Effects of Combination of Functional Electric Stimulation and Robotic Leg Movement Using Dynamic Tilt Table on Walking Characteristics in Post-Stroke Patients with Spastic Hemiplegia: A Randomized Crossover-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Design
2.2. Study Participants
2.3. Study Protocol
2.4. Intervention
2.5. Measured Variables
2.6. Sample Size Estimates
2.7. Statistical Analysis
3. Results
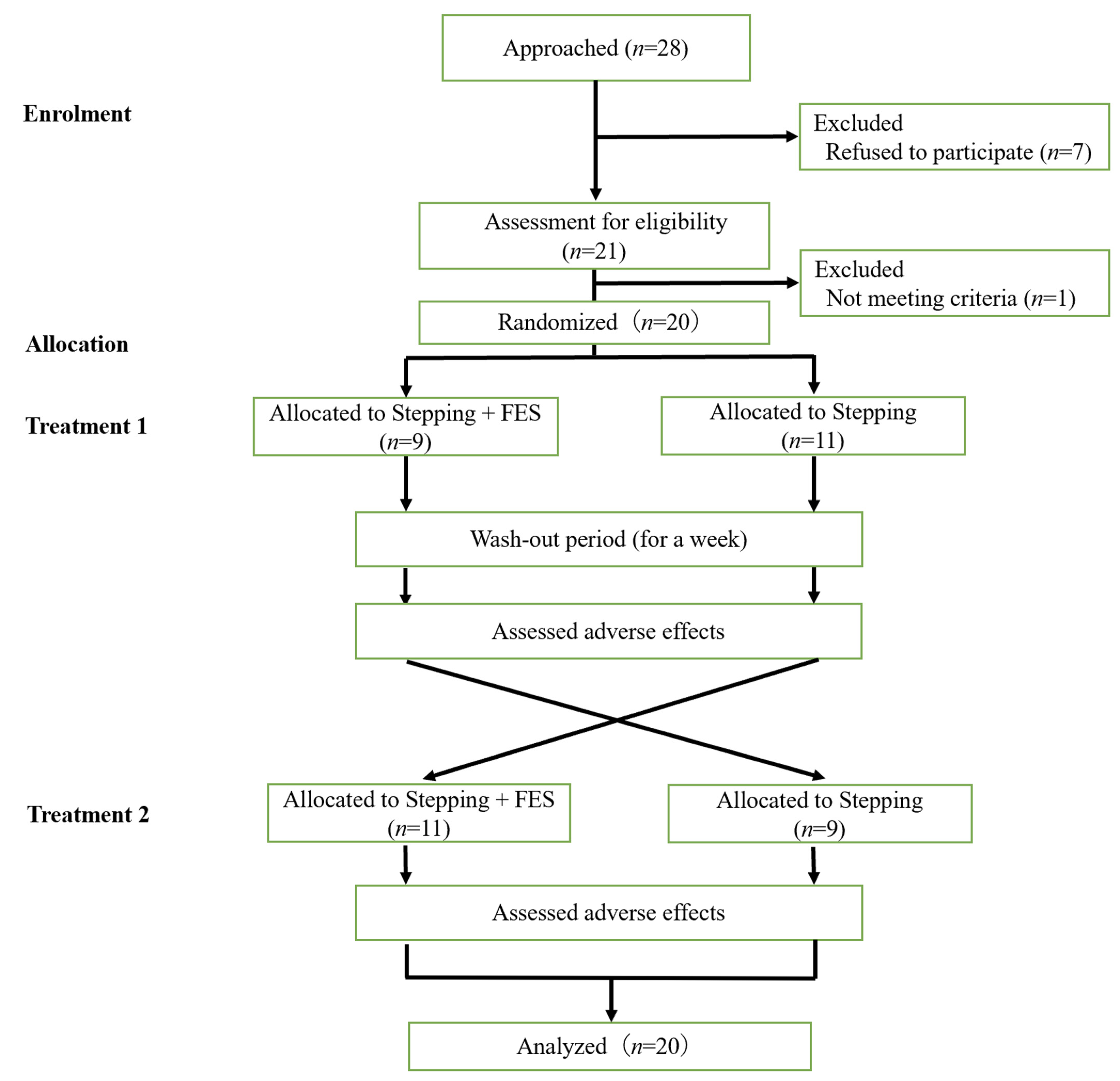
3.1. Participant Flowchart
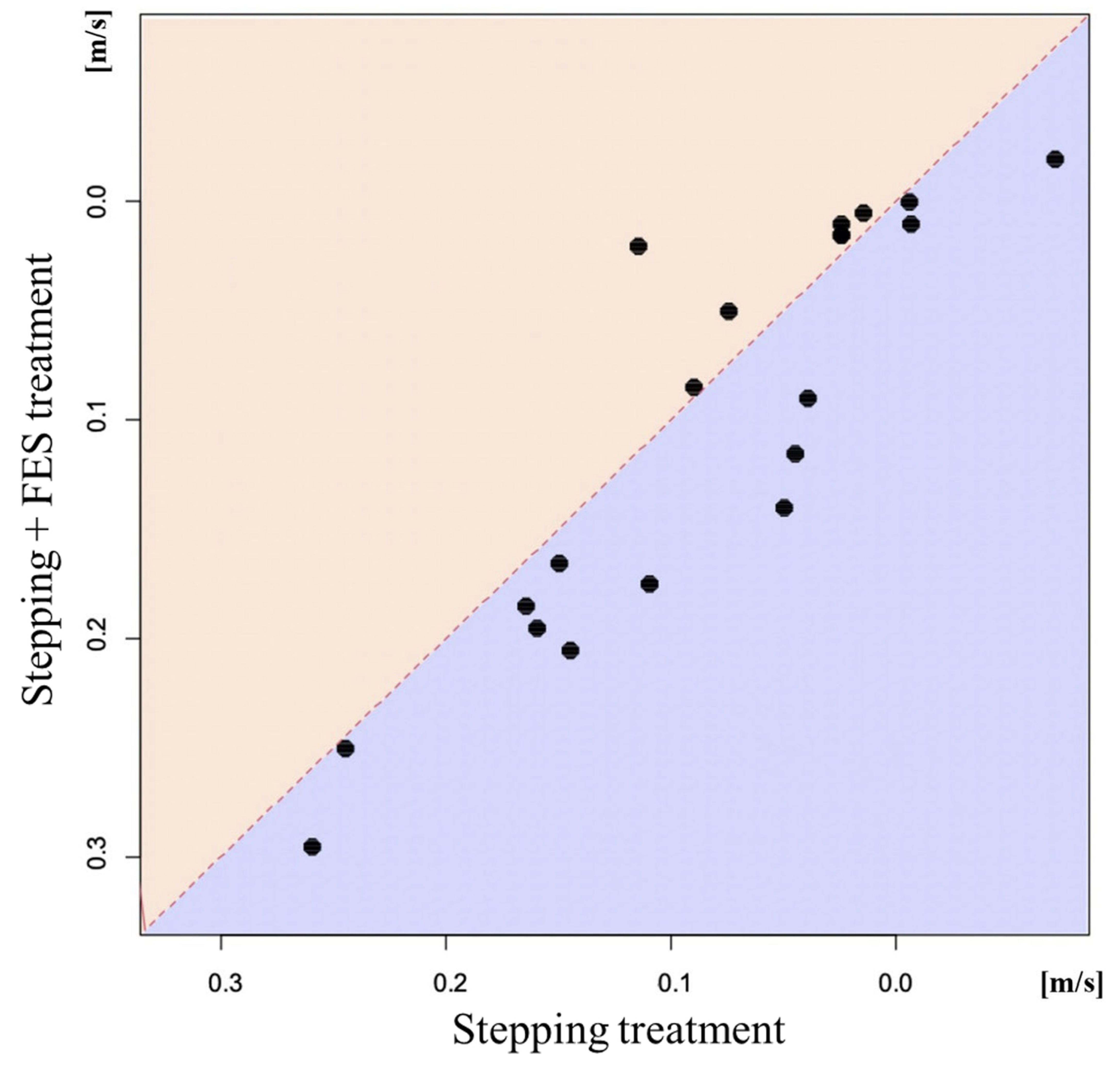
3.2. Results of 10 m Walking Test
3.3. Changes in Range of Motion, Fugl–Meyer Assessment Score, and Modified Ashworth Scale
3.4. Current Intensity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Perry, J.; Garrett, M.; Gronley, J.K.; Mulroy, S.J. Classification of walking handicap in the stroke population. Stroke 1995, 26, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Duncan, P.W.; Studenski, S.; Lai, S.M.; Richards, L.; Perera, S.; Wu, S.S. Improvements in speed-based gait classifications are meaningful. Stroke 2007, 38, 2096–2100. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.L.; Tang, P.F.; Jan, M.H. Analysis of impairments influencing gait velocity and asymmetry of hemiplegic patients after mild to moderate stroke. Arch. Phys. Med. Rehabil. 2003, 84, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Schinwelski, M.J.; Sitek, E.J.; Wąż, P.; Sławek, J.W. Prevalence and predictors of post-stroke spasticity and its impact on daily living and quality of life. Neurol. Neurochir. Pol. 2019, 53, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Dickstein, R.; Laufer, Y.; Katz, M. TENS to the posterior aspect of the legs decreases postural sway during stance. Neurosci. Lett. 2006, 393, 51–55. [Google Scholar] [CrossRef]
- Kwakkel, G.; Wagenaar, R.C.; Twisk, J.W.; Lankhorst, G.J.; Koetsier, J.C. Intensity of leg and arm training after primary middle-cerebral-artery stroke: A randomised trial. Lancet 1999, 354, 191–196. [Google Scholar] [CrossRef] [PubMed]
- van de Port, I.G.; Wood-Dauphinee, S.; Lindeman, E.; Kwakkel, G. Effects of exercise training programs on walking competency after stroke: A systematic review. Am. J. Phys. Med. Rehabil. 2007, 86, 935–951. [Google Scholar] [CrossRef] [PubMed]
- French, B.; Thomas, L.H.; Coupe, J.; McMahon, N.E.; Connell, L.; Harrison, J.; Sutton, C.J.; Tishkovskaya, S.; Watkins, C.L. Repetitive task training for improving functional ability after stroke. Cochrane Database Syst. Rev. 2016, 11, CD006073. [Google Scholar] [CrossRef]
- Veerbeek, J.M.; Koolstra, M.; Ket, J.C.; van Wegen, E.E.; Kwakkel, G. Effects of augmented exercise therapy on outcome of gait and gait-related activities in the first 6 months after stroke: A meta-analysis. Stroke 2011, 42, 3311–3315. [Google Scholar] [CrossRef]
- English, C.; Hillier, S.L.; Lynch, E.A. Circuit class therapy for improving mobility after stroke. Cochrane Database Syst. Rev. 2017, 6, CD007513. [Google Scholar] [CrossRef] [PubMed]
- Veerbeek, J.M.; van Wegen, E.; van Peppen, R.; van der Wees, P.J.; Hendriks, E.; Rietberg, M.; Kwakkel, G. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS ONE 2014, 9, e87987. [Google Scholar] [CrossRef] [PubMed]
- Kirazli, Y.; On, A.Y.; Kismali, B.; Aksit, R. Comparison of phenol block and botulinus toxin type A in the treatment of spastic foot after stroke: A randomized, double-blind trial. Am. J. Phys. Med. Rehabil. 1998, 77, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Okumura, S.; Morita, S.; Obata, K.; Furuya, K. Surgical correction of foot deformities after stroke. Clin. Orthop. Relat. Res. 1992, 282, 213–218. [Google Scholar] [CrossRef]
- Morita, S.; Muneta, T.; Yamamoto, H.; Shinomiya, K. Tendon transfer for equinovarus deformed foot caused by cerebrovascular disease. Clin. Orthop. Relat. Res. 1998, 350, 166–173. [Google Scholar] [CrossRef]
- Czell, D.; Schreier, R.; Rupp, R.; Eberhard, S.; Colombo, G.; Dietz, V. Influence of passive leg movements on blood circulation on the tilt table in healthy adults. J. Neuroeng. Rehabil. 2004, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.; Pignat, J.M.; Berney, L.; Jöhr, J.; Van de Ville, D.; Daniel, R.T.; Levivier, M.; Hirt, L.; Luft, A.R.; Grouzmann, E.; et al. Sympathetic activity and early mobilization in patients in intensive and intermediate care with severe brain injuries: A preliminary prospective randomized study. BMC Neurol. 2016, 16, 169. [Google Scholar] [CrossRef]
- Taveggia, G.; Ragusa, I.; Trani, V.; Cuva, D.; Angeretti, C.; Fontanella, M.; Panciani, P.P.; Borboni, A. Robotic tilt table reduces the occurrence of orthostatic hypotension over time in vegetative states. Int. J. Rehabil. Res. 2015, 38, 162–166. [Google Scholar] [CrossRef]
- Frazzitta, G.; Zivi, I.; Valsecchi, R.; Bonini, S.; Maffia, S.; Molatore, K.; Sebastianelli, L.; Zarucchi, A.; Matteri, D.; Ercoli, G.; et al. Effectiveness of a Very Early Stepping Verticalization Protocol in Severe Acquired Brain Injured Patients: A Randomized Pilot Study in ICU. PLoS ONE 2016, 11, e0158030. [Google Scholar] [CrossRef]
- Kuznetsov, A.N.; Rybalko, N.V.; Daminov, V.D.; Luft, A.R. Early poststroke rehabilitation using a robotic tilt-table stepper and functional electrical stimulation. Stroke Res. Treat. 2013, 2013, 946056. [Google Scholar] [CrossRef]
- Geiger, D.E.; Behrendt, F.; Schuster-Amft, C. EMG Muscle Activation Pattern of Four Lower Extremity Muscles during Stair Climbing, Motor Imagery, and Robot-Assisted Stepping: A Cross-Sectional Study in Healthy Individuals. BioMed Res. Int. 2019, 2019, 9351689. [Google Scholar] [CrossRef]
- Lo, H.C.; Tsai, K.H.; Su, F.C.; Chang, G.L.; Yeh, C.Y. Effects of a functional electrical stimulation-assisted leg-cycling wheelchair on reducing spasticity of patients after stroke. J. Rehabil. Med. 2009, 41, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Wang, J.S. The immediate effect of FES and TENS on gait parameters in patients after stroke. J. Phys. Ther. Sci. 2017, 29, 2212–2214. [Google Scholar] [CrossRef]
- Sabut, S.K.; Sikdar, C.; Mondal, R.; Kumar, R.; Mahadevappa, M. Restoration of gait and motor recovery by functional electrical stimulation therapy in persons with stroke. Disabil. Rehabil. 2010, 32, 1594–1603. [Google Scholar] [CrossRef] [PubMed]
- Salbach, N.M.; Mayo, N.E.; Higgins, J.; Ahmed, S.; Finch, L.E.; Richards, C.L. Responsiveness and predictability of gait speed and other disability measures in acute stroke. Arch. Phys. Med. Rehabil. 2001, 82, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar]
- Kim, S.J.; Cho, H.Y.; Kim, Y.L.; Lee, S.M. Effects of stationary cycling exercise on the balance and gait abilities of chronic stroke patients. J. Phys. Ther. Sci. 2015, 27, 3529–3531. [Google Scholar] [CrossRef]
- Yang, H.C.; Lee, C.L.; Lin, R.; Hsu, M.J.; Chen, C.H.; Lin, J.H.; Lo, S.K. Effect of biofeedback cycling training on functional recovery and walking ability of lower extremity in patients with stroke. Kaohsiung J. Med. Sci. 2014, 30, 35–42. [Google Scholar] [CrossRef]
- Bonnyaud, C.; Pradon, D.; Zory, R.; Bensmail, D.; Vuillerme, N.; Roche, N. Does a single gait training session performed either overground or on a treadmill induce specific short-term effects on gait parameters in patients with hemiparesis? A randomized controlled study. Top Stroke Rehabil. 2013, 20, 509–518. [Google Scholar] [CrossRef]
- Sakamoto, K.; Nakamura, T.; Uenishi, H.; Umemoto, Y.; Arakawa, H.; Abo, M.; Saura, R.; Fujiwara, H.; Kubo, T.; Tajima, F. Immediate effects of unaffected arm exercise in poststroke patients with spastic upper limb hemiparesis. Cerebrovasc. Dis. 2014, 37, 123–127. [Google Scholar] [CrossRef]
- Fujimoto, J.; Umemoto, Y.; Koike, Y.; Isida, K.; Sakamoto, K.; Tajima, F. Immediate effects of short period lower limb ergometer exercise in adolescent and young adult patients with cerebral palsy and spastic diplegia. J. Phys. Ther. Sci. 2021, 33, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Kesar, T.M.; Perumal, R.; Reisman, D.S.; Jancosko, A.; Rudolph, K.S.; Higginson, J.S.; Binder-Macleod, S.A. Functional electrical stimulation of ankle plantarflexor and dorsiflexor muscles: Effects on poststroke gait. Stroke 2009, 40, 3821–3827. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.H.; Willerslev-Olsen, M.; Conway, B.A.; Nielsen, J.B. The motor cortex drives the muscles during walking in human subjects. J. Physiol. 2012, 590, 2443–2452. [Google Scholar] [CrossRef] [PubMed]
- Khaslavskaia, S.; Ladouceur, M.; Sinkjaer, T. Increase in tibialis anterior motor cortex excitability following repetitive electrical stimulation of the common peroneal nerve. Exp. Brain Res. 2002, 145, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, J.; Tintera, J.; Gawehn, J.; Stoeter, P.; Treede, R.D. Functional MRI of human primary somatosensory and motor cortex during median nerve stimulation. Clin. Neurophysiol. 1999, 110, 47–52. [Google Scholar] [CrossRef]
- Kampe, K.K.; Jones, R.A.; Auer, D.P. Frequency dependence of the functional MRI response after electrical median nerve stimulation. Hum. Brain Mapp. 2000, 9, 106–114. [Google Scholar] [CrossRef]
- Kimberley, T.J.; Lewis, S.M.; Auerbach, E.J.; Dorsey, L.L.; Lojovich, J.M.; Carey, J.R. Electrical stimulation driving functional improvements and cortical changes in subjects with stroke. Exp. Brain Res. 2004, 154, 450–460. [Google Scholar] [CrossRef]
- Khaslavskaia, S.; Sinkjaer, T. Motor cortex excitability following repetitive electrical stimulation of the common peroneal nerve depends on the voluntary drive. Exp. Brain Res. 2005, 162, 497–502. [Google Scholar] [CrossRef]
| Factor | Overall (n = 20) | A (n = 9) | B (n = 11) |
|---|---|---|---|
| Age (years) | 73 [31, 89] | 72 [31, 84] | 74 [55, 89] |
| Sex: Male, Female | 8, 12 | 4, 5 | 4, 7 |
| Body height (m) | 1.53 [1.39, 1.74] | 1.57 [1.41, 1.74] | 1.52 [1.39, 1.73] |
| Body weight (kg) | 51.85 [43, 80] | 51.80 [44.5, 80] | 53.80 [43, 77.7] |
| Affected side: Right, Left | 10, 10 | 4, 5 | 6, 5 |
| Lesion: Hemorrhagic, Ischemic | 12, 8 | 6, 3 | 6, 5 |
| Use of orthotics (%) | 8 (40.0) | 4 (44.4) | 4 (36.4) |
| mRS score | 2.00 [1, 4] | 2.00 [1, 3] | 2.00 [1, 4] |
| MAS score on affected side (0/1/1+/2/3/4) | |||
| Knee extension | 17/2/1/0/0/0 | 6/2/1/0/0/0 | 11/0/0/0/0/0 |
| Knee flexion | 10/3/7/0/0/0 | 4/1/4/0/0/0 | 6/2/3/0/0/0 |
| Ankle dorsiflexion | 0/1/10/5/4/0 | 0/1/2/3/3/0 | 0/0/8/2/1/0 |
| Ankle plantar flexion | 19/0/0/1/0/0 | 8/0/0/1/0/0 | 11/0/0/0/0/0 |
| Stepping + FES Group | Stepping Group | Mixed Effect Model | ||||
|---|---|---|---|---|---|---|
| Mean [95%CIs] | p-Value | Mean [95%CIs] | p-Value | Coeff [95%CIs] | p-Value | |
| 10 m walking time (s) | −1.35 [−0.59, −2.12] | 0.001 | −0.59 [0.62, −1.80] | 0.318 | −0.68 [0.57, −1.88] | 0.306 |
| 10 m walking steps (step) | −1.02 [0.00, −2.05] | 0.05 | 0.17 [1.48, −1.13] | 0.783 | −1.18 [−0.09, −2.18] | 0.026 |
| 10 m walking speed (m/s) | 0.10 [0.14, 0.06] | <0.001 | 0.08 [0.12, 0.04] | <0.001 | −0.02 [−0.04, 0.00] | 0.048 |
| Cadence (step/min) | 6.69 [3.05, 10.32] | 0.001 | 7.84 [4.72, 10.97] | <0.001 | −1.38 [2.35, −5.08] | 0.461 |
| Stepping + FES Group | Stepping Group | Mixed Effect Model | ||||||
|---|---|---|---|---|---|---|---|---|
| Before | After | p Value | Before | After | p Value | 95%Coefficient Interval | p Value | |
| ROM (degree) | ||||||||
| Knee extension | 2.75 ± 3.02 | 3.75 ± 3.19 | 0.042 | 2.00 ± 4.10 | 3.00 ± 4.10 | 0.214 | 0.16 [1.51, −1.15] | 0.817 |
| Knee flexion | 148.5 ± 9.1 | 148.8 ± 9.2 | 0.666 | 147.5 ± 8.4 | 149.2 ± 7.8 | 0.015 | −1.50 [0.13, −3.38] | 0.089 |
| Ankle dorsiflexion (knee extension position) | 4.25 ± 7.12 | 10.25 ± 8.03 | <0.001 | 4.75 ± 5.50 | 10.25 ± 5.95 | <0.001 | 0.34 [2.39, −1.86] | 0.748 |
| Ankle dorsiflexion (knee flexion position) | 17.25 ± 9.52 | 24.25 ± 8.63 | <0.001 | 16.00 ± 7.88 | 22.00 ± 8.01 | <0.001 | 1.22 [3.75, −1.25] | 0.343 |
| Ankle plantar flexion | 58.75 ± 11.68 | 61.75 ± 7.48 | 0.062 | 60.25 ± 7.16 | 63.00 ± 7.68 | 0.001 | −0.18 [2.50, −2.71] | 0.892 |
| Ankle inversion | 48.25 ± 11.84 | 53.50 ± 8.44 | 0.007 | 45.25 ± 10.94 | 45.75 ± 9.22 | 0.776 | 6.39 [9.86, 2.69] | <0.001 |
| Ankle eversion | 4.75 ± 10.19 | 9.00 ± 8.37 | 0.009 | 4.50 ± 6.26 | 8.25 ± 5.20 | 0.001 | 0.74 [3.59, −1.92] | 0.612 |
| FMA Score | 23.90 ± 4.68 | 25.65 ± 4.74 | 0.001 | 24.20 ± 5.34 | 26.10 ± 4.42 | 0.001 | −0.11 [0.94, −1.17] | 0.834 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueda, K.; Umemoto, Y.; Kamijo, Y.-i.; Sakurai, Y.; Araki, S.; Ise, M.; Yoshioka, I.; Banno, M.; Mochida, S.; Iwahashi, T.; et al. Effects of Combination of Functional Electric Stimulation and Robotic Leg Movement Using Dynamic Tilt Table on Walking Characteristics in Post-Stroke Patients with Spastic Hemiplegia: A Randomized Crossover-Controlled Trial. J. Clin. Med. 2022, 11, 6911. https://doi.org/10.3390/jcm11236911
Ueda K, Umemoto Y, Kamijo Y-i, Sakurai Y, Araki S, Ise M, Yoshioka I, Banno M, Mochida S, Iwahashi T, et al. Effects of Combination of Functional Electric Stimulation and Robotic Leg Movement Using Dynamic Tilt Table on Walking Characteristics in Post-Stroke Patients with Spastic Hemiplegia: A Randomized Crossover-Controlled Trial. Journal of Clinical Medicine. 2022; 11(23):6911. https://doi.org/10.3390/jcm11236911
Chicago/Turabian StyleUeda, Koki, Yasunori Umemoto, Yoshi-ichiro Kamijo, Yuta Sakurai, Shohei Araki, Masato Ise, Izumi Yoshioka, Motohiko Banno, Satoshi Mochida, Takaya Iwahashi, and et al. 2022. "Effects of Combination of Functional Electric Stimulation and Robotic Leg Movement Using Dynamic Tilt Table on Walking Characteristics in Post-Stroke Patients with Spastic Hemiplegia: A Randomized Crossover-Controlled Trial" Journal of Clinical Medicine 11, no. 23: 6911. https://doi.org/10.3390/jcm11236911
APA StyleUeda, K., Umemoto, Y., Kamijo, Y.-i., Sakurai, Y., Araki, S., Ise, M., Yoshioka, I., Banno, M., Mochida, S., Iwahashi, T., Shimokawa, T., Nishimura, Y., & Tajima, F. (2022). Effects of Combination of Functional Electric Stimulation and Robotic Leg Movement Using Dynamic Tilt Table on Walking Characteristics in Post-Stroke Patients with Spastic Hemiplegia: A Randomized Crossover-Controlled Trial. Journal of Clinical Medicine, 11(23), 6911. https://doi.org/10.3390/jcm11236911







