Non-Invasive Myocardial Work in Patients with Severe Aortic Stenosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Timeline, Procedures, and Analysis Plan
2.3. Echocardiographic Analyses
2.4. Calculation of Non-Invasive Myocardial Work
2.5. Data Analysis and Statistics
3. Results
3.1. Study Population
3.2. Estimation of LV Pressure
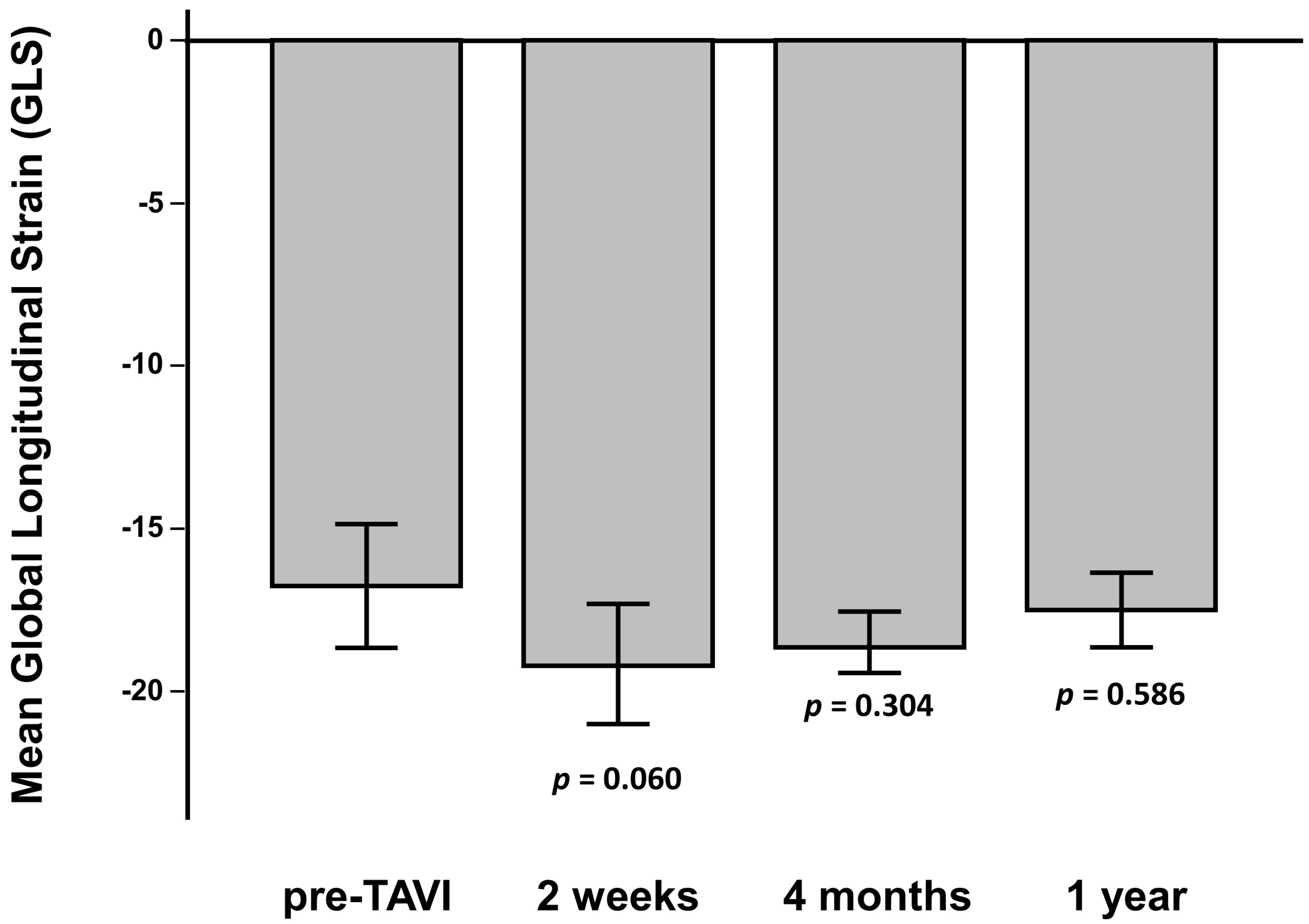
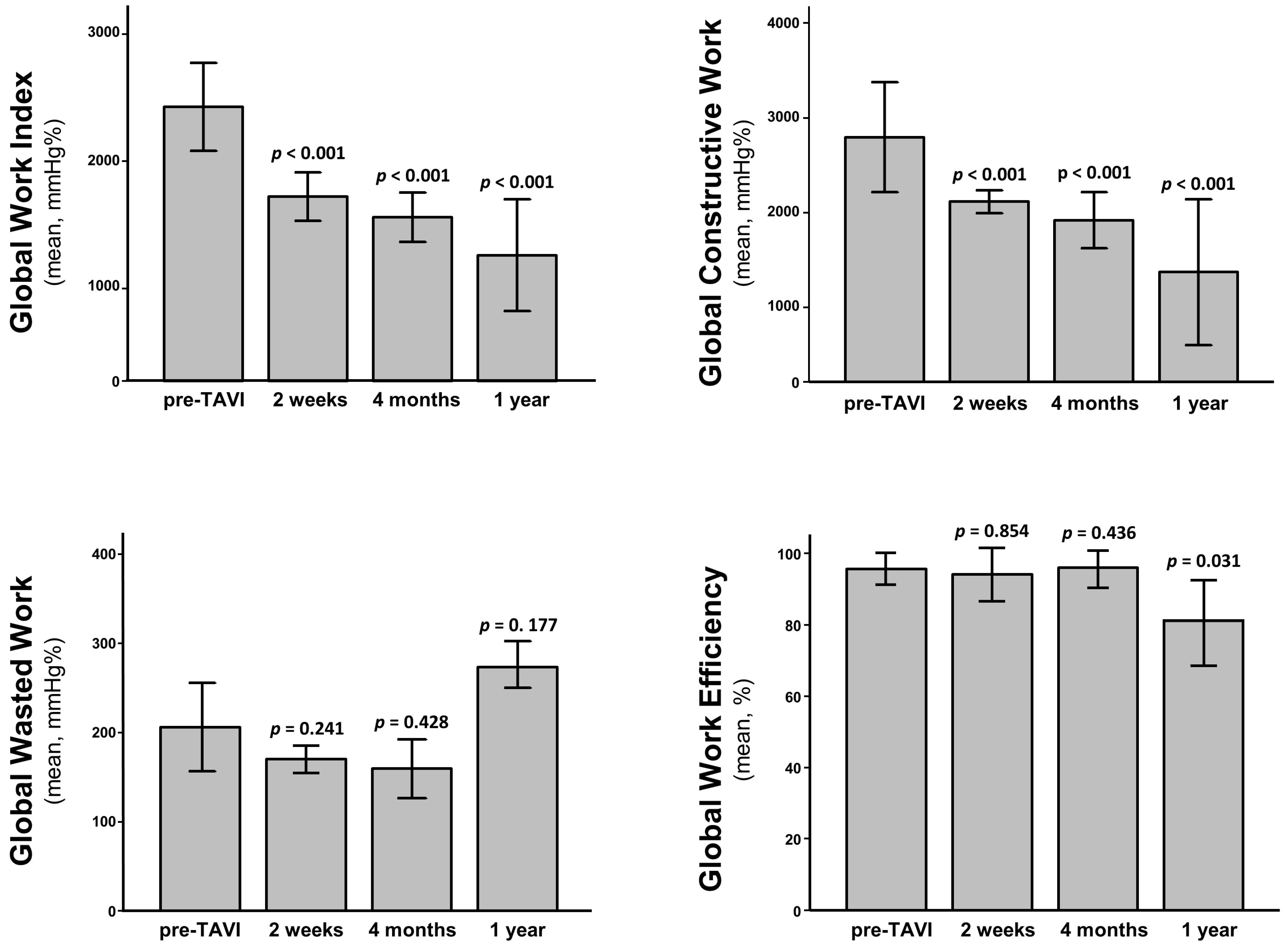
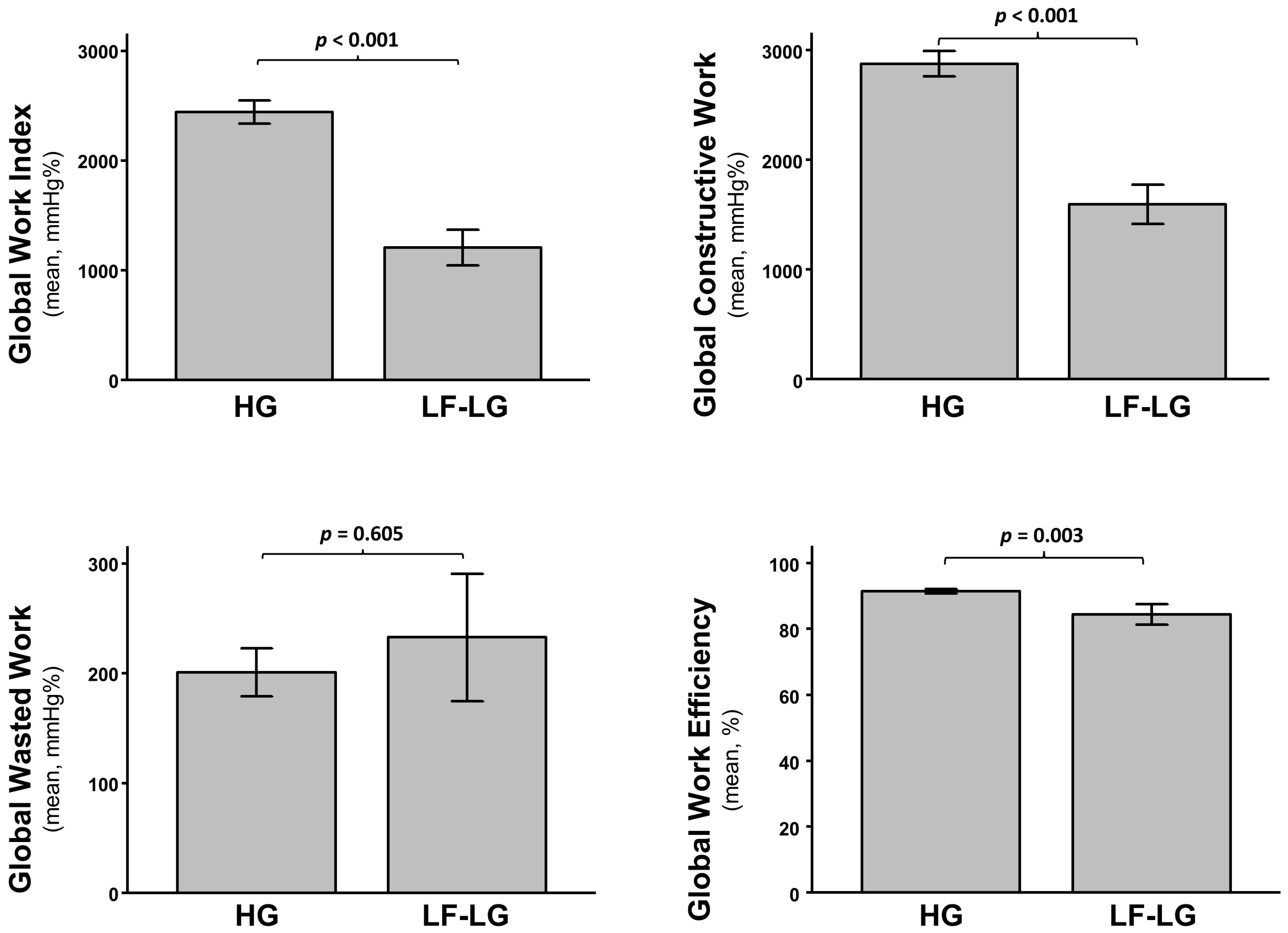
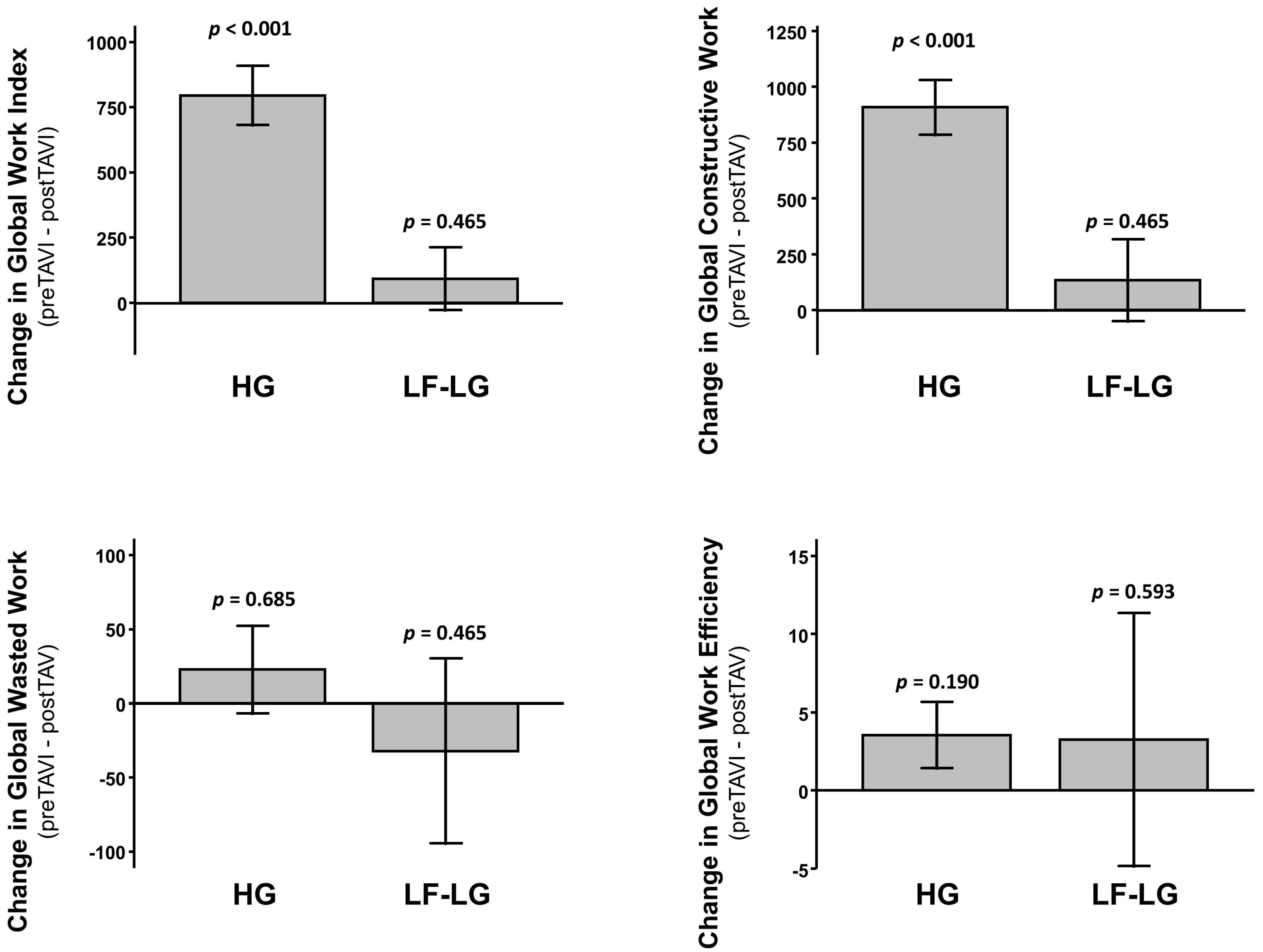
3.3. Assessment of LV Function and Mechanics
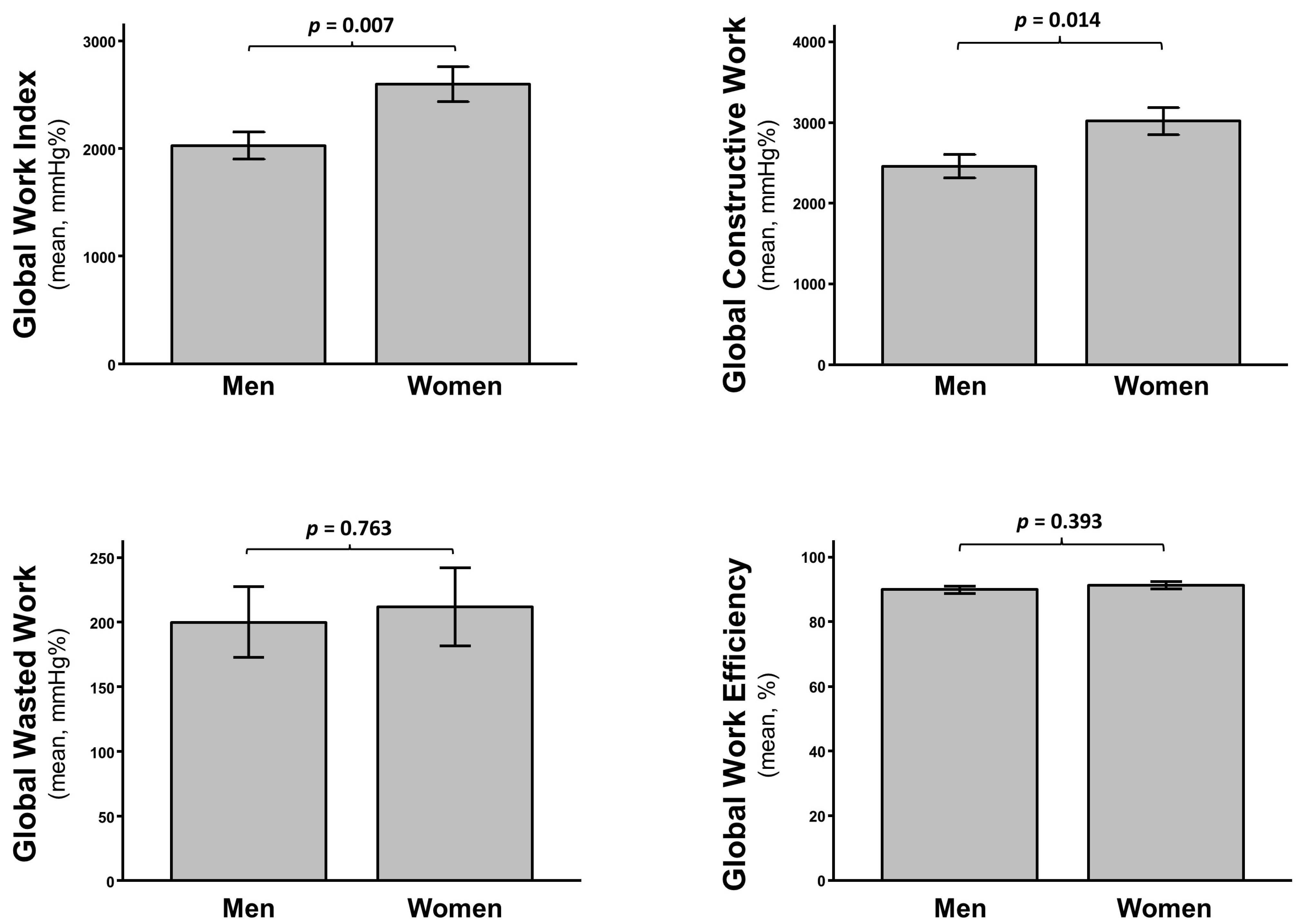
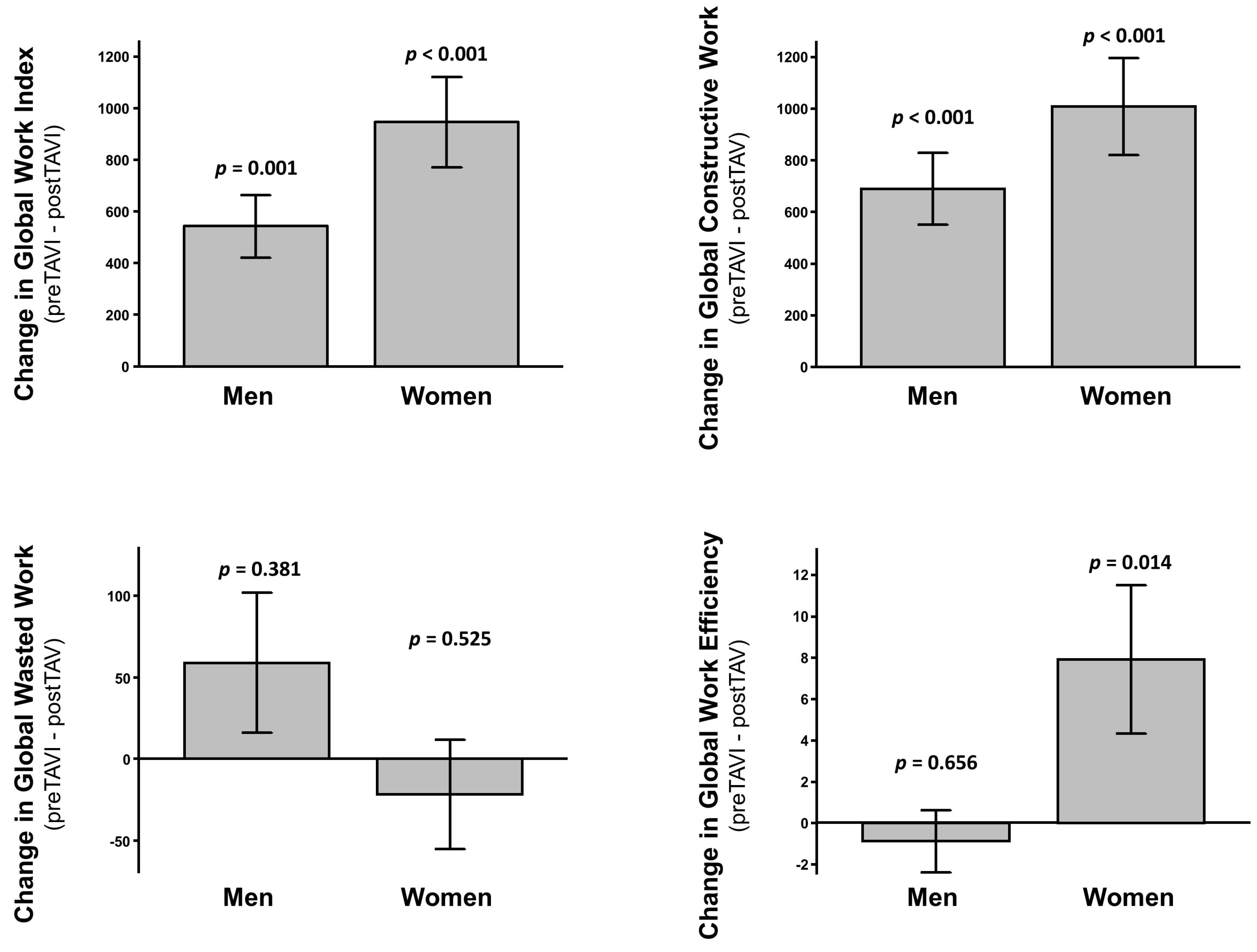
3.4. Predictors of Clinical Events
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lung, B.; Delgado, V.; Rosenhek, R.; Price, S.; Prendergast, B.; Wendler, O. Contemporary presentation and management of valvular heart disease: The EURObservational Research Programme Valvular Heart Disease II Survey. Circulation 2019, 140, 1156–1169. [Google Scholar]
- Bluemke, D.A.; Kronmal, R.A.; Lima, J.A.; Liu, K.; Olson, J.; Burke, G.L. The relationship of left ventricular mass and geometry to incident cardiovascular events: The MESA (Multi-Ethnic Study of Atherosclerosis) study. J. Am. Coll. Cardiol. 2008, 52, 2148–2155. [Google Scholar] [CrossRef] [Green Version]
- Dahl, J.S.; Magne, J.; Pellikka, P.A.; Donal, E.; Marwick, T.H. Assessment of Subclinical Left Ventricular Dysfunction in Aortic Stenosis. JACC Cardiovasc. Imaging 2019, 12, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, J.; De Rosa, S.; Leo, I.; Strangio, A.; Spaccarotella, C.; Polimeni, A.; Sorrentino, S.; Di Salvo, G.; Indolfi, C. Prediction of Significant Coronary Artery Disease Through Advanced Echocardiography: Role of Non-invasive Myocardial Work. Front. Cardiovasc. Med. 2021, 8, 719603. [Google Scholar] [CrossRef]
- Mor-Avi, V.; Lang, R.M.; Badano, L.P.; Belohlavek, M.; Cardim, N.M.; Derumeaux, G.; Galderisi, M.; Marwick, T.; Nagueh, S.F.; Sengupta, P.P.; et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J. Am. Soc. Echocardiogr. 2011, 24, 277–313. [Google Scholar] [CrossRef]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W. A novel clinical method for quantification of regional left ventricular pressure-strain loop area: A non-invasive index of myocardial work. Eur. Heart J. 2012, 33, 724–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urheim, S.; Rabben, S.I.; Skulstad, H.; Lyseggen, E.; Ihlen, H.; Smiseth, O.A. Regional myocardial work by strain Doppler echocardiography and LV pressure: A new method for quantifying myocardial function. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, 2375–2380. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Gjesdal, O. Assessment of wasted myocardial work: A novel method to quantify energy loss due to uncoordinated left ventricular contractions. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, J.; De Rosa, S.; Leo, I.; Spaccarotella, C.; Mongiardo, A.; Polimeni, A.; Sorrentino, S.; Di Salvo, G.; Indolfi, C. Non-invasive myocardial work is reduced during transient acute coronary occlusion. PLoS ONE 2020, 15, e0244397. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Bajwa, T.; Roemer, S.; Huisheree, H.; Allaqaband, S.Q.; Kroboth, S.; Perez Moreno, A.C.; Tajik, A.J.; Khandheria, B.K. Myocardial work assessment in severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Fortuni, F.; Butcher, S.C.; van der Kley, F.; Lustosa, R.P.; Karalis, I.; de Weger, A.; Priori, S.G.; van der Bijl, P.; Bax, J.J.; Delgado, V. Left ventricular myocardial work in patients with severe aortic stenosis. J. Am. Soc. Echocardiogr. 2020, 34, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, J.; De Rosa, S.; Leo, I.; Strangio, A.; La Bella, S.; Sorrentino, S.; Mongiardo, A.; Spaccarotella, C.; Polimeni, A.; Indolfi, C. Early reduction of left atrial function predicts adverse clinical outcomes in patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Open Heart 2021, 8, e001685. [Google Scholar] [CrossRef] [PubMed]
- Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Aickin, M.; Gensler, H. Adjusting for multiple testing when reporting research results: The Bonferroni vs. Holm methods. Am. J. Public Health 1996, 86, 726–728. [Google Scholar] [CrossRef] [Green Version]
- Caiazzo, G.; De Rosa, S.; Torella, D.; Spaccarotella, C.; Mongiardo, A.; Giampà, S.; Micieli, M.; Palella, E.; Gulletta, E.; Indolfi, C. Administration of a loading dose has no additive effect on platelet aggregation during the switch from ongoing clopidogrel treatment to ticagrelor in patients with acute coronary syndrome. Circ. Cardiovasc. Interv. 2014, 7, 104–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| (n = 73) | |
|---|---|
| Age (years) | 80.8 ± 6 |
| Female, n (%) | 34 (46.5) |
| Weight (kg) | 69.9 ± 12.7 |
| Height (cm) | 160.6 ± 7.6% |
| BSA (m2) | 1.76 ± 0.18 |
| BMI (kg/m2) | 26.9 ± 4.19 |
| AVA (cm2) | 0.78 ± 0.16 |
| AV mean gradient (mmHg) | 46.7 ± 15.8 |
| AV peak gradient (mmHg) | 74 ± 22.1 |
| LVEF (%) | 52.8 ± 9.3 |
| LVEDD (mm) | 50.6 ± 7.1 |
| LVESD (mm) | 34.5 ± 8.5 |
| IVSd (mm) | 14.1 ± 3 |
| LAVi (mL/m2) | 43.8 ± 8.4 |
| TAPSE (mm) | 21.5 ± 3.1 |
| PASP (mmHg) | 41.6 ± 10.9 |
| GLS (%) | −16.8 ± 5 |
| Low-Flow Low-Gradient, n (%) | 10 (13.7) |
| Hypertension | 69 (94.5) |
| Diabetes Mellitus | 17 (23.3) |
| Dyslipidemia | 45 (61.6) |
| Prior PCI, n (%) | 14 (19.2) |
| Self-Expandable Valve, n (%) | 56 (76.7) |
| Ballon-Expandable Valve, n (%) | 17 (23.3) |
| Valve Dimension (mm) | |
| 23, n (%) | 17 (23.3) |
| 25, n (%) | 1 (1.4) |
| 26, n (%) | 24 (32.9) |
| 29, n (%) | 23 (31.5) |
| 34, n (%) | 8 (10.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Rosa, S.; Sabatino, J.; Strangio, A.; Leo, I.; Romano, L.R.; Spaccarotella, C.A.; Mongiardo, A.; Polimeni, A.; Sorrentino, S.; Indolfi, C. Non-Invasive Myocardial Work in Patients with Severe Aortic Stenosis. J. Clin. Med. 2022, 11, 747. https://doi.org/10.3390/jcm11030747
De Rosa S, Sabatino J, Strangio A, Leo I, Romano LR, Spaccarotella CA, Mongiardo A, Polimeni A, Sorrentino S, Indolfi C. Non-Invasive Myocardial Work in Patients with Severe Aortic Stenosis. Journal of Clinical Medicine. 2022; 11(3):747. https://doi.org/10.3390/jcm11030747
Chicago/Turabian StyleDe Rosa, Salvatore, Jolanda Sabatino, Antonio Strangio, Isabella Leo, Letizia Rosa Romano, Carmen Anna Spaccarotella, Annalisa Mongiardo, Alberto Polimeni, Sabato Sorrentino, and Ciro Indolfi. 2022. "Non-Invasive Myocardial Work in Patients with Severe Aortic Stenosis" Journal of Clinical Medicine 11, no. 3: 747. https://doi.org/10.3390/jcm11030747
APA StyleDe Rosa, S., Sabatino, J., Strangio, A., Leo, I., Romano, L. R., Spaccarotella, C. A., Mongiardo, A., Polimeni, A., Sorrentino, S., & Indolfi, C. (2022). Non-Invasive Myocardial Work in Patients with Severe Aortic Stenosis. Journal of Clinical Medicine, 11(3), 747. https://doi.org/10.3390/jcm11030747








