Physical Activity, Psychological and Functional Outcomes in Non-Ambulatory Stroke Patients during Rehabilitation—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
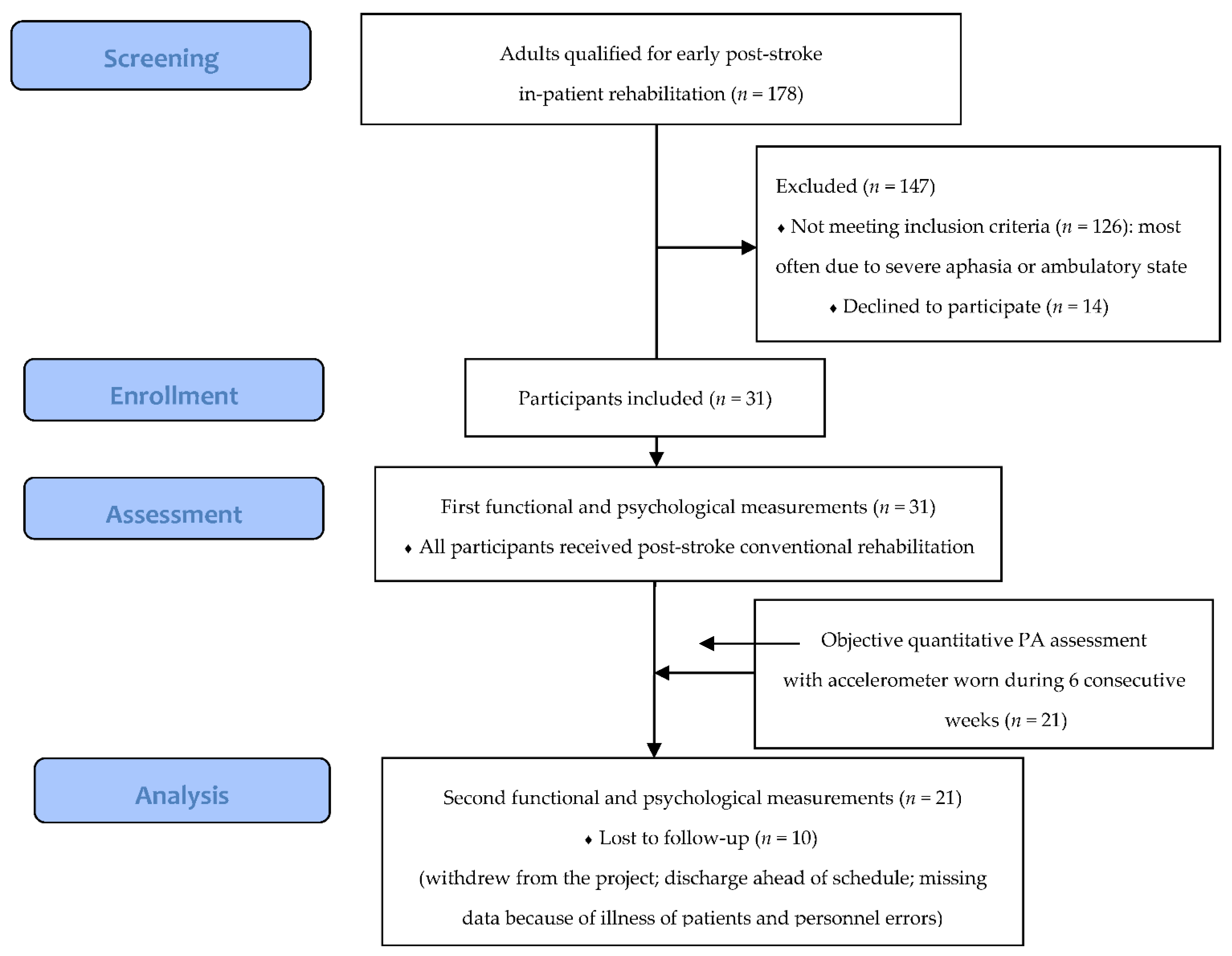
2.1. Study Design, Setting and Participants
2.2. Procedures and Outcome Measurements
- Physical activity
- 2.
- Functional outcomes
- 3.
- Psychological outcomes
2.3. Data Analysis
3. Results
3.1. Participants’ Characteristics
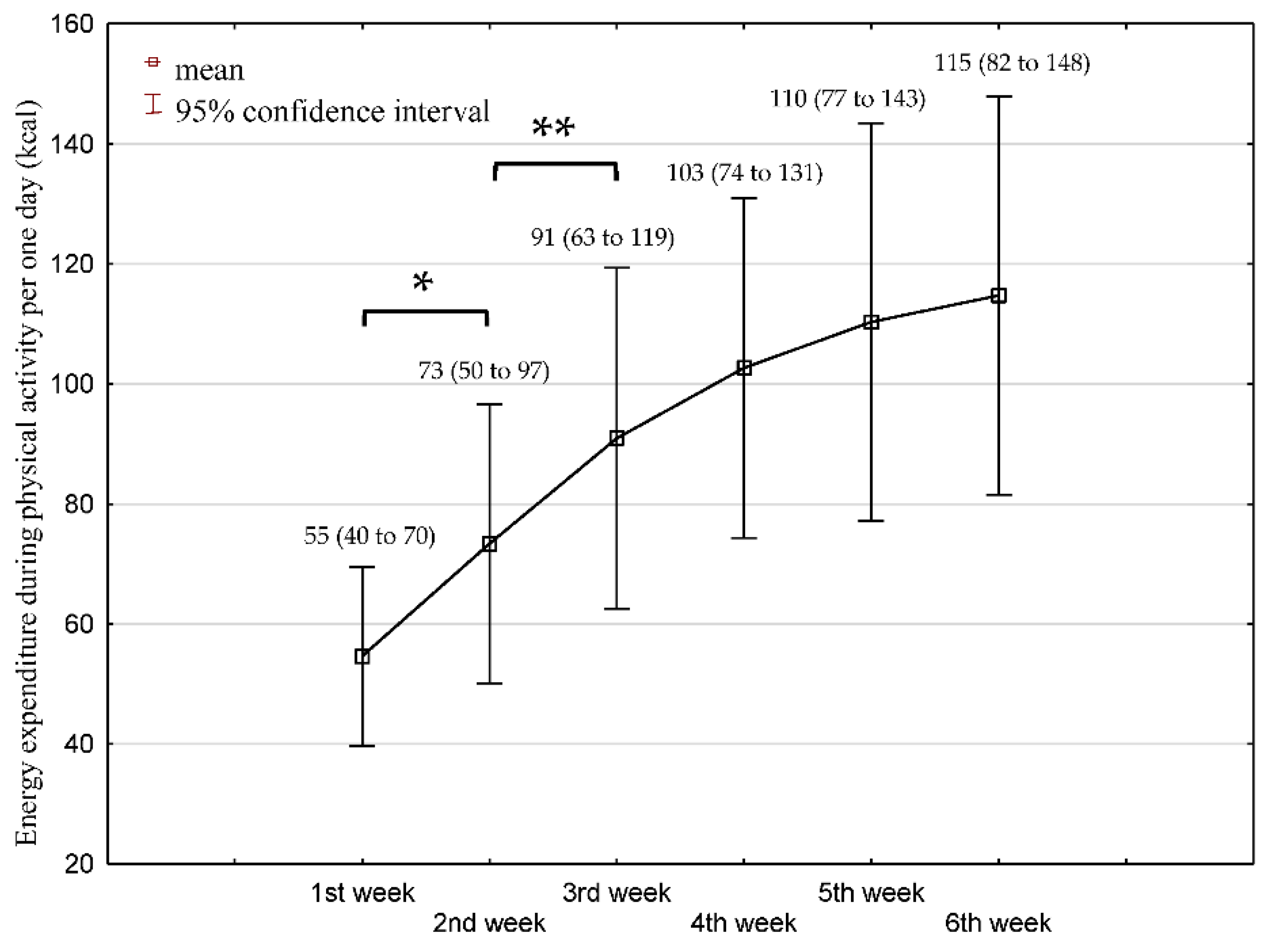
3.2. Physical Activity over 6 Weeks Rehabilitation
3.3. Functional and Psychological Outcomes at Baseline and 6 Weeks Post Rehabilitation
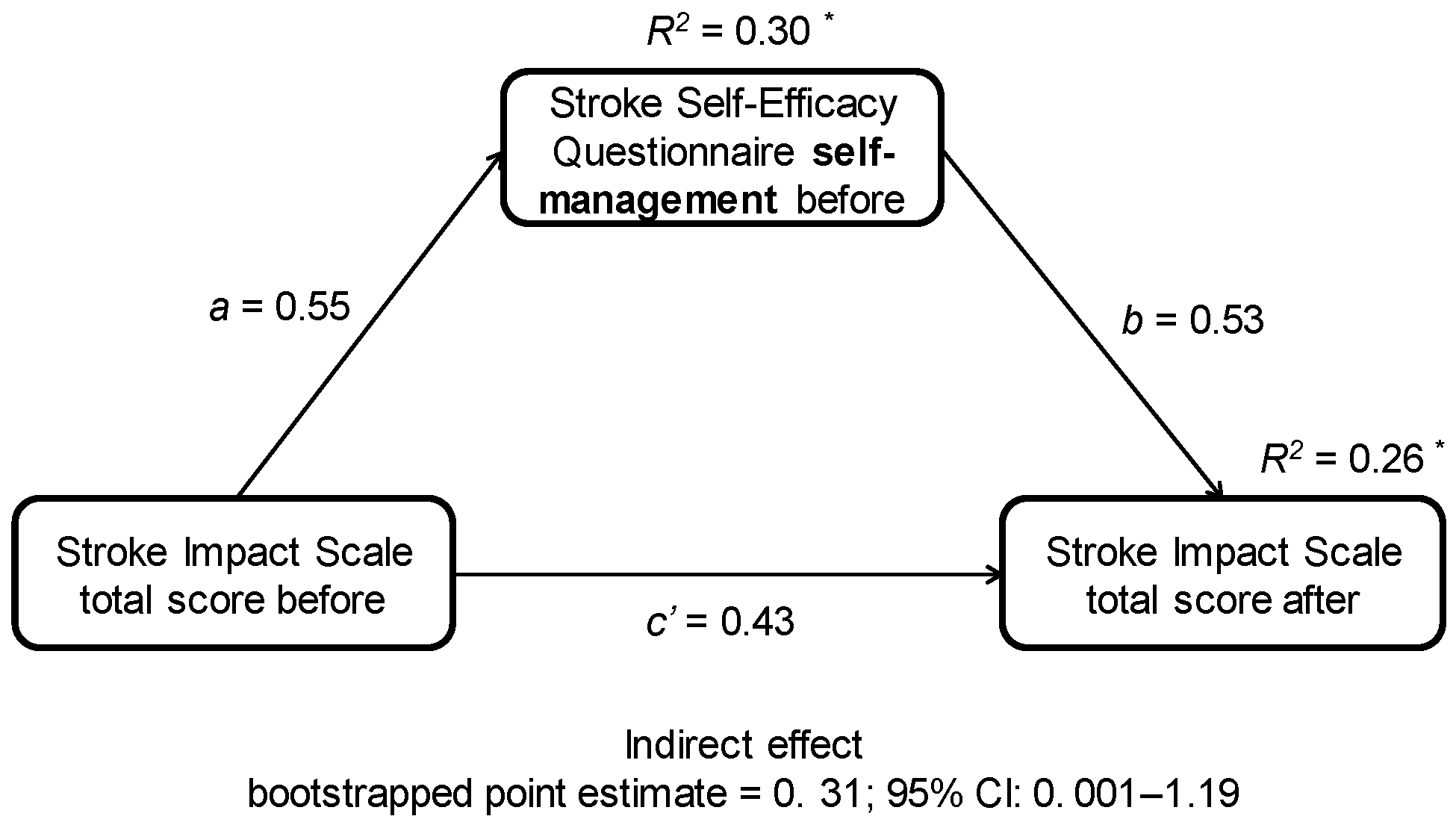
3.4. Associations between Self-Efficacy, Belief in Own Impact on Recovery, and Functional and Psychological Outcomes and PA
4. Discussion
4.1. Key Findings
4.2. Physical Activity
4.3. Functional and Psychological Outcomes during Rehabilitation
4.4. Associations between Independent and Dependent Variables
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Krishnamurthi, R.V.; Parmar, P.; Norrving, B.; Mensah, G.A.; Bennett, D.A.; Barker-Collo, S.; Moran, A.E.; Sacco, R.L.; Truelsen, T.; et al. Update on the Global Burden of Ischemic and Hemorrhagic Stroke in 1990-2013: The GBD 2013 Study. Neuroepidemiology 2015, 45, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, Regional, and National Burden of Stroke and Its Risk Factors, 1990-2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Hackett, M.L.; Yapa, C.; Parag, V.; Anderson, C.S. Frequency of Depression after Stroke. Stroke 2005, 36, 1330–1340. [Google Scholar] [CrossRef]
- Chu, C.-L.; Lee, T.-H.; Chen, Y.-P.; Ro, L.-S.; Hsu, J.-L.; Chu, Y.-C.; Chen, C.-K.; Pei, Y.-C. Recovery of Walking Ability in Stroke Patients through Postacute Care Rehabilitation. Biomed. J. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Ballester, B.R.; Maier, M.; Duff, A.; Cameirão, M.; Bermúdez, S.; Duarte, E.; Cuxart, A.; Rodríguez, S.; Mozo, R.M.S.S.; Verschure, P.F.M.J. A Critical Time Window for Recovery Extends beyond One-Year Post-Stroke. J. Neurophysiol. 2019, 122, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Mackay, C.P.; Kuys, S.S.; Brauer, S.G. The Effect of Aerobic Exercise on Brain-Derived Neurotrophic Factor in People with Neurological Disorders: A Systematic Review and Meta-Analysis. Neural Plast. 2017, 2017, 4716197. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.; Walsh, A.; Adikari, A.; Goodin, P.; Alahakoon, D.; De Silva, D.; Ong, K.L.; Nilsson, M.; Boyd, L. Finding the Intersection of Neuroplasticity, Stroke Recovery, and Learning: Scope and Contributions to Stroke Rehabilitation. Neural Plast. 2019, 2019, 5232374. [Google Scholar] [CrossRef]
- Kārkliņa, A.; Chen, E.; Bērziņa, G.; Stibrant Sunnerhagen, K. Patients’ Physical Activity in Stroke Units in Latvia and Sweden. Brain Behav. 2021, 11. [Google Scholar] [CrossRef]
- Askim, T.; Bernhardt, J.; Salvesen, Ø.; Indredavik, B. Physical Activity Early after Stroke and Its Association to Functional Outcome 3 Months Later. J. Stroke Cerebrovasc. Dis. 2014, 23, e305–e312. [Google Scholar] [CrossRef] [PubMed]
- Askim, T.; Bernhardt, J.; Churilov, L.; Fredriksen, K.R.; Indredavik, B. Changes in Physicalactivity and Related Functional and Disability Levels in the First Six Months after Stroke: A Longitudinal Follow-up Study. J. Rehabil. Med. 2013, 45, 423–428. [Google Scholar] [CrossRef]
- Thilarajah, S.; Mentiplay, B.F.; Bower, K.J.; Tan, D.; Pua, Y.H.; Williams, G.; Koh, G.; Clark, R.A. Factors Associated With Post-Stroke Physical Activity: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2018, 99, 1876–1889. [Google Scholar] [CrossRef] [PubMed]
- Church, G.; Ali, A.; Smith, C.L.; Broom, D.; Sage, K. Examining Clinical Practice Guidelines for Exercise and Physical Activity as Part of Rehabilitation for People with Stroke: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 1707. [Google Scholar] [CrossRef]
- Kubo, H.; Kanai, M.; Nozoe, M.; Inamoto, A.; Taguchi, A.; Mase, K.; Shimada, S. Different Association between Physical Activity and Physical Function According to Walking Independence in Hospital-Based Rehabilitation Program Patients with Sub-Acute Stroke. Clin. Neurol. Neurosurg. 2022, 215, 107202. [Google Scholar] [CrossRef]
- Saunders, D.H.; Mead, G.E.; Fitzsimons, C.; Kelly, P.; van Wijck, F.; Verschuren, O.; Backx, K.; English, C. Interventions for Reducing Sedentary Behaviour in People with Stroke. Cochrane Database Syst. Rev. 2021, 6, CD012996. [Google Scholar] [CrossRef]
- Field, M.J.; Gebruers, N.; Shanmuga Sundaram, T.; Nicholson, S.; Mead, G. Physical Activity after Stroke: A Systematic Review and Meta-Analysis. ISRN Stroke 2013, 2013, 464176. [Google Scholar] [CrossRef]
- Bandura, A. Perceived Self-Efficacy in Cognitive Development and Functioning.Pdf. Educ. Psychol. 1993, 28, 117–148. [Google Scholar] [CrossRef]
- Lewin, A.; Jöbges, M.; Werheid, K. The Influence of Self-Efficacy, Pre-Stroke Depression and Perceived Social Support on Self-Reported Depressive Symptoms during Stroke Rehabilitation. Neuropsychol. Rehabil. 2013, 23, 546–562. [Google Scholar] [CrossRef]
- Marks, R.; Allegrante, J.P.; Lorig, K. A Review and Synthesis of Research Evidence for Self-Efficacy-Enhancing Interventions for Reducing Chronic Disability: Implications for Health Education Practice (Part II). Health Promot. Pract. 2005, 6, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Volz, M.; Voelkle, M.C.; Werheid, K. General Self-Efficacy as a Driving Factor of Post-Stroke Depression: A Longitudinal Study. Neuropsychol. Rehabil. 2019, 29, 1426–1438. [Google Scholar] [CrossRef]
- Korpershoek, C.; van der Bijl, J.; Hafsteinsdottir, T.B. Self-Efficacy and Its Influence on Recovery of Patients with Stroke: A Systematic Review. J. Adv. Nurs. 2011, 67, 1876–1894. [Google Scholar] [CrossRef]
- Torrisi, M.; De Cola, M.C.; Buda, A.; Carioti, L.; Scaltrito, M.V.; Bramanti, P.; Manuli, A.; De Luca, R.; Calabrò, R.S. Self-Efficacy, Poststroke Depression, and Rehabilitation Outcomes: Is There a Correlation? J. Stroke Cerebrovasc. Dis. 2018, 27, 3208–3211. [Google Scholar] [CrossRef] [PubMed]
- Nott, M.; Wiseman, L.; Seymour, T.; Pike, S.; Cuming, T.; Wall, G. Stroke Self-Management and the Role of Self-Efficacy. Disabil. Rehabil. 2021, 43, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Jones, F.; Riazi, A. Self-Efficacy and Self-Management after Stroke: A Systematic Review. Disabil. Rehabil. 2010, 33, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, D.; Johnston, M. Perceived Control Predicting the Recovery of Individual-Specific Walking Behaviours Following Stroke: Testing Psychological Models and Constructs. Br. J. Health Psychol. 2008, 13, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Zirk, M.; Storm, V. Subjective Stroke Impact and Depressive Symptoms: Indications for a Moderating Role of Health-Related Locus of Control. Front. Psychiatry 2019, 10, 918. [Google Scholar] [CrossRef]
- Lei, T.T.; Han, H.M.; Liu, X.J. Multiple Mediation Effects of Health Locus of Control and Hope on the Relationship between Stroke Patients’ Social Support and Self-Management. Front. Nurs. 2020, 7, 49–57. [Google Scholar] [CrossRef]
- Rosenstock, I.M.; Strecher, V.J.; Becker, M.H. Social Learning Theory and the Health Belief Model. Health Educ. Behav. 1988, 15, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Mohd Zulkifly, M.F.; Ghazali, S.E.; Che Din, N.; Desa, A.; Raymond, A.A. The Ability of Recovery Locus of Control Scale (RLOC) and Post-Traumatic Stress Symptoms (PTSS) to Predict the Physical Functioning of Stroke Patients. Malaysian J. Med. Sci. 2015, 22, 31–41. [Google Scholar]
- Lloyd, M.; Skelton, D.A.; Mead, G.E.; Williams, B.; van Wijck, F. Physical Fitness Interventions for Nonambulatory Stroke Survivors: A Mixed-Methods Systematic Review and Meta-Analysis. Brain Behav. 2018, 8, e01000. [Google Scholar] [CrossRef]
- Holden, M.K.; Gill, K.M.; Magliozzi, M.R.; Nathan, J.; Piehl-Baker, L. Clinical Gait Assessment in the Neurologically Impaired Reliability and Meaningfulness. Phys. Ther. 1984, 64, 35–40. [Google Scholar] [CrossRef]
- Balogun, J.A.; Martin, D.A.; Clendenin, M.A. Calorimetric Validation of the Caltrac® Accelerometer during Level Walking. Phys. Ther. 1989, 69, 501–509. [Google Scholar] [CrossRef]
- Gebruers, N.; Vanroy, C.; Truijen, S.; Engelborghs, S.; De Deyn, P.P. Monitoring of Physical Activity after Stroke: A Systematic Review of Accelerometry-Based Measures. Arch. Phys. Med. Rehabil. 2010, 91, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Plasqui, G.; Westerterp, K.R. Accelerometers: An Evaluation against Doubly Labeled Water. Obesity 2007, 15, 2371–2379. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Blum, L.; Korner-Bitensky, N. Usefulness of the Berg Balance Scale in Stroke Rehabilitation: A Systematic Review. Phys. Ther. 2008, 88, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Vellone, E.; Savini, S.; Fida, R.; Dickson, V.V.; Melkus, G.D.E.; Carod-Artal, F.J.; Rocco, G.; Alvaro, R. Psychometric Evaluation of the Stroke Impact Scale 3.0. J. Cardiovasc. Nurs. 2015, 30, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.L.; Stistrup, R.D.; Schjøtt, C.S.; Madsen, J.; Vinther, A. Absolute and Relative Reliability of the Timed “Up & Go” Test and “30second Chair-Stand” Test in Hospitalised Patients with Stroke. PLoS ONE 2016, 11, e0165663. [Google Scholar] [CrossRef]
- Fulk, G.D.; Echternach, J.L.; Nof, L.; O’Sullivan, S. Clinometric Properties of the Six-Minute Walk Test in Individuals Undergoing Rehabilitation Poststroke. Physiother. Theory Pract. 2008. [CrossRef]
- Partridge, C.; Johnston, M. Perceived Control of Recovery from Physical Disability: Measurement and Prediction. Br. J. Clin. Psychol. 1989, 28, 53–59. [Google Scholar] [CrossRef]
- Jones, F.; Partridge, C.; Reid, F. The Stroke Self-Efficacy Questionnaire: Measuring Individual Confidence in Functional Performance after Stroke. J. Clin. Nurs. 2008. [CrossRef]
- Schwarzer, R.; Jerusalem, M. Generalized Self-Efficacy Scale. J. Weinman, S. Wright, M. Johnston, Meas. Health Psychol. A user’s portfolio. Causal Control. Beliefs 1995, 35, 37. [Google Scholar]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Wichowicz, H.M.; Wieczorek, D. Badanie Przesiewowe Depresji Poudarowej z Użyciem Hospital Anxiety and Depression Scale (HADS). Psychiatr. Pol. 2011, 45, 505–514. [Google Scholar] [PubMed]
- Felton, B.J.; Revenson, T.A.; Hinrichsen, G.A. AIS-Acceptance of Illness Scale. Meas. tools Promot. Health Psychol. 2001, 158, 167. [Google Scholar]
- Mukaka, M.M. Statistics Corner: A Guide to Appropriate Use of Correlation Coefficient in Medical Research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar] [PubMed]
- Haeuber, E.; Shaughnessy, M.; Forrester, L.W.; Coleman, K.L.; Macko, R.F. Accelerometer Monitoring of Home- and Community-Based Ambulatory Activity after Stroke. Arch. Phys. Med. Rehabil. 2004, 85, 1997–2001. [Google Scholar] [CrossRef]
- Fini, N.A.; Holland, A.E.; Keating, J.; Simek, J.; Bernhardt, J. How Physically Active Are People Review and Quantitative Synthesis. Phys. Ther. 2017, 97, 707–717. [Google Scholar] [CrossRef]
- Rand, D.; Eng, J.J.; Tang, P.F.; Hung, C.; Jeng, J.S. Daily Physical Activity and Its Contribution to the Health-Related Quality of Life of Ambulatory Individuals with Chronic Stroke. Health Qual. Life Outcomes 2010, 8, 80. [Google Scholar] [CrossRef]
- Kunkel, D.; Fitton, C.; Burnett, M.; Ashburn, A. Physical Inactivity Post-Stroke: A 3-Year Longitudinal Study. Disabil. Rehabil. 2015, 37, 304–310. [Google Scholar] [CrossRef]
- Hokstad, A.; Indredavik, B.; Bernhardt, J.; Ihle-Hansen, H.; Salvesen, O.; Seljeseth, Y.M.; Schüler, S.; Engstad, T.; Askim, T. Hospital Differences in Motor Activity Early after Stroke: A Comparison of 11 Norwegian Stroke Units. J. Stroke Cerebrovasc. Dis. 2015, 24, 1333–1340. [Google Scholar] [CrossRef]
- Kramer, S.F.; Churilov, L.; Kroeders, R.; Pang, M.Y.C.; Bernhardt, J. Changes in Activity Levels in the First Month after Stroke. J. Phys. Ther. Sci. 2013, 25, 599–604. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eng, J.J.; Reime, B. Exercise for Depressive Symptoms in Stroke Patients: A Systematic Review and Meta-Analysis. Clin. Rehabil. 2014, 28, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Stone, A.J. Combined Aerobic and Resistance Training for Cardiorespiratory Fitness, Muscle Strength, and Walking Capacity after Stroke: A Systematic Review and Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2020, 29, 104498. [Google Scholar] [CrossRef] [PubMed]
- Borschmann, K.; Pang, M.Y.C.; Bernhardt, J.; Iuliano-Burns, S. Stepping towards Prevention of Bone Loss after Stroke: A Systematic Review of the Skeletal Effects of Physical Activity after Stroke. Int. J. Stroke 2012, 7, 330–335. [Google Scholar] [CrossRef]
- Chen, M.; De Rimmer, J.H. Effects of Exercise on Quality of Life in Stroke Survivors: A Meta-Analysis. Stroke 2011, 42, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, R.; Wondergem, R.; Otten, C.; Pisters, M.F. Effect of Aerobic Training on Vascular and Metabolic Risk Factors for Recurrent Stroke: A Meta- Analysis. Disabil. Rehabil. 2021, 43, 2084–2091. [Google Scholar] [CrossRef]
- Lord, S.E.; McPherson, K.; McNaughton, H.K.; Rochester, L.; Weatherall, M. Community Ambulation after Stroke: How Important and Obtainable Is It and What Measures Appear Predictive? Arch. Phys. Med. Rehabil. 2004, 85, 234–239. [Google Scholar] [CrossRef]
- Veerbeek, J.M.; Van Wegen, E.; Van Peppen, R.; Van Der Wees, P.J.; Hendriks, E.; Rietberg, M.; Kwakkel, G. What Is the Evidence for Physical Therapy Poststroke? A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e87987. [Google Scholar] [CrossRef]
- Castiglia, S.F.; Galeoto, G.; Lauta, A.; Palumbo, A.; Tirinelli, F.; Viselli, F.; Santilli, V.; Sacchetti, M.L. The Culturally Adapted Italian Version of the Barthel Index (IcaBI): Assessment of Structural Validity, Inter-Rater Reliability and Responsiveness to Clinically Relevant Improvements in Patients Admitted to Inpatient Rehabilitation Centers. Funct. Neurol. 2017, 32, 221. [Google Scholar] [CrossRef]
- Song, M.-J.; Lee, J.-H.; Shin, W.-S. Minimal Clinically Important Difference of Berg Balance Scale Scores in People with Acute Stroke. Phys. Ther. Rehabil. Sci. 2018, 7, 102–108. [Google Scholar] [CrossRef]
- Fryer, C.; Luker, J.; Mcdonnell, M.; Hillier, S. Self Management Programmes for Quality of Life in People with Stroke. Cochrane Database Syst. Rev. 2016, 8, CD010442. [Google Scholar] [CrossRef] [PubMed]
- Pucciarelli, G.; Brugnera, A.; Greco, A.; Petrizzo, A.; Simeone, S.; Vellone, E.; Alvaro, R. Stroke Disease-Specific Quality of Life Trajectories after Rehabilitation Discharge and Their Sociodemographic and Clinical Associations: A Longitudinal, Multicentre Study. J. Adv. Nurs. 2021, 77, 1856–1866. [Google Scholar] [CrossRef]
- Skoglund, E.; Westerlind, E.; Persson, H.C.; Sunnerhagen, K.S. Self-Perceived Impact of Stroke: A Longitudinal Comparison between One and Five Years Post-Stroke. J. Rehabil. Med. 2019, 51, 660–664. [Google Scholar] [CrossRef]
- Paolucci, S.; Iosa, M.; Coiro, P.; Venturiero, V.; Savo, A.; De Angelis, D.; Morone, G. Post-Stroke Depression Increases Disability More than 15% in Ischemic Stroke Survivors: A Case-Control Study. Front. Neurol. 2019, 10, 926. [Google Scholar] [CrossRef]
- Yu, H.L.; Cao, D.X.; Liu, J. Effect of a Novel Designed Intensive Patient Care Program on Cognitive Impairment, Anxiety, Depression as Well as Relapse Free Survival in Acute Ischemic Stroke Patients: A Randomized Controlled Study. Neurol Res. 2019, 41, 857–866. [Google Scholar] [CrossRef]
- Szczepańska-Gieracha, J.; Mazurek, J. The Role of Self-Efficacy in the Recovery Process of Stroke Survivors. Psychol. Res. Behav. Manag. 2020, 13, 897–906. [Google Scholar] [CrossRef]
- Gutierrez, D.; Wing, J.J.; Drum, E.; Boden-Albala, B. Abstract WP81: The Association between Health-Related Locus Of Control And Post-Stroke Disability, Quality Of Life, And Depression. Stroke 2022, 53, AWP81. [Google Scholar] [CrossRef]
- Hamzah, A. Sugiyanto Strengthening of Health Locus of Control Could Increase the Independence of Post Stroke Patients in Implementing the Daily Activities at Home. J. Nurs. Care 2014, 3, 1–4. [Google Scholar] [CrossRef]
- Minshall, C.; Ski, C.F.; Apputhurai, P.; Thompson, D.R.; Castle, D.J.; Jenkins, Z.; Knowles, S.R. Exploring the Impact of Illness Perceptions, Self-Efficacy, Coping Strategies, and Psychological Distress on Quality of Life in a Post-Stroke Cohort. J. Clin. Psychol. Med. Settings 2020 281 2020, 28, 174–180. [Google Scholar] [CrossRef]
- Rogowska, A.M.; Zmaczyńska-Witek, B.; Mazurkiewicz, M.; Kardasz, Z. The Mediating Effect of Self-Efficacy on the Relationship between Health Locus of Control and Life Satisfaction: A Moderator Role of Movement Disability. Disabil. Health J. 2020, 13, 100923. [Google Scholar] [CrossRef]
- Kobylańska, M.; Kowalska, J.; Neustein, J.; Mazurek, J.; Wójcik, B.; Bełza, M.; Cichosz, M.; Szczepańska-Gieracha, J. The Role of Biopsychosocial Factors in the Rehabilitation Process of Individuals with a Stroke. Work 2019, 61, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Guzek, Z.; Kowalska, J. Analysis of the Degree of Acceptance of Illness among Patients after a Stroke: An Observational Study. Clin. Interv. Aging 2020, 15, 2063–2072. [Google Scholar] [CrossRef]
- van Mierlo, M.L.; van Heugten, C.M.; Post, M.W.M.; de Kort, P.L.M.; Visser-Meily, J.M.A. Life Satisfaction Post Stroke: The Role of Illness Cognitions. J. Psychosom. Res. 2015, 79, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Amaricai, E.; Poenaru, D. V The Post-Stroke Depression and Its Impact on Functioning in Young and Adult Stroke Patients of a Rehabilitation Unit. J. Ment. Health 2016, 25, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Alghwiri, A.A. The Correlation between Depression, Balance, and Physical Functioning Post Stroke. J. Stroke Cerebrovasc. Dis. 2016, 25, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.C.; Balraj, E.; DiSanzo, B.L.; Ivey, F.M.; Hafer-Macko, C.E.; Treuth, M.S.; Ryan, A.S. Validating Accelerometry as a Measure of Physical Activity and Energy Expenditure in Chronic Stroke. Top. Stroke Rehabil. 2017, 24, 18–23. [Google Scholar] [CrossRef] [PubMed]
| INSTRUCTION: Rate what your recovery depends on, on a scale of 1 to 5. Where 1 is the least important factor and 5 is the most important factor. You can only assign each grade once. | |
| from me (my commitment, motivation, strategies for coping with stroke) iloc | Rate: … |
| from a doctor, physical therapist, psychologist and other forms of therapy eloc | Rate: … |
| from a family, relatives, my surroundings eloc | Rate: … |
| from a disease progression eloc | Rate: … |
| other (please specify if there are any other factors not listed on the scale) | Rate: … |
| Age (Years) | Gender (Female/Male) | Time since Stroke (Days) | Hemiplegic Side (R/L) | Ischemic/ Hemorrhagic Stroke | Mini-Mental State Examination | Before/during the COVID-19 Pandemic |
|---|---|---|---|---|---|---|
| 72.3 | 18/13 | 33.3 | 13/18 | 29/2 | 25.6 | 11/20 |
| ±6.5 | 58.1% | ±25.9 | 41.9% | 93.5% | ±3.1 | 35.5% |
| Outcome | Baseline (Mean ± SD) | Post 6 Weeks (Mean ± SD) | Change (Mean ± SD) | p-Value | Change CI (95%) |
|---|---|---|---|---|---|
| Barthel Index | 41.7 (17.3) | 79.6 (17.9) | 37.8 (19.6) | <0.001 | (29.3; 46.3) |
| Trunk Control Test | 66.6 (26.9) | 88.7 (16.2) | 22.2 (21.7) | <0.001 | (12.8; 31.5) |
| Berg Balance Scale | 10.8 (9.4) | 34.3 (12.3) | 23.6 (11) | <0.001 | (18.2; 27.7) |
| HADS | |||||
| anxiety | 7.6 (3.2) | 6.6 (3.5) | −1 (3.2) | 0.165 | (−2.4; 0.4) |
| depression | 6.1 (4.2) | 5.3 (4.3) | −0.8 (2.7) | 0.234 | (−2.1; 0.4) |
| total score | 13.8 (6.2) | 12 (6.6) | −1.8 (4.6) | 0.098 | (−3.9; 0.3) |
| SSEQ | |||||
| activities | 4 (3.8) | 13.5 (5.7) | 9.5 (6) | <0.001 | (6.8; 12.2) |
| self-management | 8.8 (4.4) | 10.5 (3.9) | 1.7 (3.3) | 0.036 | (0.1; 3.2) |
| total score | 12.9 (6.1) | 23.8 (9) | 10.9 (6.4) | <0.001 | (7.3; 14.5) |
| Acceptance of Illness Scale | 23.3 (7.9) | 27 (7.9) | 3.7 (1.6) | 0.131 | (−1.2; 8.5) |
| Belief in own impact on recovery (from 1 to 5) | 4 (1.3) | 4.5 (1.1) | 0.5 (0.9) | 0.059 | (0; 0.9) |
| Belief in external impact on recovery (from 1 to 5) | 2.7 (0.3) | 2.7 (0.3) | −0.1 (0.3) | 0.272 | (−02; 0.1) |
| GSES | 33.1 (6) | 30.9 (7.6) | −2.2 (6.4) | 0.139 | (−5.1; 0.7) |
| Stroke Impact Scale | |||||
| total score | 341.4 (61.1) | 504.6 (118.2) | 163.1 (99.5) | <0.001 | (117.8; 208.4) |
| Strength | 25.8 (20.8) | 57.1 (22.5) | 31.3 (19.9) | <0.001 | (22.6; 39.9) |
| Memory and thinking | 73.2 (18.3) | 86.5 (13.8) | 13.3 (16) | 0.002 | (6.2; 20.5) |
| Emotions | 67.3 (18.3) | 75.1 (16.5) | 7.8 (12.4) | 0.01 | (2.3; 13.3) |
| Communication | 80.5 (22.3) | 89.5 (15.1) | 8.9 (21.8) | 0.068 | (−0.7; 18.6) |
| ADL | 22.4 (13.4) | 54.6 (21.2) | 32.2 (24.7) | <0.001 | (21.5; 42.8) |
| Mobility | 14.1 (8.4) | 54.1 (24.6) | 40.1 (24.5) | <0.001 | (29.5; 50.7) |
| Hand function | 8.7 (12.9) | 32.4 (33.9) | 23.7 (27.1) | <0.001 | (12; 35.4) |
| Participation | 45.9 (26.9) | 44.5 (27.3) | −1.4 (20.2) | 0.745 | (−10.4; 7.5) |
| Perception of recovery (%) | 23.9 (16.7) | 57.6 (14.9) | 33.7 (21.1) | <0.001 | (24.6; 42.8) |
| Variables | Stroke Self-Efficacy Questionnaire | Belief in Own Impact on Recovery | General Self- Efficacy Scale | Stroke Impact Scale Total Score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Score | Activity | Self-Management | ||||||||||||
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | Δ | 1 | 2 | Δ | |
| Belief in own impact on recovery (1) | 0.32 | 0.64 ** | −0.02 | 0.56 * | 0.55 ** | 0.75 *** | - | 0.56 * | 0.37 * | 0.32 | −0.06 | 0.17 | 0.58 ** | 0.41 |
| Belief in own impact on recovery (2) | 0.12 | 0.23 | −0.30 | 0.13 | 0.49 * | 0.40 | 0.56 * | - | 0.36 | −0.03 | −0.510 * | 0.06 | 0.20 | 0.05 |
| Stroke Impact Scale total score (1) | 0.37 * | 0.38 | 0.23 | 0.29 | 0.29 | 0.39 | 0.17 | 0.06 | 0.33 | 0.47 * | 0.33 | - | 0.54 * | 0.03 |
| Stroke Impact Scale total score (2) | 0.65 ** | 0.72 *** | 0.19 | 0.69 *** | 0.62 ** | 0.64 ** | 0.58 ** | 0.20 | 0.19 | 0.58 ** | 0.51 * | 0.54 * | - | 0.86 *** |
| Barthel Index (1) | 0.20 | 0.01 | 0.24 | 0.02 | 0.03 | 0.02 | 0.07 | 0.38 | 0.09 | −0.13 | −0.10 | 0.45 * | 0.04 | −0.18 |
| Barthel Index (2) | 0.43 * | 0.56 * | 0.19 | 0.61 ** | 0.40 | 0.52 * | 0.34 | 0.20 | 0.19 | 0.13 | 0.13 | 0.33 | 0.77 *** | 0.58 ** |
| Barthel Index Δ | 0.18 | 0.46 | −0.12 | 0.48 * | 0.20 | 0.27 | 0.05 | −0.02 | 0.13 | 0.16 | 0.17 | 0.03 | 0.51 * | 0.68 *** |
| Berg Balance Scale Δ | 0.24 | 0.47 | −0.02 | 0.35 | 0.24 | 0.32 | −0.07 | −0.01 | 0.29 | 0.23 | 0.20 | 0.13 | 0.53 * | 0.54 * |
| Time Up & Go | 0.13 | −0.48 | 0.12 | −0.55 * | 0.06 | −0.37 | 0.08 | 0.08 | −0.01 | −0.43 | −0.67 ** | −0.15 | −0.59 * | −0.53 * |
| 6-Minute Walk Test | 0.18 | 0.54 | 0.11 | 0.61 ** | 0.13 | 0.43 | 0.26 | 0.06 | −0.07 | 0.33 | 0.58 * | 0.42 | 0.69 ** | 0.60 * |
| HADS Depression subscale (2) | −0.12 | −0.34 | 0.44 * | −0.31 | −0.52 * | −0.45 * | −0.44 * | −0.48 * | −0.41 | −0.53 * | −0.09 | −0.32 | −0.44 * | −0.36 |
| HADS Depression subscale Δ | −0.40 | −0.36 | 0.00 | −0.37 | −0.56 ** | −0.35 | −0.43 | −0.32 | −0.44 * | −0.22 | −0.04 | −0.16 | −0.43 | −0.41 |
| Acceptance of Illness Scale (1) | 0.48 ** | 0.36 | 0.37 | 0.26 | 0.32 | 0.56 ** | 0.14 | 0.05 | 0.24 | 0.40 | 0.12 | 0.38 * | 0.34 | 0.05 |
| Acceptance of Illness Scale (2) | −0.10 | 0.22 | −0.37 * | 0.24 | 0.17 | 0.32 | 0.33 | 0.45 * | 0.16 | 0.06 | −0.15 | −0.24 | −0.10 | 0.02 |
| mean daily physical activity Δ 6th-1st week | 0.02 | 0.36 | −0.24 | 0.32 | 0.27 | 0.31 | 0.15 | 0.49 * | 0.08 | 0.15 | 0.08 | 0.10 | 0.33 | 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Błaszcz, M.; Prucnal, N.; Wrześniewski, K.; Pasiut, S.; Mika, P.; Kucia, M.; Stach, B.; Woźniak, M.; Mirek, E. Physical Activity, Psychological and Functional Outcomes in Non-Ambulatory Stroke Patients during Rehabilitation—A Pilot Study. J. Clin. Med. 2022, 11, 7260. https://doi.org/10.3390/jcm11247260
Błaszcz M, Prucnal N, Wrześniewski K, Pasiut S, Mika P, Kucia M, Stach B, Woźniak M, Mirek E. Physical Activity, Psychological and Functional Outcomes in Non-Ambulatory Stroke Patients during Rehabilitation—A Pilot Study. Journal of Clinical Medicine. 2022; 11(24):7260. https://doi.org/10.3390/jcm11247260
Chicago/Turabian StyleBłaszcz, Marcin, Nina Prucnal, Krzysztof Wrześniewski, Szymon Pasiut, Piotr Mika, Małgorzata Kucia, Beata Stach, Marcin Woźniak, and Elżbieta Mirek. 2022. "Physical Activity, Psychological and Functional Outcomes in Non-Ambulatory Stroke Patients during Rehabilitation—A Pilot Study" Journal of Clinical Medicine 11, no. 24: 7260. https://doi.org/10.3390/jcm11247260
APA StyleBłaszcz, M., Prucnal, N., Wrześniewski, K., Pasiut, S., Mika, P., Kucia, M., Stach, B., Woźniak, M., & Mirek, E. (2022). Physical Activity, Psychological and Functional Outcomes in Non-Ambulatory Stroke Patients during Rehabilitation—A Pilot Study. Journal of Clinical Medicine, 11(24), 7260. https://doi.org/10.3390/jcm11247260







