Hypophosphatemia on ICU Admission Is Associated with an Increased Length of Stay in the ICU and Time under Mechanical Ventilation
Abstract
:1. Introduction
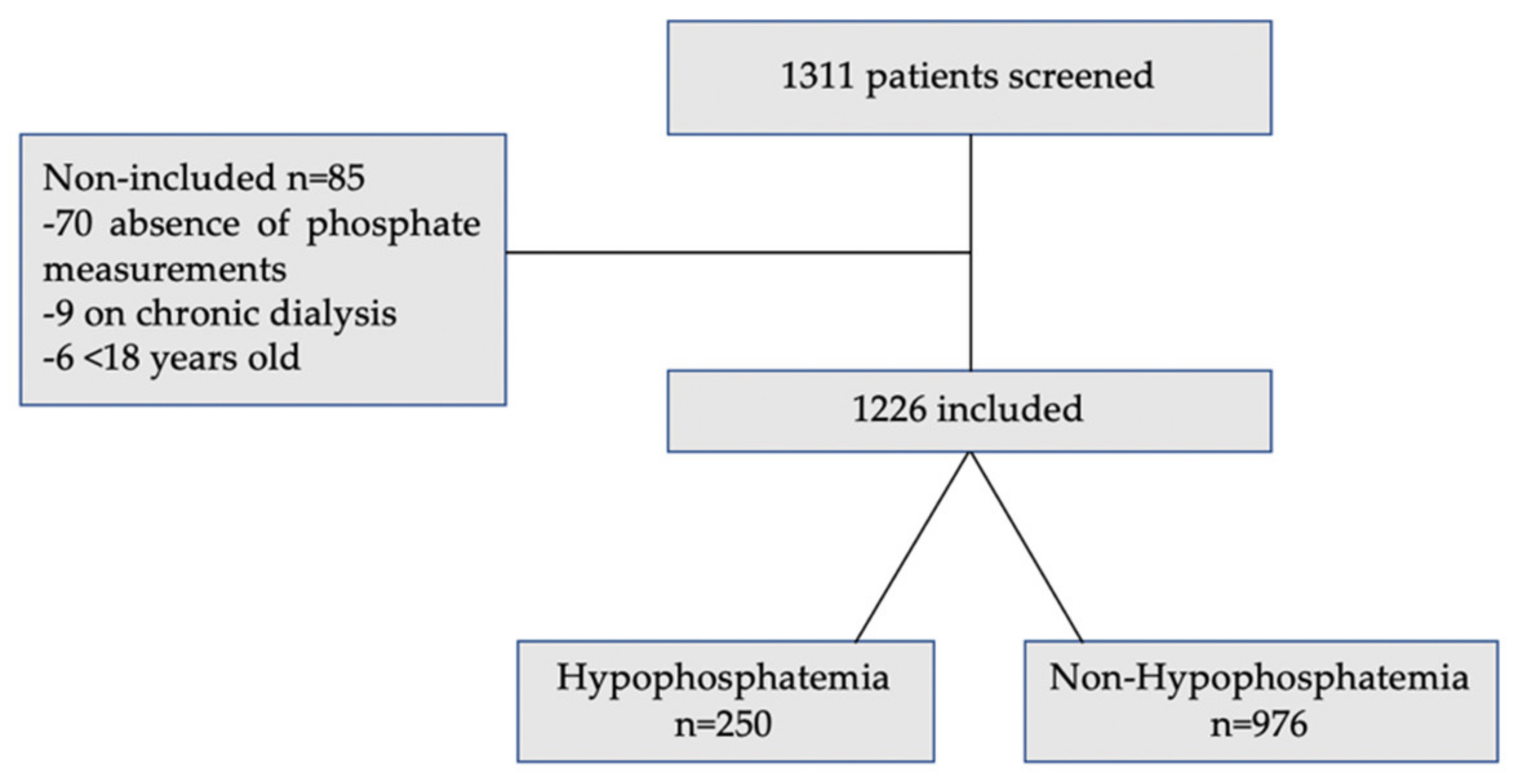
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Study Population Characteristics
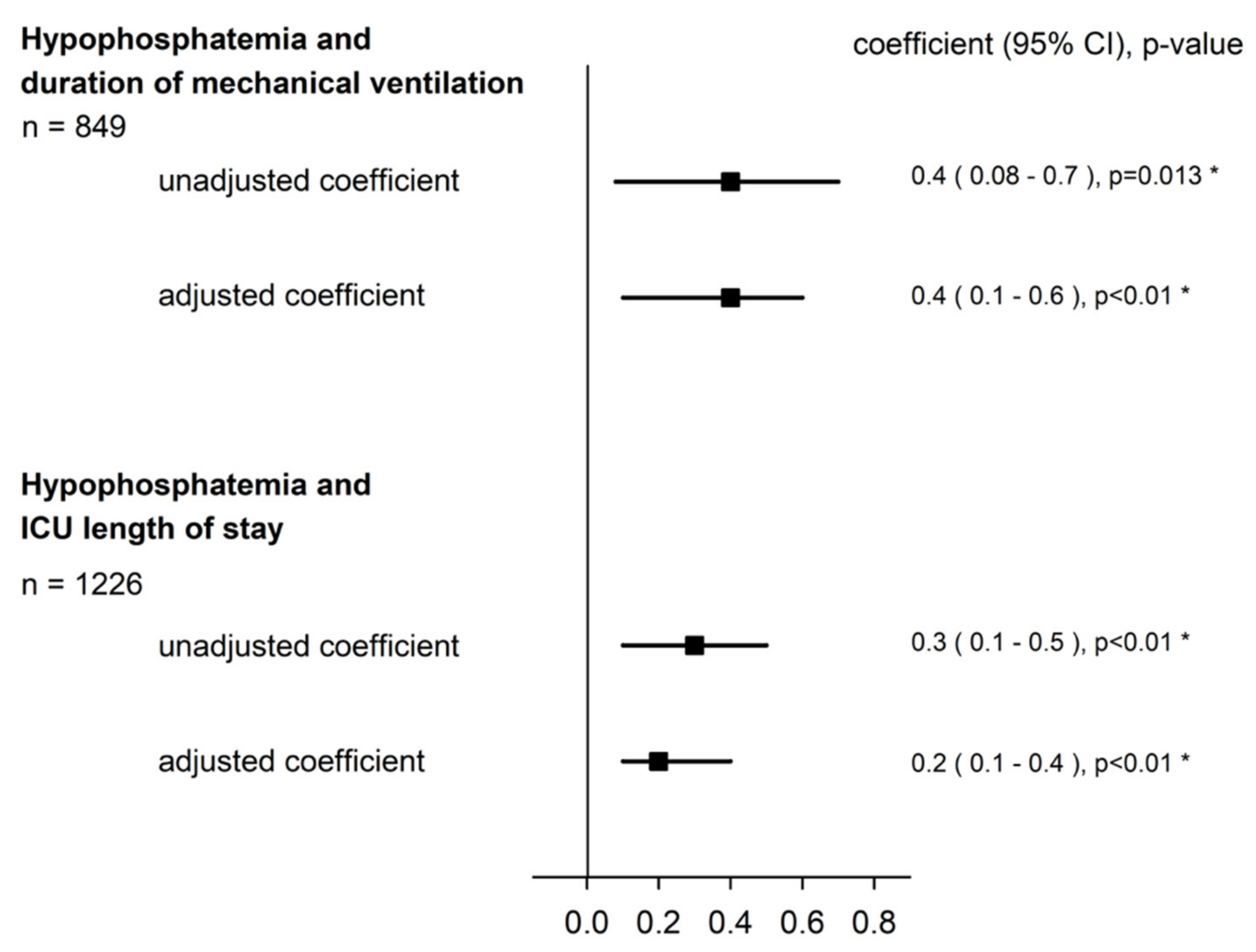
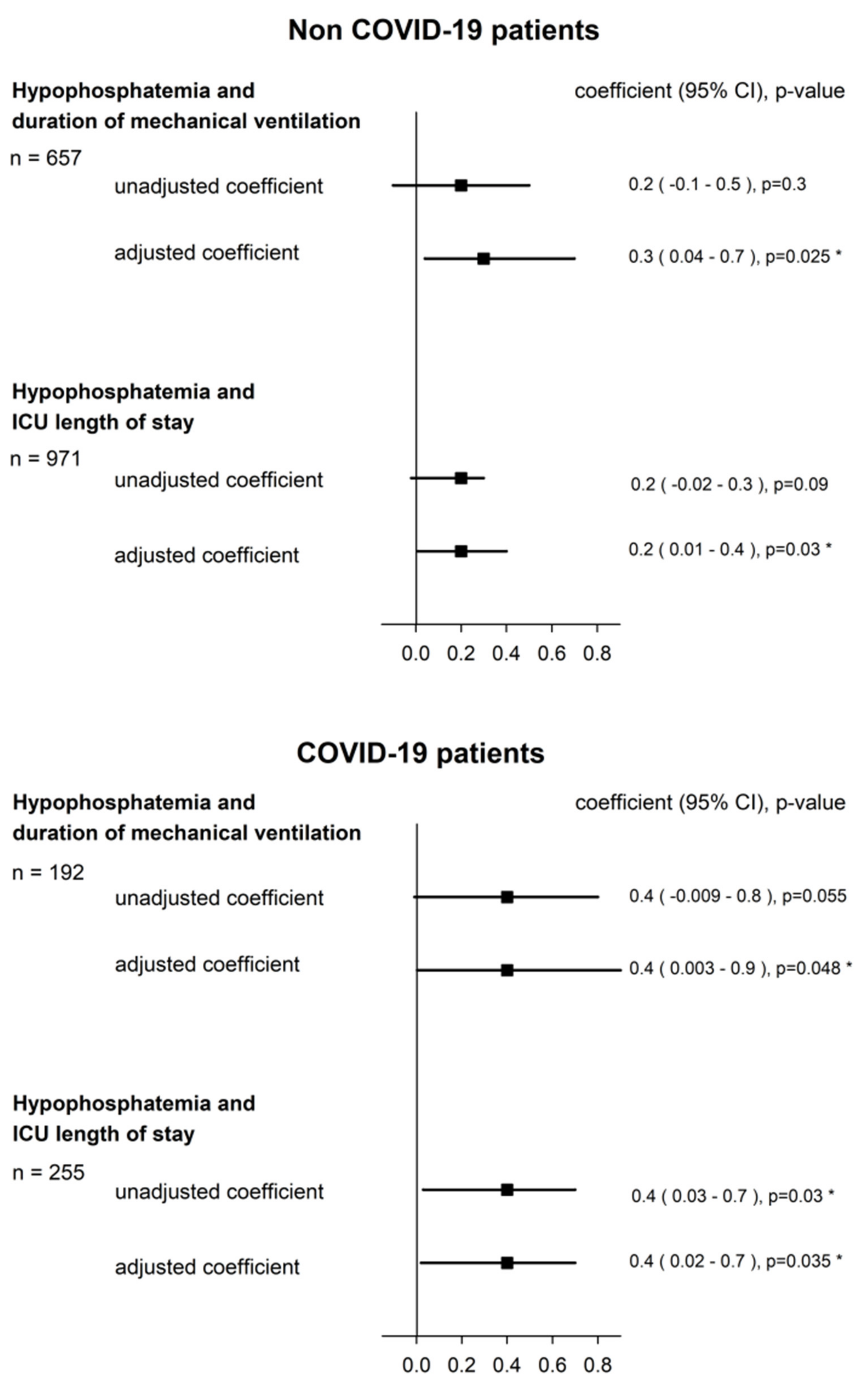
3.2. Hypophosphatemia and Duration of Mechanical Ventilation
3.3. Hypophosphatemia and ICU Length of Stay
3.4. Hypophosphatemia and Mortality in the ICU
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Total n = 1226 | Non-COVID-19 n = 971 | COVID-19 n = 255 | p Value | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Gender male, n (%) | 807 (65.8%) | 620 (63.9%) | 187 (73.3%) | <0.01 * |
| Age, mean (SD) | 61.2 (15.1) | 60.5 (0.5) | 64 (0.7) | <0.01 * |
| BMI, mean (SD) | 26.8 (7) | 26.1 (0.2) | 29.7 (0.6) | <0.01 * |
| APACHE II, mean (SD) | 24.2 (8.6) | 24.3 (8.5) | 23.7 (9.1) | 0.3 |
| SAPSII, mean (SD) | 50.6 (20) | 49.7 (0.6) | 53.8 (0.6) | <0.01 * |
| eGFR <60 mL/min at ICU admission, n (%) | 354 (29%) | 305 (31.4%) | 49 (19.2%) | <0.01 * |
| Phosphatemia on ICU admission, mmol/L mean (SD) | 1.12 (0.46) | 1.2 (0.02) | 1.02 (0.1) | <0.01 * |
| Prevalence of hypophosphatemia on ICU admission, mean (SD) | 250 (20.4%) | 183 (18.9%) | 67 (26.3%) | <0.01 * |
| Ionized calcemia on ICU admission, mmol/L mean (SD) | 1.14 (0.1) | 1.15 (0.03) | 1.11 (0.005) | <0.01 * |
| Intubated patients during ICU stay, n (%) | 856 (69.8%) | 664 (68.4%) | 192 (75.3%) | <0.01 * |
| Weaning ventilator failure, n (%) | 91 (10.6%) | 61 (6.3%) | 30 (11.8%) | <0.01 * |
| New tracheotomy during ICU stay, n (%) | 49 (4%) | 24 (2.5%) | 25 (9.8%) | <0.01 * |
| Infections during the first 48 h of ICU stay, n (%) | 526 (42.9%) | 293(30.1%) | 233 (91.4%) | <0.01 * |
| New infections during ICU stay, n (%) | 48 (3.9%) | 42 (4.3%) | 6 (2.4%) | 0.14 |
| Hemodiafiltration during ICU stay, n (%) | 50 (4.1%) | 43 (4.4%) | 7 (2.8%) | 0.2 |
| Study outcomes | ||||
| Ventilation time in days, mean (SD) | 6 (9.5) | 3.8 (6.4) | 13.2 (13.7) | <0.01 * |
| ICU length of stay, mean (SD) | 6 (8.8) | 4.4 (6.4) | 12 (13) | <0.01 * |
| Death in the ICU, n (%) | 156 (12.7%) | 96 (9.9%) | 60 (23.5%) | <0.01 * |
| Univariable Analysis, n = 849 | Days of Mechanical Ventilation Regression Coefficient, (95% CI) | p Value |
| Non-hypophosphatemia | Ref. | |
| Hypophosphatemia | 0.4 (0.08–0.7) | 0.013 * |
| Multivariable Analysis, n = 849 | Days of Mechanical Ventilation Regression Coefficient, (95% CI) | p Value |
| Non-hypophosphatemia | Ref. | |
| Hypophosphatemia | 0.4 (0.1–0.6) | <0.01 * |
| Gender male | 0.1 (−0.1–0.3) | 0.4 |
| SAPS II | 0.03 (0.02–0.03) | <0.01 * |
| Infection within the first 48 h | 0.8 (0.6–1.1) | <0.01 * |
| Reason for ICU admission | ||
| - Abdominal disease | Ref. | NA |
| - Septic shock | 0.2 (−0.4–0.7) | 0.5 |
| - Cardiovascular disease | −0.3 (−0.7–0.03) | 0.07 |
| - Respiratory failure | 1.2 (0.8–1.6) | <0.01 * |
| - Neurological disease | 0.2 (−0.2–0.5) | 0.3 |
| - Others (hematologic, ENT, or metabolic disease) | −0.2 (−0.8–0.3) | 0.4 |
| Non-COVID-19 Patients (n = 657) | COVID-19 Patients (n = 192) | |||
|---|---|---|---|---|
| Univariable Analysis | Days of Mechanical Ventilation Regression Coefficient, (95% CI) | p | Days of Mechanical Ventilation Regression Coefficient, (95% CI) | p |
| Non-hypoP (≥0.8 mmol/L) | Ref. | Ref. | ||
| HypoP (<0.8 mmol/L) | 0.2 (−0.1–0.5) | 0.3 | 0.4 (−0.009–0.8) | 0.055 |
| Multivariable Analysis | Days of Mechanical Ventilation, Regression Coefficient, (95% CI) | p | Days of Mechanical Ventilation, Regression Coefficient, (95% CI) | p |
| Non-hypoP (≥0.8 mmol/L) | Ref. | Ref. | ||
| HypoP (<0.8 mmol/L) | 0.3 (0.04–0.7) | 0.025 * | 0.4 (0.003–0.9) | 0.048 * |
| Gender male | 0.09 (−0.2–0.4) | 0.5 | 0.07 (−0.4–0.5) | 0.7 |
| SAPS II | 0.04 (0.03–0.04) | <0.01 * | 0.003 (−0.007–0.1) | 0.6 |
| Infections within the first 48 h | 0.8 (0.5–1.1) | <0.01 * | 0.9 (0.2–1.5) | <0.01 * |
| Reason for ICU admission | ||||
| - Abdominal disease | Ref. | NA | ||
| - Septic shock | 0.1 (−0.5–0.7) | 0.7 | ||
| - Cardiovascular disease | −0.3 (−0.7–0.05) | 0.09 | ||
| - Respiratory failure | 0.4 (−0.2–0.9) | 0.2 | ||
| - Neurological disease | 0.2 (−0.1–0.6) | 0.2 | ||
| - Others (hematologic, ENT or metabolic disease) | −0.2 (−0.8–0.3) | 0.4 |
| Univariable Analysis, n = 1226 | ICU Length of Stay in Days Regression Coefficient, (95% CI) | p Value |
|---|---|---|
| Non-hypophosphatemia | Ref. | |
| Hypophosphatemia | 0.3 (0.1–0.5) | <0.01 * |
| Multivariable Analysis, n = 1226 | ICU Length of Stay in Days Regression Coefficient, (95% CI) | p Value |
| Non-hypophosphatemia | Ref. | |
| Hypophosphatemia | 0.2 (0.1–0.4) | <0.01 * |
| Gender male | 0.03 (−0.1–0.2) | 0.7 |
| SAPS II | 0.02 (0.01–0.02) | <0.01 * |
| Infections within the first 48 h | 0.5 (0.3–0.6) | <0.01 * |
| Reason for ICU admission | ||
| - Abdominal disease | Ref. | NA |
| - Septic shock | −0.09 (−0.4–0.2) | 0.6 |
| - Cardiovascular disease | −0.007 (−0.2–0.2) | 0.9 |
| - Respiratory failure | 0.6 (0.4–0.8) | <0.01 * |
| - Neurological disease | 0.1 (−0.8–0.3) | 0.2 |
| - Others (hematologic, ENT, or metabolic disease) | −0.05 (−0.3–0.3) | 0.8 |
| Non-COVID-19 Patients (n = 971) | COVID-19 (n = 255) | |||
|---|---|---|---|---|
| Univariable Analysis | ICU Length of Stay in Days Regression Coefficient, (95% CI) | p | ICU Length of Stay in Days Regression Coefficient, (95% CI) | p |
| Non-hypoP (≥0.8 mmol/L) | Ref. | Ref. | ||
| HypoP (<0.8 mmol/L) | 0.2 (−0.02–0.3) | 0.09 | 0.4 (0.03–0.7) | 0.03 * |
| Multivariable Analysis | ICU Length of Stay in Days Regression Coefficient, (95% CI) | p | ICU Length of Stay in Days Regression Coefficient, (95% CI) | p |
| Non-hypoP (≥0.8 mmol/L) | Ref. | Ref. | ||
| HypoP (<0.8 mmol/L) | 0.2 (0.01–0.4) | 0.03 * | 0.4 (0.02–0.7) | 0.035 * |
| Gender male | −0.02 (−0.2–0.1) | 0.8 | 0.07 (−0.2–0.4) | 0.6 |
| SAPS II | 0.02 (0.01–0.02) | <0.01 * | 0.01 (0.004–0.02) | <0.01 * |
| Infections within the first 48 h | 0.4 (0.2–0.6) | <0.01 * | 0.5 (0.02–1) | 0.04 * |
| Reason for ICU admission | ||||
| - Abdominal disease | Ref. | |||
| - Septic shock | −0.07 (−0.4–0.2) | 0.7 | ||
| - Cardiovascular disease | −0.02 (−0.2–0.2) | 0.8 | ||
| - Respiratory failure | −0.07 (−0.4–0.2) | 0.6 | ||
| - Neurological disease | 0.1 (−0.09–0.3) | 0.2 | ||
| - Others (hematologic, ENT, or metabolic disease) | −0.07 (−0.4–0.2) | 0.6 |
References
- Demirjian, S.; Teo, B.W.; Guzman, J.A.; Heyka, R.J.; Paganini, E.P.; Fissell, W.H.; Schold, J.D.; Schreiber, M.J. Hypophosphatemia during continuous hemodialysis is associated with prolonged respiratory failure in patients with acute kidney injury. Nephrol. Dial. Transplant. 2011, 26, 3508–3514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wadsworth, R.; Siddiqui, S. Phosphate homeostasis in critical care. BJA Educ. 2016, 16, 305–309. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.; Fourrier, F. Hypophosphatemia in the ICU. Reanimation 2003, 12, 280–287. [Google Scholar] [CrossRef]
- Sin, J.C.K.; King, L.; Ballard, E.; Llewellyn, S.; Laupland, K.B.; Tabah, A. Hypophosphatemia and Outcomes in ICU: A Systematic Review and Meta-Analysis. J. Intensiv. Care Med. 2020, 36, 1025–1035. [Google Scholar] [CrossRef]
- Blaser, A.R.; Gunst, J.; Ichai, C.; Casaer, M.P.; Benstoem, C.; Besch, G.; Dauger, S.; Fruhwald, S.M.; Hiesmayr, M.; Joannes-Boyau, O.; et al. Hypophosphatemia in critically ill adults and children—A systematic review. Clin. Nutr. 2020, 40, 1744–1754. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, C.; Chen, L.; Zhang, X.; Kou, Q. Impact of hypophosphatemia on outcome of patients in intensive care unit: A retrospective cohort study. BMC Anesthesiol. 2019, 19, 86. [Google Scholar] [CrossRef]
- Broman, M.; Wilsson, A.M.J.; Hansson, F.; Klarin, B. Analysis of Hypo- and Hyperphosphatemia in an Intensive Care Unit Cohort. Anesthesia Analg. 2017, 124, 1897–1905. [Google Scholar] [CrossRef]
- Sharma, S.; Kelly, Y.P.; Palevsky, P.M.; Waikar, S.S. Intensity of Renal Replacement Therapy and Duration of Mechanical Ventilation: Secondary Analysis of the Acute Renal Failure Trial Network Study. Chest 2020, 158, 1473–1481. [Google Scholar] [CrossRef]
- Tan, B.; Chew, N.W.; Lee, G.K.; Jing, M.; Goh, Y.; Yeo, L.L.; Zhang, K.; Chin, H.-K.; Ahmad, A.; Khan, F.A.; et al. Psychological Impact of the COVID-19 Pandemic on Health Care Workers in Singapore. Ann. Intern. Med. 2020, 173, 317–320. [Google Scholar] [CrossRef] [Green Version]
- van Kempen, T.A.T.G.; Deixler, E. SARS-CoV-2: Influence of phosphate and magnesium, moderated by vitamin D, on energy (ATP) metabolism and on severity of COVID-19. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E2–E6. [Google Scholar] [CrossRef]
- Javdani, F.; Parsa, S.; Shakeri, H.; Hatami, N.; Kalani, N.; Haghbeen, M.; Raufi, R.; Abbasi, A.; Keshavarz, P.; Hashemi, S.A. Phosphate levels and pulmonary damage in COVID-19 patients based on CO-RADS scheme: Is there any link between parathyroid gland and COVID-19? MedRxiv 2020. preprint. [Google Scholar] [CrossRef]
- Boles, J.-M.; Bion, J.; Connors, A.F.; Herridge, M.; Marsh, B.; Melot, C.; Pearl, R.; Silverman, H.; Stanchina, M.; Vieillard-Baron, A.; et al. Weaning from mechanical ventilation. Eur. Respir. J. 2007, 29, 1033–1056. [Google Scholar] [CrossRef]
- Federspiel, C.K.; Itenov, T.S.; Thormar, K.; Liu, K.D.; Bestle, M.H. Hypophosphatemia and duration of respiratory failure and mortality in critically ill patients. Acta Anaesthesiol. Scand. 2018, 62, 1098–1104. [Google Scholar] [CrossRef]
- Berger, M.; Appelberg, O.; Reintam-Blaser, A.; Ichai, C.; Joannes-Boyau, O.; Casaer, M.; Schaller, S.; Gunst, J.; Starkopf, J.; Abel, A.; et al. Prevalence of hypophosphatemia in the ICU—Results of an international one-day point prevalence survey. Clin. Nutr. 2021, 40, 3615–3621. [Google Scholar] [CrossRef]
- Suzuki, S.; Egi, M.; Schneider, A.; Bellomo, R.; Hart, G.K.; Hegarty, C. Hypophosphatemia in critically ill patients. J. Crit. Care 2013, 28, 536.e9–536.e19. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Shi, Y.; Cao, G.; Meng, F.; Zhu, W.; Yang, G. Effect of hypophosphatemia on the withdrawal of mechanical ventilation in patients with acute exacerbations of chronic obstructive pulmonary disease. Biomed. Rep. 2016, 4, 413–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Craddock, P.R.; Yawata, Y.; VanSanten, L.; Gilberstadt, S.; Silvis, S.; Jacob, H.S. Acquired Phagocyte Dysfunction. A Complication of the Hypophosphatemia of Parenteral Hyperalimentation. N. Engl. J. Med. 1974, 290, 1244–1248. [Google Scholar] [CrossRef]
- Xue, X.; Ma, J.; Zhao, Y.; Zhao, A.; Liu, X.; Guo, W.; Yan, F.; Wang, Z.; Guo, Y.; Fan, M. Correlation between hypophosphatemia and the severity of Corona Virus Disease 2019 patients. MedRxiv 2020. [Google Scholar] [CrossRef]
- Lemon, S.J.; Zack, S.D.; Voils, S.A. No difference in mechanical ventilation–free hours in critically ill patients who received intravenous, oral, or enteral phosphate replacement. J. Crit. Care 2017, 39, 31–35. [Google Scholar] [CrossRef]
- Geerse, D.A.; Bindels, A.J.; Kuiper, M.A.; Roos, A.N.; Spronk, P.E.; Schultz, M.J. Treatment of hypophosphatemia in the intensive care unit: A review. Crit. Care 2010, 14, R147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Total n = 1226 | Non-HypoP (>0.8 mmol/L) n= 976 | HypoP (≤0.8 mmol/L) n = 250 | p Value | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Gender male, n (%) | 807 (65.8%) | 640 (65.6%) | 167 (66.8%) | 0.7 |
| Age, mean (SD) | 61.2 (15.1) | 61.6 (15.2) | 59.8 (14.7) | 0.09 |
| BMI, mean (SD) | 26.8 (7) | 26.8 (6.5) | 27.1 (9) | 0.6 |
| APACHE II, mean (SD) | 24.2 (8.6) | 24.4 (8.7) | 23.4 (8.2) | 0.1 |
| SAPS II, mean (SD) | 50.6 (20) | 50.9 (20.3) | 49.4 (18.4) | 0.3 |
| Reason for ICU admission, n (%) | <0.01 * | |||
| - Septic shock | 83 (6.8%) | 67 (6.9%) | 16 (6.4%) | |
| - Cardiovascular disease | 301 (24.6%) | 253 (26%) | 48 (19.2%) | |
| - Respiratory failure | 300 (24.4%) | 224 (23%) | 76 (30.3%) | |
| - Neurological disease | 335 (27.3%) | 258 (26.4%) | 77 (30.8%) | |
| - Abdominal disease | 148 (12.1%) | 129 (13.2%) | 19 (7.6%) | |
| - Others (hematologic, ENT, or metabolic disease) | 59 (4.8%) | 45 (4.6%) | 14 (5.6%) | |
| eGFR <60 mL/min at ICU admission, n (%) | 354 (28.9%) | 319 (32.8%) | 35 (14.1%) | <0.01 * |
| Phosphatemia on ICU admission, mmol/L mean (SD) | 1.12 (0.46) | 1.3 (0.4) | 0.6 (0.1) | <0.01 * |
| Ionized calcemia on ICU admission, mmol/L mean (SD) | 1.14 (0.1) | 1.15 (0.1) | 1.13 (0.09) | <0.01 * |
| Intubated patients during ICU stay, n (%) | 856 (69.8%) | 670 (68.7%) | 186 (74.4%) | 0.08 |
| Weaning ventilator failure, n (%) | 91 (10.6%) | 75 (11.2%) | 16 (8.6%) | 0.5 |
| New tracheotomy during ICU stay, n (%) | 49 (4%) | 35 (3.6%) | 14 (5.6%) | 0.1 |
| Infections during the first 48 h of ICU stay, n (%) | 526 (42.9%) | 414 (42.4%) | 112 (44.8%) | 0.5 |
| New infections during ICU stay, n (%) | 48 (3.9%) | 33 (3.4%) | 15 (6%) | 0.06 |
| Hemodiafiltration during ICU stay, n (%) | 50 (4.1%) | 44 (4.5%) | 6 (2.4%) | 0.1 |
| COVID-19 patients, n (%) | 255 (20.8%) | 188 (19.3%) | 67 (26.8%) | <0.01 * |
| Study outcomes | ||||
| Ventilation time in days, mean (SD) | 6 (9.5) | 5.6 (8.9) | 7.4 (11.2) | 0.02 * |
| ICU length of stay, mean (SD) | 6 (8.8) | 5.6 (8.3) | 7.4 (10.4) | <0.01 * |
| Death in the ICU, n (%) | 156 (12.7%) | 126 (12.9%) | 30 (12%) | 0.7 |
| n = 1226 | Mortality in the ICU, Odds Ratio (95% CI) | p Value |
|---|---|---|
| Hypophosphatemia | 1.09 (0.69–1.72) | 0.7 |
| Gender male | 1.55 (1.02–2.35) | 0.04 * |
| SAPS II | 1.05 (1.04–1.07) | <0.01 * |
| Infections within the first 48 h | 2.14 (1.46–3.13) | <0.01 * |
| Intubation | 1.68 (0.89–3.15) | 0.11 |
| Reason for ICU admission | <0.01 * | |
| - Abdominal disease | 1.0 (Reference) | NA |
| - Septic shock | 1.32 (0.5–3.5) | 0.57 |
| - Cardiovascular disease | 2.85 (1.31–6.21) | <0.01 * |
| - Respiratory failure | 3.56 (1.68–7.54) | <0.01 * |
| - Neurological disease | 2.83 (1.30–6.17) | <0.01 * |
| - Others (hematologic, ENT, or metabolic disease) | 0.63 (0.13–3.07) | 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wozniak, H.; Dos Santos Rocha, A.; Beckmann, T.S.; Larpin, C.; Buetti, N.; Quintard, H.; Pugin, J.; Heidegger, C.P. Hypophosphatemia on ICU Admission Is Associated with an Increased Length of Stay in the ICU and Time under Mechanical Ventilation. J. Clin. Med. 2022, 11, 581. https://doi.org/10.3390/jcm11030581
Wozniak H, Dos Santos Rocha A, Beckmann TS, Larpin C, Buetti N, Quintard H, Pugin J, Heidegger CP. Hypophosphatemia on ICU Admission Is Associated with an Increased Length of Stay in the ICU and Time under Mechanical Ventilation. Journal of Clinical Medicine. 2022; 11(3):581. https://doi.org/10.3390/jcm11030581
Chicago/Turabian StyleWozniak, Hannah, André Dos Santos Rocha, Tal Sarah Beckmann, Christophe Larpin, Niccolò Buetti, Hervé Quintard, Jérôme Pugin, and Claudia Paula Heidegger. 2022. "Hypophosphatemia on ICU Admission Is Associated with an Increased Length of Stay in the ICU and Time under Mechanical Ventilation" Journal of Clinical Medicine 11, no. 3: 581. https://doi.org/10.3390/jcm11030581
APA StyleWozniak, H., Dos Santos Rocha, A., Beckmann, T. S., Larpin, C., Buetti, N., Quintard, H., Pugin, J., & Heidegger, C. P. (2022). Hypophosphatemia on ICU Admission Is Associated with an Increased Length of Stay in the ICU and Time under Mechanical Ventilation. Journal of Clinical Medicine, 11(3), 581. https://doi.org/10.3390/jcm11030581







