Usefulness of Selected Peripheral Blood Counts in Predicting Death in Patients with Severe and Critical COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Clinical Data
2.4. Laboratory Data
2.5. Outcome
2.6. Statistical Analysis
3. Results
3.1. Peripheral Blood Leukocyte Parameters
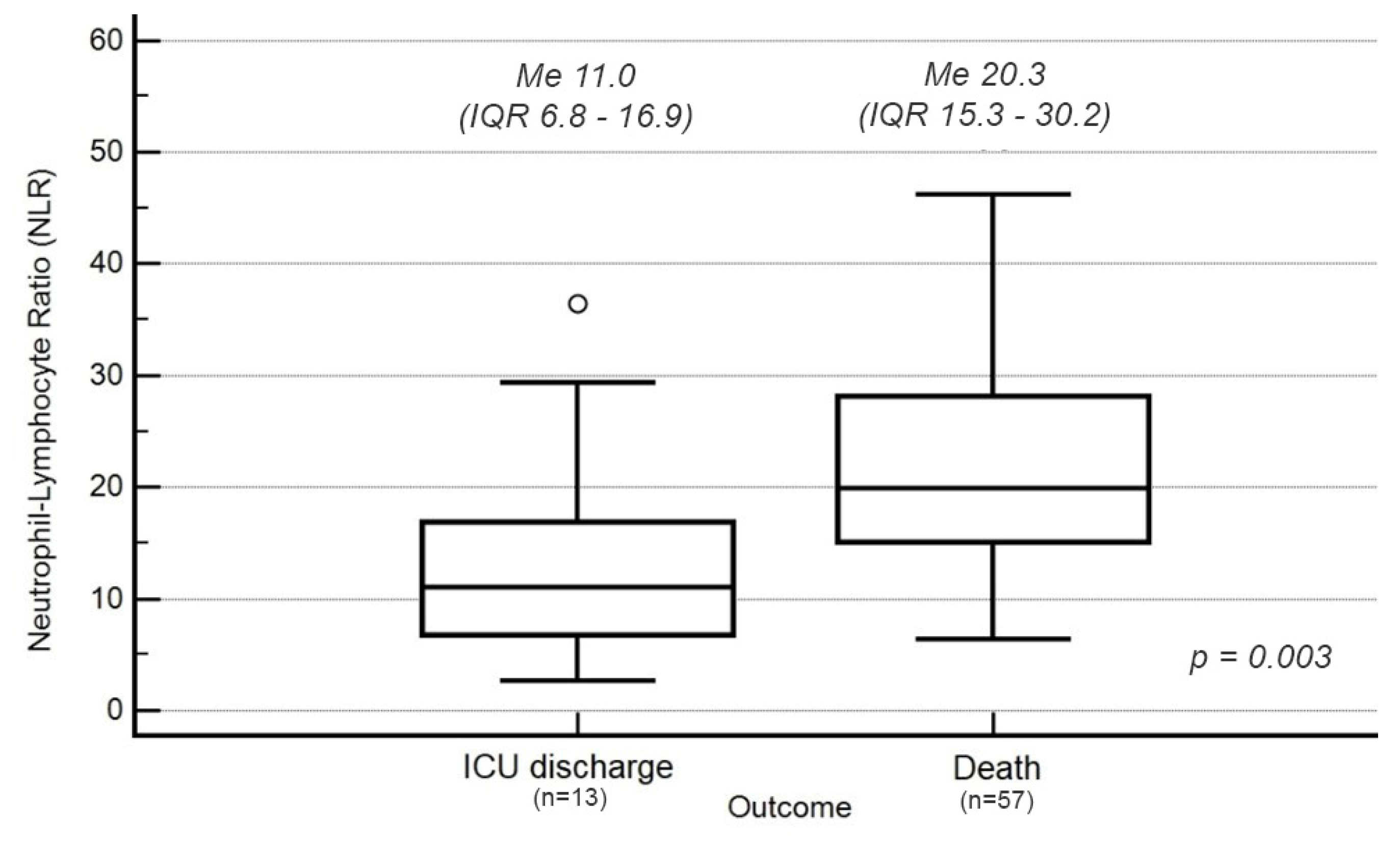
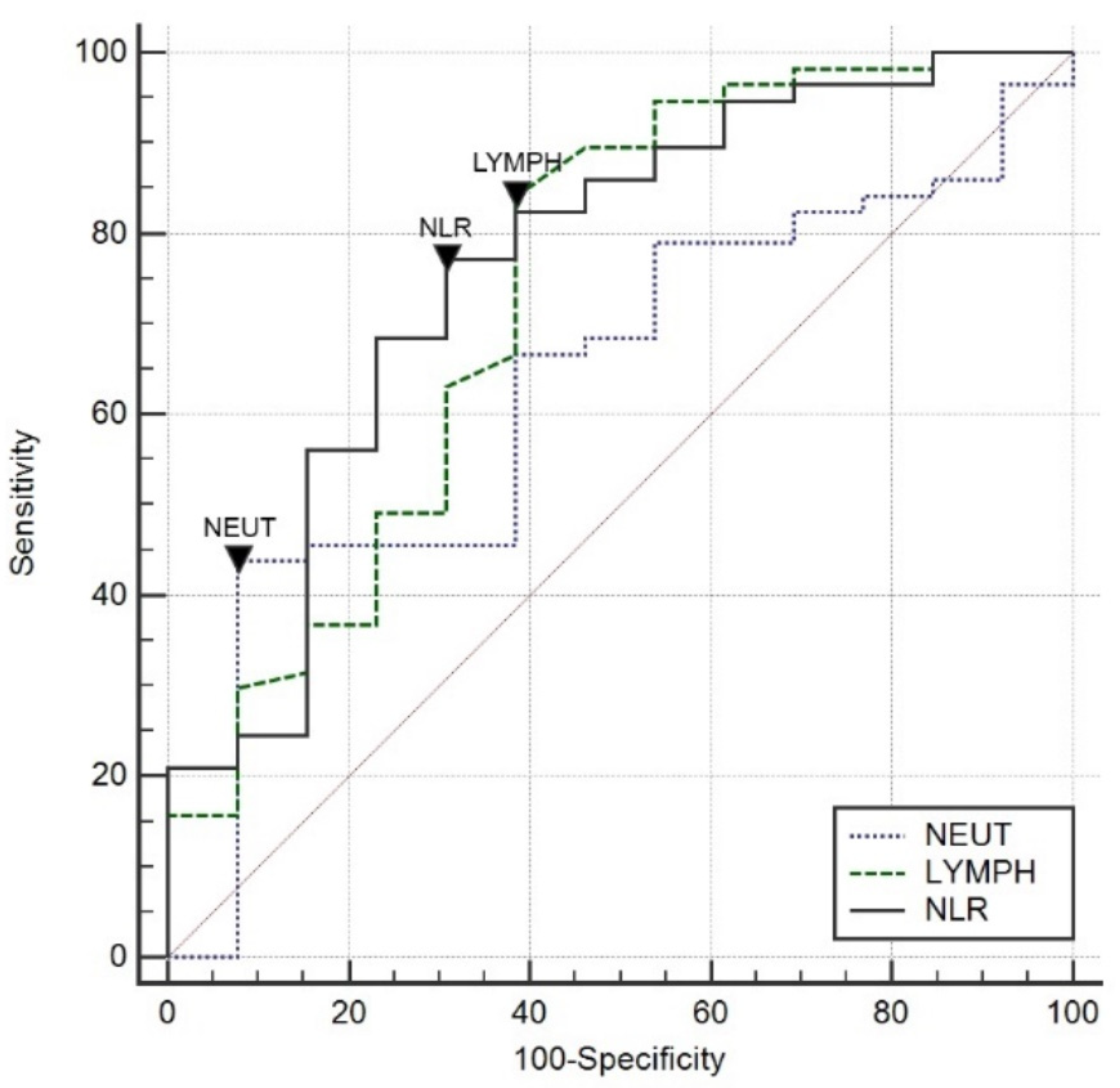
3.2. Neutrophil–Lymphocyte Ratio (NLR)
3.3. Red Cell Distribution Width (RDW)
3.4. Logistic Regression Model
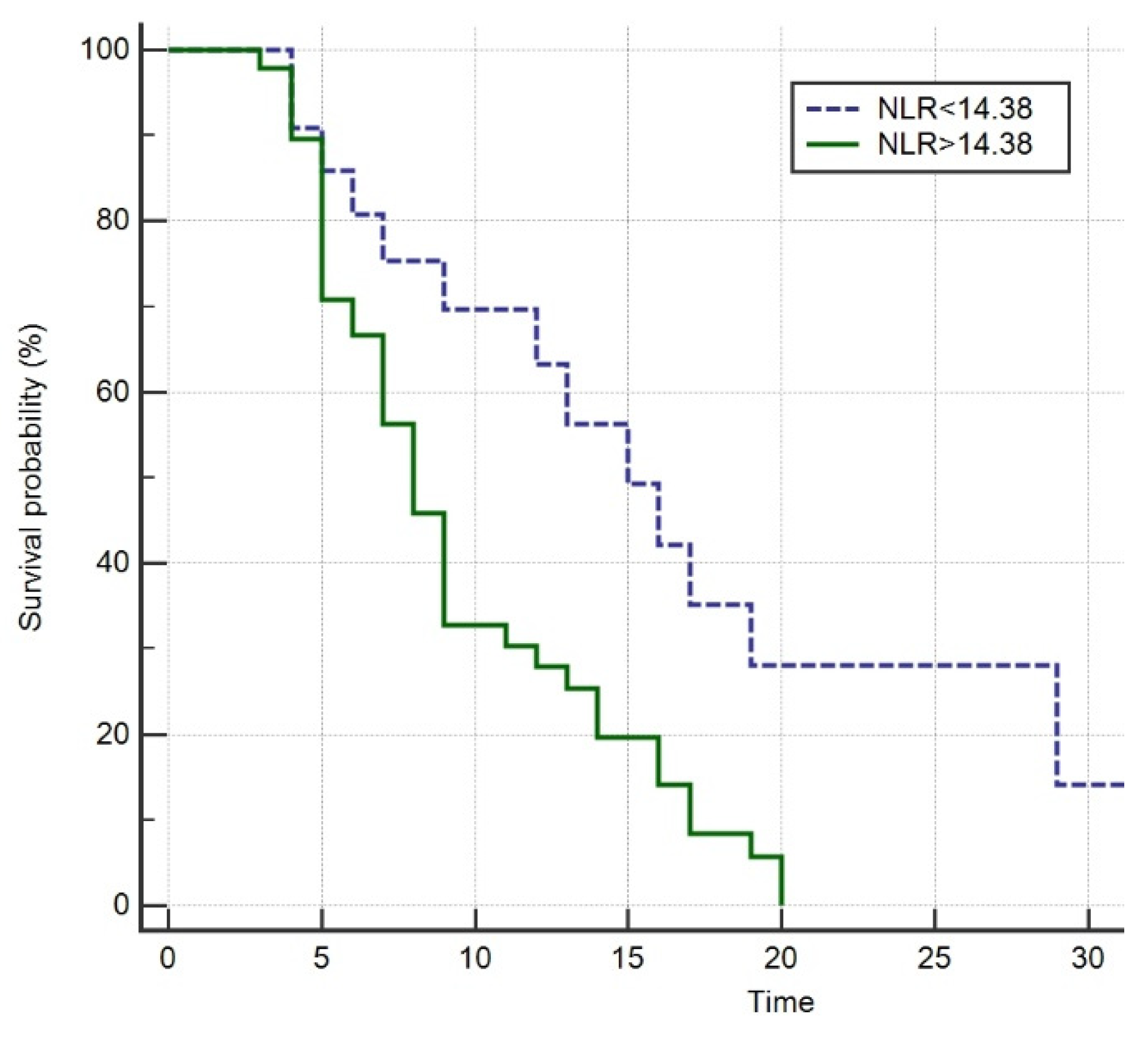
3.5. Survival Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chan, J.F.W.; Kok, K.H.; Zhu, Z.; Chu, H.; To, K.K.W.; Yuan, S.; Yuen, K.Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Available online: http://www.covid19.who.int (accessed on 30 January 2022).
- Mena, G.; Martinez, P.P.; Mahmud, A.S.; Marquet, P.A.; Buckee, C.O.; Santillana, M. Socioeconomic status determines COVID-19 incidence and related mortality in Santiago, Chile. Science 2021, 372, eabg5298. [Google Scholar] [CrossRef]
- Keddie, S.; Ziff, O.; Chou, M.K.; Taylor, R.L.; Heslegrave, A.; Garr, E.; Lakdawala, N.; Church, A.; Ludwig, D.; Manson, J.; et al. Laboratory biomarkers associated with COVID-19 severity and management. Clin. Immunol. 2020, 221, 108614. [Google Scholar] [CrossRef] [PubMed]
- Samprathi, M.; Jayashree, M. Biomarkers in COVID-19, An Up-To-Date Review. Front. Pediatr. 2021, 8, 607647. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.Y.; Feng, S.D.; Chen, G.P.; Wu, J.N. Predictive value of the neutrophil to lymphocyte ratio for disease deterioration and serious adverse outcomes in patients with COVID-19, a prospective cohort study. BMC Infect. Dis. 2021, 21, 80. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Cai, T.; Fan, L.; Lou, K.; Hua, X.; Huang, Z. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int. J. Infect. Dis. 2020, 95, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Y.; Wang, J.; Wang, S. Association between red blood cell distribution width and long-term mortality in acute respiratory failure patients. Sci. Rep. 2020, 10, 21185. [Google Scholar] [CrossRef]
- Pluta, M.; Klocek, T.; Krzych, Ł.J. Diagnostic accuracy of red blood cell distribution width in predicting in-hospital mortality in patients undergoing high-risk gastrointestinal surgery. Anaesthesiol. Intensive Ther. 2018, 50, 277–282. [Google Scholar] [CrossRef]
- Ustawa z Dnia 5 Grudnia 1996, r. o Zawodzie Lekarza; Rozdz.4 (Tekst Jedn. Dz.U. 1997 nr 28 Poz. 152). Available online: http://isap.sejm.gov.pl/ (accessed on 15 December 2021). (In Polish)
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [Green Version]
- COVID-19 Treatment Guidelines Panel, National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 20 November 2021).
- Nates, J.L.; Nunnally, M.; Kleinpell, R.; Blosser, S.; Goldner, J.; Birriel, B. ICU Admission, Discharge, and Triage Guidelines: A Framework to Enhance Clinical Operations, Development of Institutional Policies, and Further Research. Crit. Care Med. 2016, 44, 1553–1602. [Google Scholar] [CrossRef] [Green Version]
- Farkas, J. PulmCrit: Neutrophil–Lymphocyte Ratio (NLR): Free Upgrade to Your WBC. Available online: https://emcrit.org/pulmcrit/nlr/ (accessed on 1 October 2020).
- Liu, Y.; Du, X.; Chen, J.; Jin, Y.; Peng, L.; Wang, H.H.X. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020, 81, e6–e12. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, Q.; Huang, C.; Shi, C.; Wang, L.; Shi, N. CT quantification of pneumonia lesions in early days predicts progression to severe illness in a cohort of COVID-19 patients. Theranostics 2020, 10, 5613–5622. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.-P.; Liu, J.-P.; Tao, W.-Q.; Li, H.-M. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int. Immunopharmacol. 2020, 84, 106504. [Google Scholar] [CrossRef]
- Shang, W.; Dong, J.; Ren, Y.; Tian, M.; Li, W.; Hu, J. The value of clinical parameters in predicting the severity of COVID-19. J. Med. Virol. 2020, 92, 2188–2192. [Google Scholar] [CrossRef]
- Yan, X.; Li, F.; Wang, X.; Yan, J.; Zhu, F.; Tang, S. Neutrophil to lymphocyte ratio as prognostic and predictive factor in patients with coronavirus disease 2019, A retrospective cross-sectional study. J. Med. Virol. 2020, 92, 2573–2581. [Google Scholar] [CrossRef]
- Ulloque-Badaracco, J.R.; Ivan Salas-Tello, W.; Al-kassab-Córdova, A.; Alarcón-Braga, E.A.; Benites-Zapata, V.A.; Maguiña, J.L.; Hernandez, A.V. Prognostic value of neutrophil-to-lymphocyte ratio in COVID-19 patients: A systematic review and meta-analysis. Int. J. Clin. Pract. 2021, 75, e14596. [Google Scholar] [CrossRef]
- Kaushik, R.; Gupta, M.; Sharma, M.; Jash, D.; Jain, N.; Sinha, N. Diagnostic and Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Early and Late Phase of Sepsis. Indian J. Crit. Care Med. 2018, 22, 660–663. [Google Scholar] [CrossRef]
- Huang, Z.; Fu, Z.; Huang, W.; Huang, K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: A meta-analysis. Am. J. Emerg. Med. 2020, 38, 641–647. [Google Scholar] [CrossRef]
- Falzone, E.; Pasquier, P.; Hoffmann, C.; Barbier, O.; Boutonnet, M.; Salvadori, A. Triage in military settings. Anaesth. Crit. Care Pain Med. 2017, 36, 43–51. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Kalemaki, D.; Tzagkarakis, E.; Lydakis, C. Pitfalls in studies of eosinopenia and neutrophil-to-lymphocyte count ratio. Infect. Dis. 2018, 50, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Polish Diagnostic, Therapeutic and Organisational Recommendations for the Care of Individuals Infected with SARS-CoV-2 or Exposed to a SARS-CoV-2 Infection. Available online: http://aotm.gov.pl (accessed on 14 December 2021).


| Parameter | Survival 1 | Death | p |
|---|---|---|---|
| (n = 13) | (n = 57) | ||
| Age (years) | |||
| Median (IQR) | 57 (53–67) | 67 (61–72) | 0.01 |
| Sex | |||
| male, n (%) | 4 (31%) | 43 (75%) | 0.002 |
| female, n (%) | 9 (69%) | 14 (25%) | 0.002 |
| Past medical history | |||
| Obesity, n (%) | 8 (61%) | 37 (65%) | 0.8 |
| Hypertension, n (%) | 8 (61%) | 32 (56%) | 0.7 |
| Diabetes, n (%) | 2 (15%) | 11 (19%) | 0.7 |
| Chronic kidney disease, n (%) | 2 (15%) | 6 (11%) | 0.6 |
| COPD, n (%) | 1 (8%) | 5 (9%) | 0.9 |
| CAD, n (%) | 4 (31%) | 14 (25%) | 0.7 |
| Hypothyroidism, n (%) | 1 (8%) | 3 (5%) | 0.7 |
| Stroke, n (%) | - | 2 (4%) | - |
| Duration of ICU hospitalization (days) | |||
| Median (IQR) | 11 (8–15) | 8 (5–13) | 0.3 |
| % lung injury 2 | |||
| Median (IQR) | 70 (26–79) | 80 (70–90) | 0.03 |
| Pulmonary embolism, n (%) | 1 (8%) | 6 (10%) | 0.7 |
| ICU admission priority 3 | |||
| 1, n (%) | 11 (85%) | 47 (82%) | <0.001 |
| 2, n (%) | 2 (15%) | 10 (18%) | 0.02 |
| Ventilation | |||
| HFNOT, n (%) | 8 (62%) | 31 (54%) | <0.001 |
| NIV, n (%) | 11 (85%) | 35 (61%) | <0.001 |
| IMV, n (%) | 8 (62%) | 56 (98%) | <0.001 |
| Selected arterial blood gas parameters 4 | |||
| pH, Median (IQR) | 7.37 (7.35–7.44) | 7.35 (7.27–7.42) | 0.1 |
| pO2, Median (IQR) | 75 (63–84) | 67 (51–88) | 0.5 |
| pCO2, Median (IQR) | 40 (32–50) | 39 (32–50) | 0.9 |
| % SaO2, Median (IQR) | 94 (90–96) | 91 (86–96) | 0.2 |
| Time from hospital admission to intubation (days) | |||
| Median (IQR) | 2.5 (1.5–4) | 4.5 (3–10) | 0.1 |
| Prone position, n (%) | 12 (92%) | 45 (79%) | 0.3 |
| Pharmacotherapy 5 | |||
| NMBA, n (%) | 7 (54%) | 48 (84%) | 0.02 |
| Dexamethasone, n (%) | 13 (100%) | 57 (100%) | <0.001 |
| Remdesivir, n (%) | 4 (31%) | 13 (23%) | 0.1 |
| Tocilizumab, n (%) | 4 (31%) | 15 (26%) | 0.7 |
| Pharmacol. support of the cardiovasc. system | |||
| Adrenaline, n (%) | 1 (8%) | 46 (81%) | <0.001 |
| Norepinephrine, n (%) | 8 (62%) | 57 (100%) | <0.001 |
| Argipressin, n (%) | 1 (8%) | 26 (46%) | 0.01 |
| Dopamine, n (%) | - | 5 (9%) | - |
| Dobutamine, n (%) | 1 (8%) | 11 (19%) | 0.3 |
| Milrinone, n (%) | - | 2 (4%) | - |
| Extracorporeal Therapies | |||
| TPE, n (%) | 2 (15%) | 5 (9%) | 0.5 |
| CRRT, n (%) | 3 (23%) | 25 (44%) | 0.2 |
| Cytokine adsorbers, n (%) | 1 (8%) | 7 (12%) | 0.6 |
| Parameter | All (n = 70) Me (IQR) | Survival 1 (n = 13) Me (IQR) | Death (n = 57) Me (IQR) | p |
|---|---|---|---|---|
| WBC (×109 L−1) | 13.0 (9.3–16.7) | 11.3 (9.1–15.7) | 13.1 (9.3–19.1) | 0.4 |
| RBC (×1012 L−1) | 4.1 (3.6–4.7) | 4.1 (3.7–5.0) | 4.1 (3.5–4.7) | 0.3 |
| HGB (g dL−1) | 12.7 (10.9–14.1) | 13.3 (10.5–15.3) | 12.6 (11.1–14.0) | 0.3 |
| Hematocrit (%) | 37 (34–43) | 40 (31–45) | 37 (35–43) | 0.9 |
| MCV (fL) | 91.1 (87–98) | 89 (85–91) | 92 (88–98) | 0.02 |
| MCH (pg) | 30 (29–32) | 30 (29–31) | 31 (30–33) | 0.05 |
| MCHC (g dL−1) | 34 (33–34) | 34 (33–34) | 34 (33–34) | 0.8 |
| PLT (×106 L−1) | 226 (176–305) | 254 (226–370) | 210 (168–298) | 0.07 |
| LYMPH (%) | 4.8 (3.2–6.8) | 7.3 (5.4–11.7) | 4.5 (3.0–5.9) | 0.003 |
| LYMPH (×106 L−1) | 0.60 (0.42–0.87) | 1.0 (0.5–1.4) | 0.5 (0.4–0.8) | 0.007 |
| MONO (%) | 3.45 (2.5–4.9) | 4.7 (3.1–5.2) | 3.3 (2.4–4.6) | 0.1 |
| MONO (×106 L−1) | 0.43 (0.27–0.63) | 0.5 (0.4–0.7) | 0.4 (0.2–0.6) | 0.2 |
| NEUT (%) | 89.2 (85.1–91.7) | 81.8 (80.2–89.6) | 89.8 (87.4–92.2) | 0.005 |
| NEUT (×106 L−1) | 11.5 (7.9–15.2) | 9.0 (7.5–12.8) | 11.7 (8.5–17.9) | 0.1 |
| EOS (%) | 0.0 (0.0–0.1) | 0.0 (0.0–0.2) | 0.0 (0.0–0.0) | 0.3 |
| EOS (×106 L−1) | 0.0 (0.0–0.01) | 0.0 (0.0–0.0) | 0.0 (0.0–0.1) | 0.4 |
| BASO (%) | 0.2 (0.1–0.2) | 0.2 (0.1–0.3) | 0.1 (0.1–0.2) | 0.2 |
| BASO (×106 L−1) | 0.02 (0.01–0.04) | 0.02 (0.01–0.04) | 0.02 (0.01–0.03) | 0.7 |
| RDW-SD (fL) | 46.9 (42.9–49.8) | 43.9 (40.9–47.3) | 48.1 (43.1–50.5) | 0.01 |
| PCT (%) | 0.24 (0.20–0.33) | 0.27 (0.24–0.41) | 0.23 (0.20–0.33) | 0.1 |
| MPV (fL) | 10.8 (10.2–11.7) | 10.5 (9.7–11.4) | 10.8 (10.2–11.7) | 0.3 |
| PDW (%) | 12.8 (11.1–14.4) | 12.1 (10.8–13.6) | 12.9 (11.3–15) | 0.4 |
| Variable | Group | p | |||
|---|---|---|---|---|---|
| Normal | Mild | Moderate | Sever | ||
| Stress | Stress | Stress | Stress | ||
| NLR < 6 | NLR 6–9 | NLR 9–18 | NLR > 8 | ||
| n, (%) | 2 (3%) | 8 (11%) | 23 (33%) | 37 (53%) | <0.001 |
| Age (years) | |||||
| Median (IQR) | 54 (54–78) | 62 (56–69) | 67 (60–71) | 65 (61–73) | 0.7 |
| Sex | |||||
| male, n (%) | - | 5 (7%) | 16 (23%) | 25 (36%) | <0.001 |
| female, n (%) | 2 (3%) | 3 (4%) | 7 (10%) | 11 (16%) | 0.03 |
| Duration of hospitalization in ITU (days) | |||||
| Median (IQR) | 15 (4–26) | 12 (8–14) | 12 (6–17) | 8 (5–9) | 0.2 |
| % lung injury 1 | |||||
| Median (IQR) | 75 (60–90) | 75 (65–85) | 85 (70–90) | 80 (70–80) | 0.4 |
| Pulmonary embolism, n (%) | - | 1 (1%) | 3 (4%) | 3 (4%) | 0.8 |
| ICU admission priority 2 | |||||
| 1, n (%) | 2 (3%) | 5 (7%) | 21 (30%) | 30 (43%) | <0.001 |
| 2, n (%) | - | 3 (4%) | 2 (3%) | 7 (10%) | 0.2 |
| Ventilation | |||||
| HFNOT, n (%) | 2 (3%) | 5 (7%) | 13 (19%) | 19 (27%) | 0.6 |
| NIV, n (%) | 2 (3%) | 5 (7%) | 15 (21%) | 24 (34%) | 0.8 |
| IMV, n (%) | 1 (1%) | 6 (9%) | 21 (30%) | 36 (51%) | 0.03 |
| Prone position, n (%) | 2 (3%) | 8 (11%) | 18 (26%) | 29 (41%) | 0.4 |
| Pharmacol. support of the cardiovasc. system | |||||
| Adrenaline, n (%) | - | 5 (7%) | 15 (21%) | 27 (39%) | 0.2 |
| Norepinephrine, n (%) | 1 (1%) | 6 (9%) | 21 (30%) | 37 (53%) | 0.006 |
| Argipressin, n (%) | - | 2 (3%) | 11 (16%) | 14 (20%) | 0.4 |
| Dopamine, n (%) | - | - | 2 (3%) | 3 (4%) | 0.8 |
| Dobutamine, n (%) | - | 2 (3%) | 2 (3%) | 8 (11%) | 0.5 |
| Milrinone, n (%) | - | - | - | 2 (3%) | 0.6 |
| Extracorporeal therapies | |||||
| TPE, n (%) | - | 1 (1%) | 3 (4%) | 3 (4%) | 0.9 |
| CRRT, n (%) | - | 4 | 7 (10%) | 17 (24%) | 0.4 |
| Cytokine adsorbers, n (%) | - | 3 (4%) | 1 (1%) | 4 (6%) | 0.08 |
| Death before discharge from ICU | - | 5 (7%) | 18 (26%) | 34 (49%) | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pluta, M.P.; Zachura, M.N.; Winiarska, K.; Kalemba, A.; Kapłan, C.; Szczepańska, A.J.; Krzych, Ł.J. Usefulness of Selected Peripheral Blood Counts in Predicting Death in Patients with Severe and Critical COVID-19. J. Clin. Med. 2022, 11, 1011. https://doi.org/10.3390/jcm11041011
Pluta MP, Zachura MN, Winiarska K, Kalemba A, Kapłan C, Szczepańska AJ, Krzych ŁJ. Usefulness of Selected Peripheral Blood Counts in Predicting Death in Patients with Severe and Critical COVID-19. Journal of Clinical Medicine. 2022; 11(4):1011. https://doi.org/10.3390/jcm11041011
Chicago/Turabian StylePluta, Michał P., Mateusz N. Zachura, Katarzyna Winiarska, Alicja Kalemba, Cezary Kapłan, Anna J. Szczepańska, and Łukasz J. Krzych. 2022. "Usefulness of Selected Peripheral Blood Counts in Predicting Death in Patients with Severe and Critical COVID-19" Journal of Clinical Medicine 11, no. 4: 1011. https://doi.org/10.3390/jcm11041011
APA StylePluta, M. P., Zachura, M. N., Winiarska, K., Kalemba, A., Kapłan, C., Szczepańska, A. J., & Krzych, Ł. J. (2022). Usefulness of Selected Peripheral Blood Counts in Predicting Death in Patients with Severe and Critical COVID-19. Journal of Clinical Medicine, 11(4), 1011. https://doi.org/10.3390/jcm11041011







