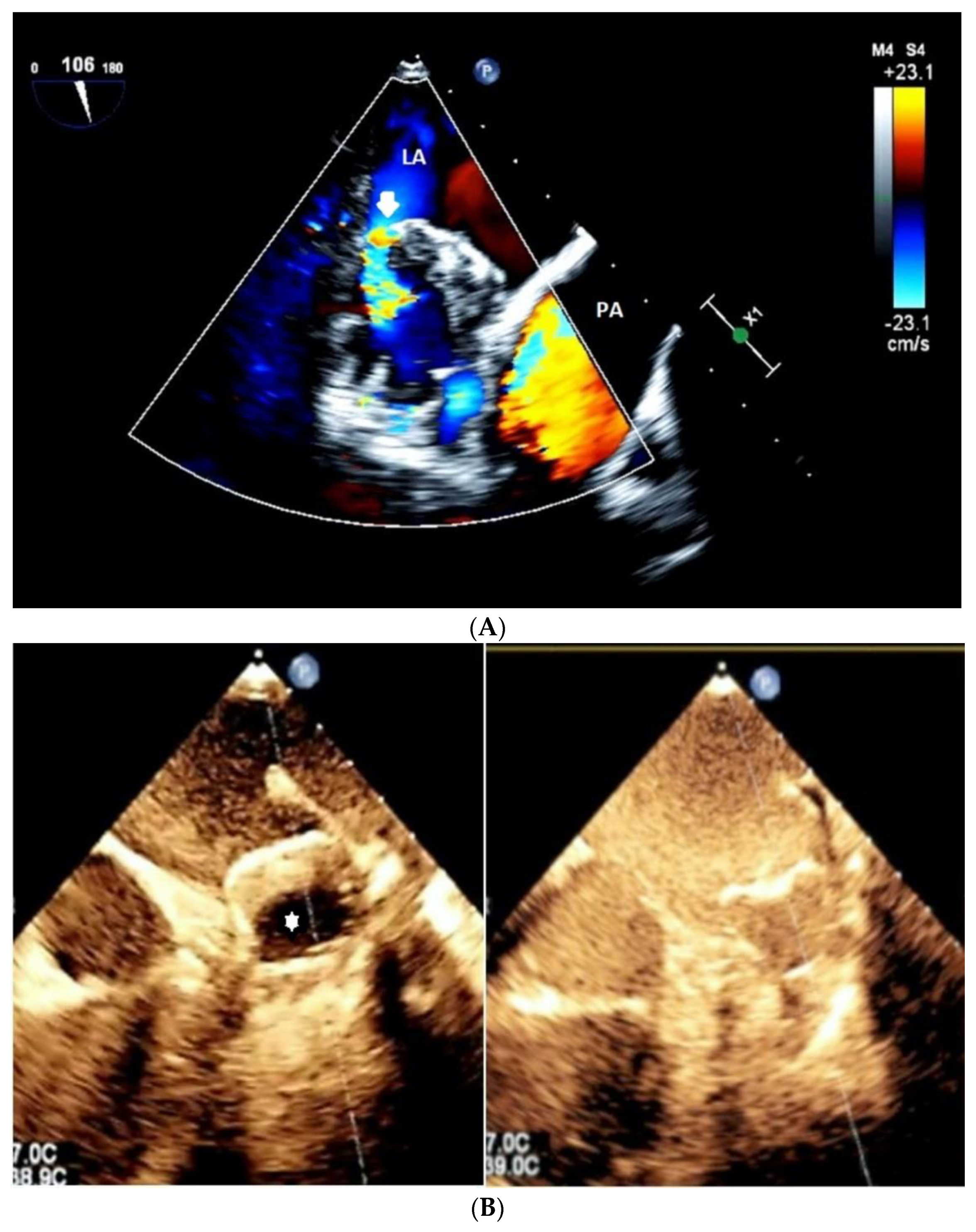
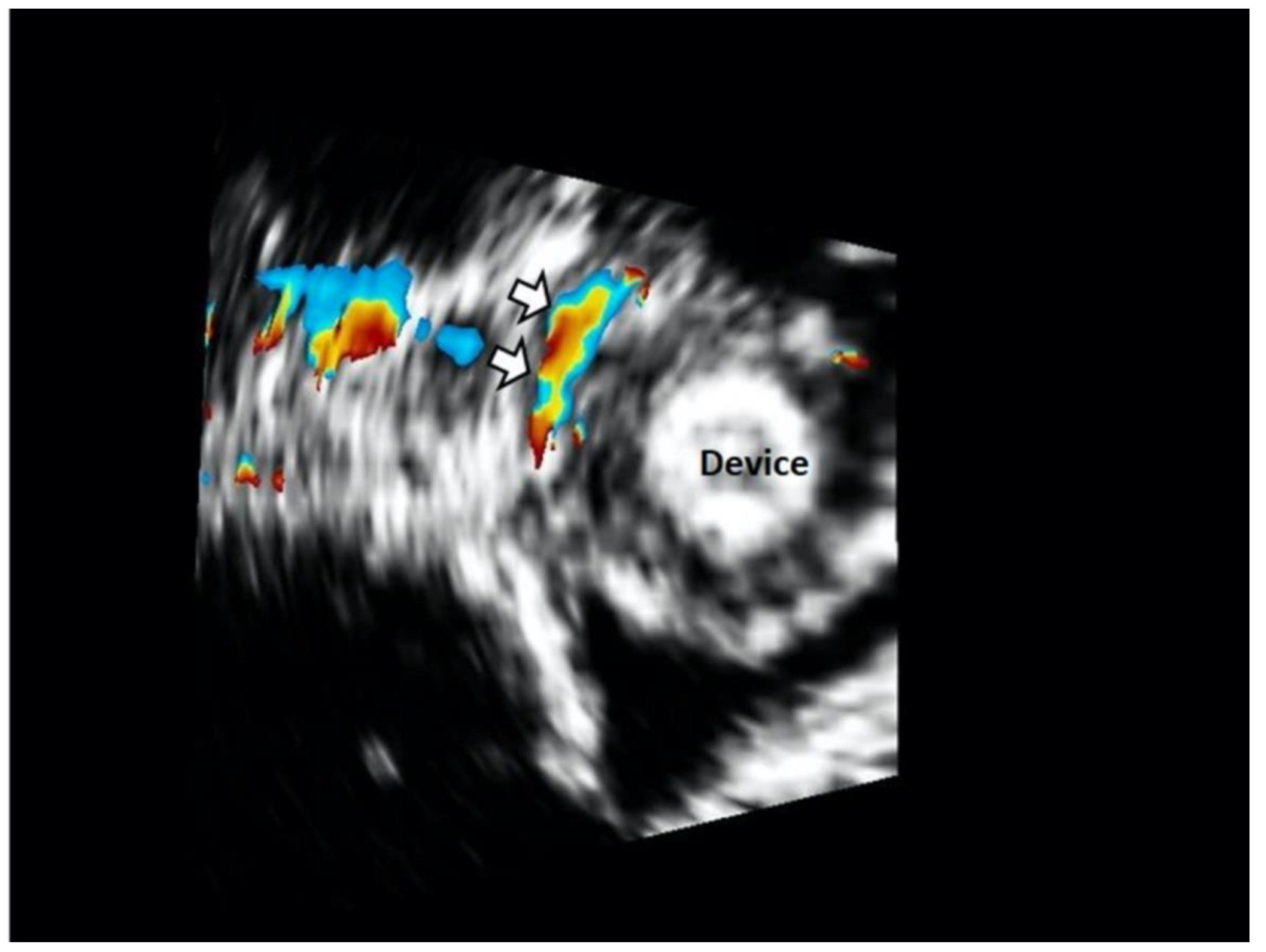

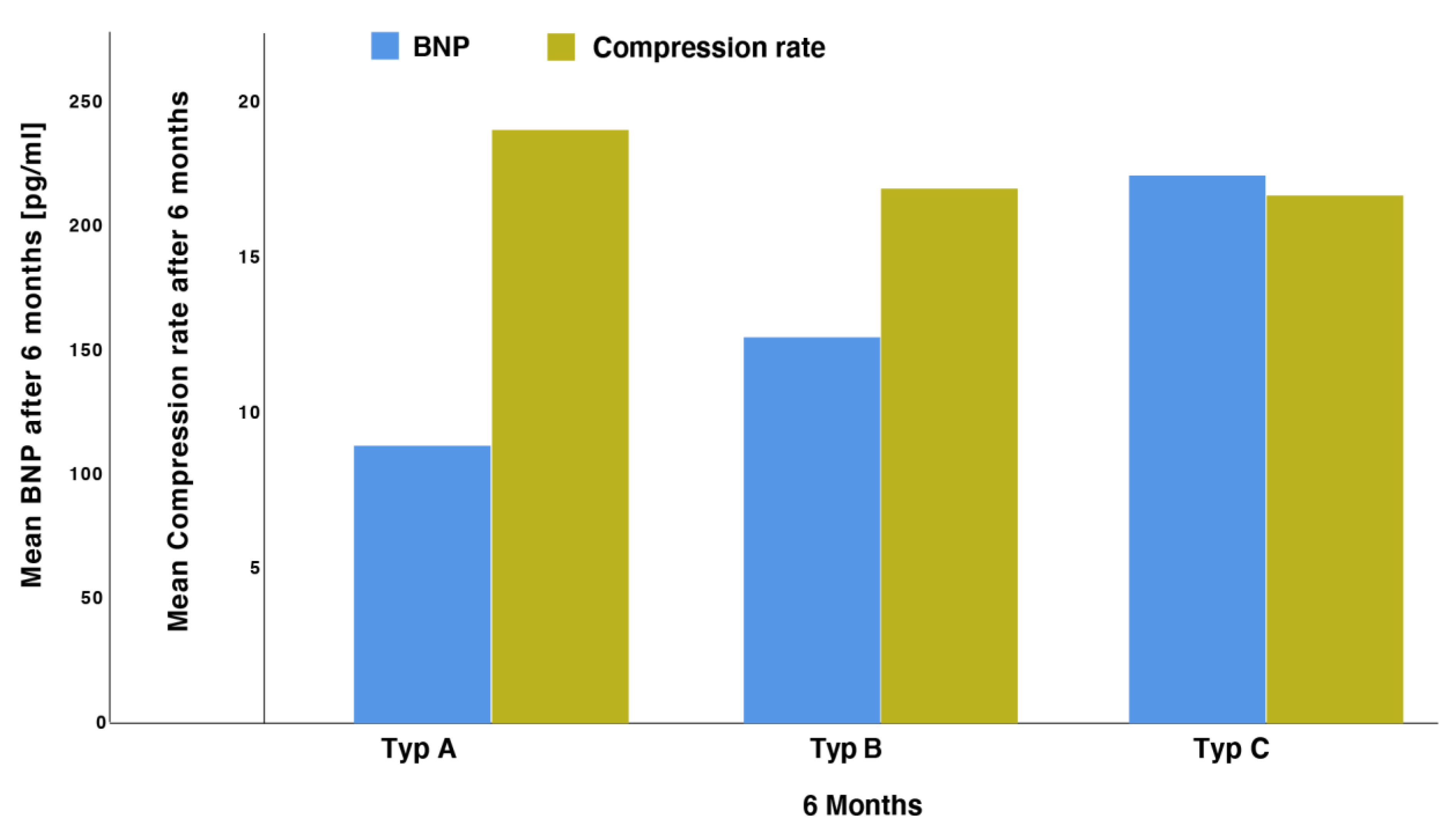
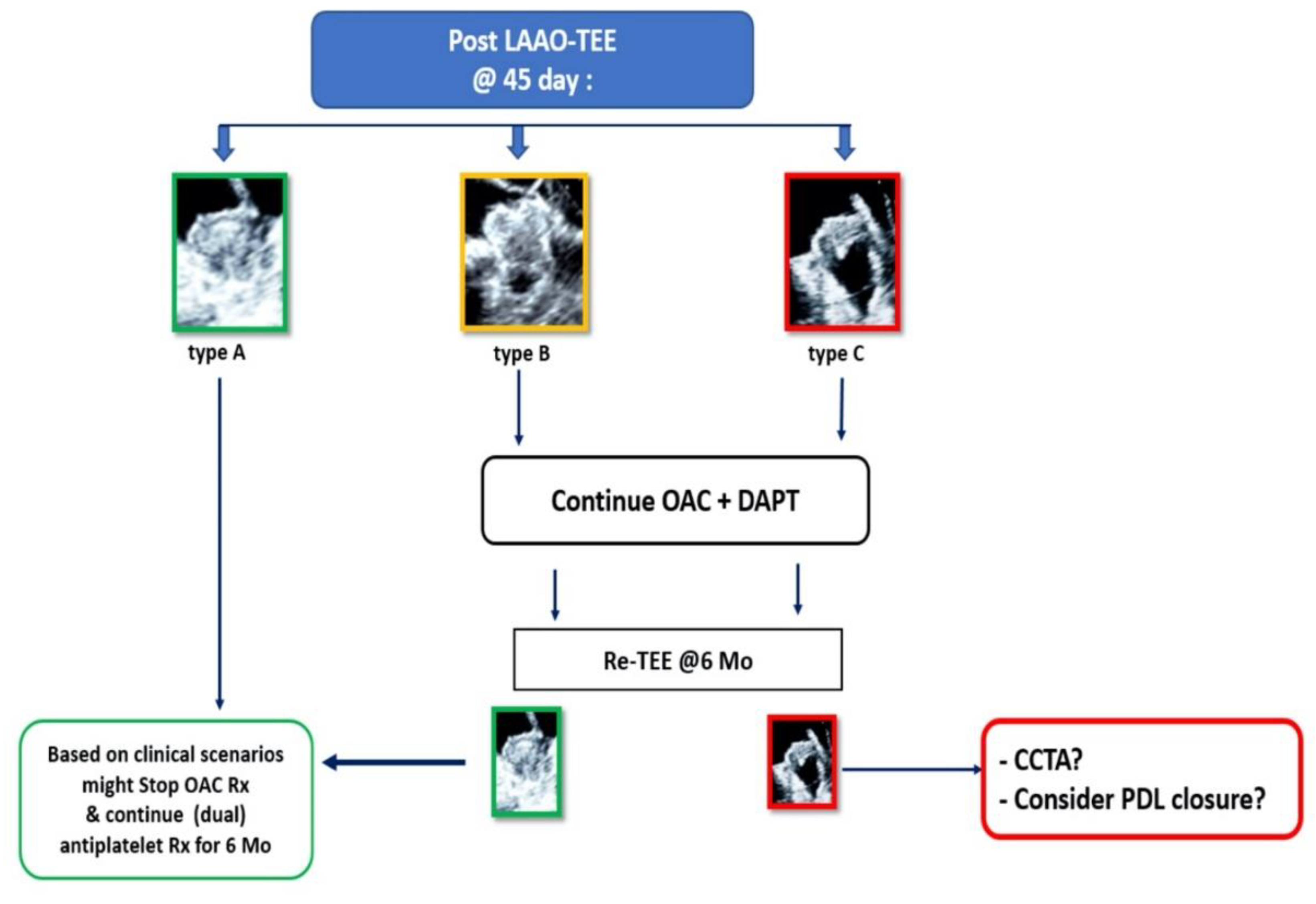
| Study Population (N = 72) | |
|---|---|
| Demographics | |
| Age (years, mean ± SD) | 73 ± 8.1 |
| Male (N (%)) | 48 (65) |
| Female (N (%)) | 25 (35) |
| BMI (kg/m2, mean ± SD) | 28.8 ± 4.8 |
| CHA2DS2VASc Score (mean ± SD) | 3.9 ± 0.9 |
| HAS-BLED score (mean ± SD) | 4.9 ± 0.8 |
| Atrial fibrillation | |
| Paroxysmal (N (%)) | 36 (49) |
| Persistent (N (%)) | 7 (10) |
| Permanent (N (%)) | 31 (42) |
| Anticoagulation until day 45 | |
| Vitamin K antagonist (N (%)) | 5 (7) |
| LMWH or NOAC (N (%)) | 18 (24) |
| DAPT (N (%)) | 18 (24) |
| Vitamin K antagonist or LMWH or NOAC + antiplatelet therapy (N (%)) | 33 (45) |
| Laboratory data | |
| Hemoglobin (mmol/dL; mean ± SD) | 7.6 ± 1.6 |
| eGFR (mL/min/1.73 m2; mean ± SD) | 56.9 ± 23.4 |
| BNP (pg/mL; n = 47, mean ± SD) | 171.5 ± 110.9 |
| Echocardiographic parameters | |
| LVEF ((%) n = 62) | 58.1 ± 14.1 |
| Left atrial diameter (mm, n = 50, mean ± SD) | 46.3 ± 8.2 |
| TRPG (mmHg, n = 33, mean ± SD) | 35.2 ± 13.4 |
| Procedural Data | |
| Occluder Size (mm, mean ± SD) | 26.3 ± 3.8 |
| Type of Occluder (N (%)) | |
| Watchman device | 66 (91.5) |
| LAmbre ™ | 6 (8.5) |
| Parameters | AUC | CI (95%) | p-Value |
|---|---|---|---|
| Orifice area at baseline | 0.78 | 0.61–0.95 | 0.019 |
| Leakage size after 45 days | 0.75 | 0.59–0.91 | 0.008 |
| Mean compression rate after 45 days | 0.47 | 0.27–0.68 | 0.79 |
| Occluder size | 0.77 | 0.60–0.93 | 0.005 |
| CHA2DS2VASc score at baseline | 0.56 | 0.37–0.75 | 0.57 |
| HAS–BLED score at baseline | 0.52 | 0.35–0.71 | 0.80 |
| BNP at baseline | 0.57 | 0.38–0.75 | 0.51 |
| BNP after 45 days | 0.61 | 0.42–0.79 | 0.31 |
| Sphericity index at baseline | 0.56 | 0.33–0.79 | 0.12 |
| Parameters | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR (CI) | p-Value | OR (CI) | p-Value | |
| Atrial Fibrillation | 0.73 (0.20–2.63) | 0.627 | - | - |
| Maximal ostial diameter in mm (2D) | 1.19 (1.01–1.41) | 0.043 | 0.98 (0.70–1.37) | 0.898 |
| Average ostial diameter in mm (2D) | 1.30 (1.03–1.63) | 0.026 | 1.04 (0.95–1.14) | 0.411 |
| 3D perimeter in mm | 1.06 (1.00–1.13) | 0.043 | 1.22 (0.77–1.94) | 0.402 |
| 3D orifice area in cm2 | 1.73 (0.98–3.05) | 0.058 | - | - |
| 3D area derived diameter in mm | 1.19 (0.99–1.44) | 0.063 | - | - |
| 3D minimal ostial diameter in mm | 1.18 (0.98–1.42) | 0.076 | - | - |
| 3D maximal ostial diameter in mm | 1.12 (0.97–1.29) | 0.121 | - | - |
| 3D average ostial diameter in mm | 1.19 (0.99–1.43) | 0.061 | - | - |
| Ostial sphericity index | 0.29 (0.02–5.76) | 0.418 | - | - |
| Average LAA depth in mm | 1.12 (0.96–1.30) | 0.149 | - | - |
| Maximal compression rate | 0.94 (0.86–1.02) | 0.154 | - | - |
| Average compression rate | 0.95 (0.86–1.04) | 0.252 | - | - |
| Implanted occluder device size in mm | 1.23 (1.03–1.48) | 0.023 | 1.01 (0.76–1.33) | 0.963 |
| Age in years | 1.04 (0.96–1.12) | 0.315 | - | - |
| Sex | 0.71 (0.23–2.12) | 0.556 | - | - |
| BMI in kg/m2 | 0.99 (0.88–1.11) | 0.808 | - | - |
| BSA in m2 | 1.20 (0.09–15.47) | 0.887 | - | - |
| eGFR in mL/min/kg | 0.99 (0.97–1.01) | 0.451 | - | - |
| Hb in mmol/L | 1.01 (0.71–1.44) | 0.964 | - | - |
| BNP in pg/mL | 1.00 (0.99–1.00) | 0.582 | - | - |
| LVEF in% | 0.99 (0.95–1.03) | 0.588 | - | - |
| LVEDD in mm | 0.94 (0.84–1.05) | 0.258 | - | - |
| TR-PPG in mmHg | 1.01 (0.96–1.07) | 0.624 | - | - |
| Type C | Outcome | |
|---|---|---|
| No PDL (n = 44) | PDL (n = 27) | |
| No (n = 54) | 44 | 10 |
| Yes (n = 17) | 0 | 17 |
| Sensitivity | 100% | |
| Specificity | 63% | |
| PPV | 81.5% | |
| NPV | 100% | |
| PLR | 2.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamadanchi, A.; Ijuin, S.; Haertel, F.; Bekfani, T.; Westphal, J.; Franz, M.; Moebius-Winkler, S.; Schulze, P.C. A Novel Echocardiographic-Based Classification for the Prediction of Peri-Device Leakage following Left Atrial Appendage Occluder Implantation. J. Clin. Med. 2022, 11, 1059. https://doi.org/10.3390/jcm11041059
Hamadanchi A, Ijuin S, Haertel F, Bekfani T, Westphal J, Franz M, Moebius-Winkler S, Schulze PC. A Novel Echocardiographic-Based Classification for the Prediction of Peri-Device Leakage following Left Atrial Appendage Occluder Implantation. Journal of Clinical Medicine. 2022; 11(4):1059. https://doi.org/10.3390/jcm11041059
Chicago/Turabian StyleHamadanchi, Ali, Shun Ijuin, Franz Haertel, Tarek Bekfani, Julian Westphal, Marcus Franz, Sven Moebius-Winkler, and P. Christian Schulze. 2022. "A Novel Echocardiographic-Based Classification for the Prediction of Peri-Device Leakage following Left Atrial Appendage Occluder Implantation" Journal of Clinical Medicine 11, no. 4: 1059. https://doi.org/10.3390/jcm11041059
APA StyleHamadanchi, A., Ijuin, S., Haertel, F., Bekfani, T., Westphal, J., Franz, M., Moebius-Winkler, S., & Schulze, P. C. (2022). A Novel Echocardiographic-Based Classification for the Prediction of Peri-Device Leakage following Left Atrial Appendage Occluder Implantation. Journal of Clinical Medicine, 11(4), 1059. https://doi.org/10.3390/jcm11041059






