Secretoneurin as a Novel Biomarker of Cardiovascular Episodes: Are We There Yet? A Narrative Review
Abstract
1. Introduction
- Provide independent information about the risk and prognosis of the studied disease;
- Account for a significant proportion of the identified risk;
- At best, be useful for stratifying the disease into clinically relevant categories;
- Be easily reproducible with low inter-sample variation, high sensitivity, and high specificity.
2. Basic Properties of Secretoneurin
3. Biological Effects of Secretoneurin
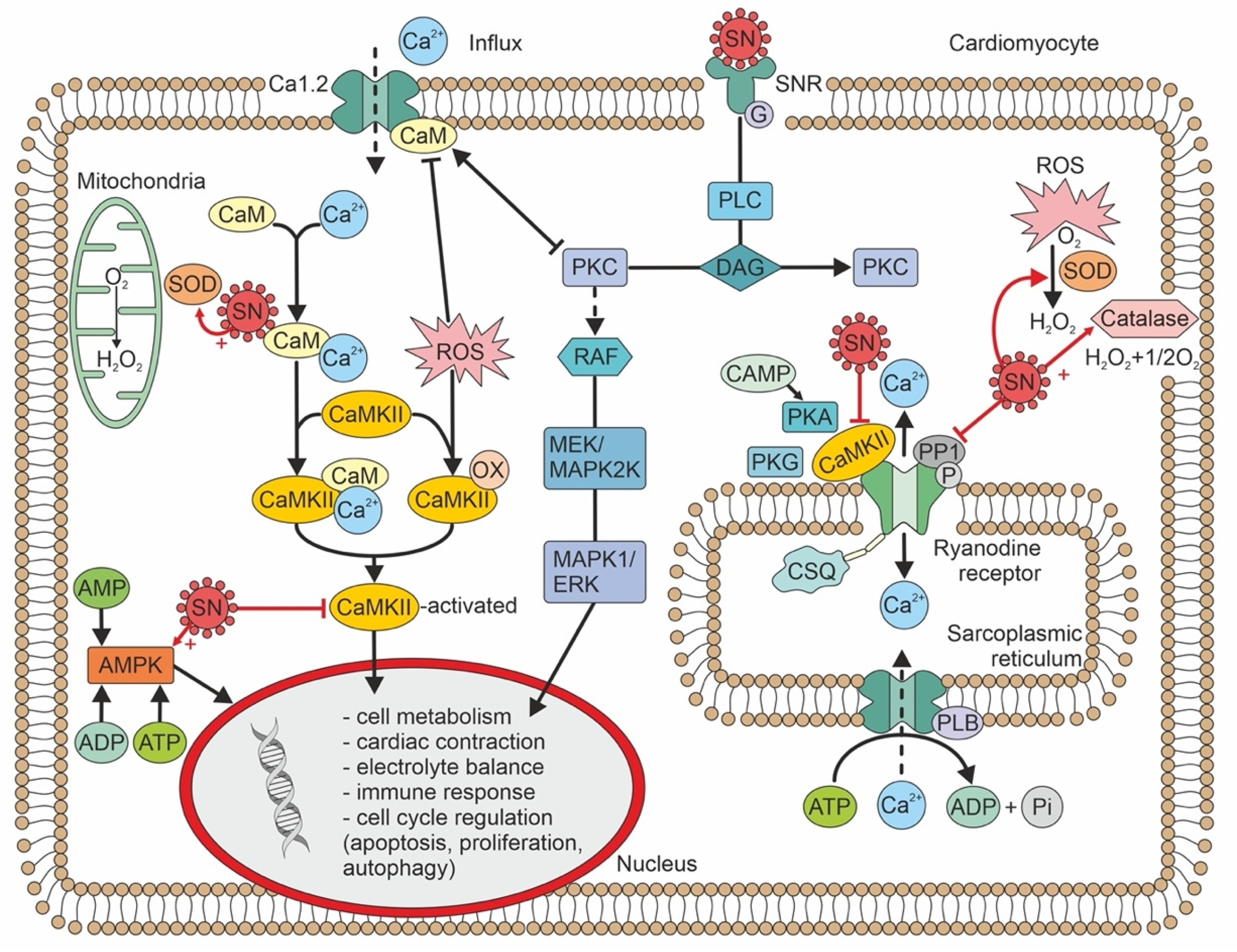
4. Cellular Pathophysiology of Secretoneurin
5. Plasma Secretoneurin Determination/Normal Range
6. Clinical Utility of Secretoneurin
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, M.E. Will Secretoneurin be the next big thing. J. Am. Coll. Cardiol. 2015, 65, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.P.; Marx, S.O. Secretoneurin to the rescue? Maybe or Maybe not? Circ. Arrhythm. Electrophysiol. 2019, 12, e007298. [Google Scholar] [CrossRef] [PubMed]
- Ottesen, A.H.; Louch, W.E.; Carlson, C.R.; Landsverk, O.J.; Kurola, J.; Johansen, R.F.; Moe, M.K.; Aronsen, J.M.; Høiseth, A.D.; Jarstadmarken, H.; et al. Secretoneurin is a novel prognostic cardiovascular biomarker associated with cardiomyocyte calcium handling. J. Am. Coll. Cardiol. 2015, 65, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Ottesen, A.H.; Carlson, C.R.; Eken, O.S.; Sadredini, M.; Myhre, P.L.; Shen, X.; Dalhus, B.; Laver, D.R.; Lunde, P.K.; Kurola, J.; et al. Secretoneurin is an Endogenous CaMKII Inhibitor that Attenuates Ca2+-Dependent Arrhythmia. Circ. Arrhythm. Electrophysiol. 2019, 12, 007045. [Google Scholar] [CrossRef] [PubMed]
- Trudeau, V.L.; Martyniuk, C.J.; Zhao, E.; Hu, H.; Volkoff, H.; Decatur, W.A.; Basak, A. Is secretoneurin a new hormone? Gen. Comp. Endocrinol. 2012, 175, 10–18. [Google Scholar] [CrossRef]
- Zhao, E.; Hu, H.; Trudeau, L.V. Secretoneurin as a hormone regulator in the pituitary. Regul. Pept. 2010, 165, 117–122. [Google Scholar] [CrossRef]
- Kirchmair, R.; Hogue-Angeletti, R.; Gutierrez, J.; Fischer-Colbrie, R.; Winkler, H. Secretineurin: A neuropeptide generated in brain, adrenal medulla and other endocrine tissues by proteolytic processing of secretogranin II (chromogranin C). Neuroscience 1993, 53, 359–365. [Google Scholar] [CrossRef]
- Zhao, E.; Basak, A.; Crump, K.; Trudeau, V.L. Proteolytic processing and differential distribution of secretogranin-II in goldfish. Gen. Comp. Endocrinol. 2006, 146, 100–107. [Google Scholar] [CrossRef]
- Morvan, J. Tooze SA. Discovery and progress in our understanding of the regulated secretory pathway in neuronedocrine cells. Histochem. Cell Biol. 2008, 129, 243–252. [Google Scholar] [CrossRef][Green Version]
- Pertl, C.; Kaufmann, W.; Amann, R.; Heinemann, A.; Ebeleseder, K.; Polansky, R.; Kim, S. Secretoneurin, a novel neuropeptide, in the human dental pulp. Arch. Oral Biol. 1998, 43, 361–365. [Google Scholar] [CrossRef]
- Jansson, A.M.; Røsjø, H.; Omland, T.; Karlsson, T.; Hartford, M.; Flyvbjerg, A.; Caidahl, K. Prognostic value of circulation chromogranin A levels in acute coronary syndromes. Eur. Heart J. 2009, 30, 25–32. [Google Scholar] [CrossRef]
- Røsjø, H.; Masson, S.; Latini, R.; Flyvbjerg, A.; Milani, V.; La Rovere, M.T.; Revera, M.; Mezzani, A.; Tognoni, G.; Tavazzi, L.; et al. Prognostic value of chromogranin A in chornic heart failure: Data from the GISSI-Heart failure trial. Eur. Heart J. Fail. 2010, 12, 549–556. [Google Scholar] [CrossRef]
- Røsjø, H.; Husberg, C.; Dahl, M.B.; Stridsberg, M.; Sjaastad, I.; Finsen, A.V.; Carlson, C.R.; Øie, E.; Omland, T.; Christensen, G. Chromogranin B in heart failure: A putative cardiac biomarker expressed in the failing myocardium. Circ. Heart Fail. 2010, 3, 503–511. [Google Scholar] [CrossRef]
- Fischer-Colbrie, R.; Kirchmair, R.; Kahler, C.M.; Wiedermann, C.J.; Saria, A. Secretoneurin: A new player in angiogenesis and chemotaxis linking nerves, blood vessels and the immune system. Curr. Pept. Sci. 2005, 6, 373–385. [Google Scholar] [CrossRef]
- Steiner, R.; Colbrie-Fischer, R.; Bletsa, A.; Laimer, J.; Troger, J. Secretoneurin and PE-11 immunoreactivity in the human dental pulp. Arch. Oral Biol. 2018, 86, 13–17. [Google Scholar] [CrossRef]
- Mitchell, K.; Mikwar, M.; Da Fonte, D.; Lu, C.; Tao, B.; Peng, D.; Erandani, W.U.; Hu, W.; Trudeau, V.L. Secretoneurin is a secretogranin-2 derived hormonal peptide in vertebrate neuroendocrine systems. Gen. Comp. Endocrinol. 2020, 299, 113588. [Google Scholar] [CrossRef]
- Kirchmair, R.; Gander, R.; Egger, M.; Hanley, A.; Silver, M.; Ritsch, A.; Murayama, T.; Kaneider, N.; Sturm, W.; Kearny, M.; et al. The neuropeptide secretoneurin acts as a direct angiogenic cytokine in vitro and in vivo. Circulation 2004, 109, 777–783. [Google Scholar] [CrossRef]
- Schgoer, W.; Theurl, M.; Albrecht-Schgoer, K.; Jonach, V.; Koller, B.; Lener, D.; Franz, W.M.; Kirchmair, R. Secretoneurin gene therapy improves blood flow in an ischemia model in type 1 diabetic mice by enhancing therapeutic neovascularization. PLoS ONE 2013, 8, e74029. [Google Scholar] [CrossRef][Green Version]
- Albrecht-Schgoer, K.; Schgoer, W.; Holfeld, J.; Theurl, M.; Wiedemann, D.; Steger, C.; Gupta, R.; Semsroth, S.; Fischer-Colbrie, R.; Beer, A.G.; et al. The angiogenic factor secretoneurin induces coronary angiogenesis in a model of myocardial infarction by stimulation of vascular endothelial growth factor signaling in endothelial cells. Circulation 2012, 126, 2491–2501. [Google Scholar] [CrossRef]
- Assefa, F.; Lim, J.; Kim, J.A.; Ihn, H.J.; Lim, S.; Nam, S.H.; Bae, Y.C.; Park, E.K. Secretoneurin, a neuropeptide, enhances bone regeneration in a mouse calcarial bone defect model. Tissue Eng. Regen. Med. 2021, 18, 315–324. [Google Scholar] [CrossRef]
- Kahler, C.M.; Schratzberger, P.; Wiedermann, C.J. Response of vascular smooth muscle cells to the neuropeptide secretoneurin. A functional role for migration and proliferation in vitro. Arter. Thromb. Vasc. Biol. 1997, 17, 2029–2035. [Google Scholar] [CrossRef]
- Tanaka, K.; Honda, M.; Takabate, T. Redox regulation of MAPK pathways and cardiac hypertrophy in adult rat cardiac myocyte. J. Am. Coll. Cardiol. 2001, 37, 676–685. [Google Scholar] [CrossRef]
- Cingolani, H.E.; Ennis, I.L.; Aiello, E.A.; Pérez, N.G. Role of autocrine/paracrine mechanisms in response to myocardial strain. Pflug. Arch. 2011, 462, 29–38. [Google Scholar] [CrossRef]
- Chen, H.-L.; Liu, Y.; Jiang, W.; Wang, X.-X.; Yuan, G.-L.; Zhao, Y.-L.; Yu, C. Secretoneurin suppresses cardiac hypertrophy through suppression of oxidant stress. Eur. J. Pharmacol. 2018, 822, 13–24. [Google Scholar] [CrossRef]
- Chan, C.K.Y.; Vanhoutte, P.M. Secretoneurin facilitates endothelium-dependent relaxations in porcine coronary arteries. Am. J. Physiol. Circ. Physiol. 2011, 300, H1159–H1165. [Google Scholar] [CrossRef]
- Kähler, C.M.; Schratzberger, P.; Kaufmann, G.; Hochleitner, B.; Bechter, O.; Götsch, C.; Wöll, E.; Marschang, P.; Herold, M.; Wiedermann, C.J. Transendothelial migration of leukocytes and signaling in response to neuropeptide secretoneurin. Regul. Pept. 2002, 105, 35–46. [Google Scholar] [CrossRef]
- Reinisch, N.; Kirchmair, R.; Kähler, C.M.; Hogue-Angeletti, R.; Fischer-Colbrie, R.; Winkler, H.; Wiedermann, C.J. Attraction of human monocytes by the neuropeptide secretoneurin. FEBS Lett. 1993, 334, 41–44. [Google Scholar] [CrossRef]
- Zhao, E.; Zhang, D.; Basak, A.; Trudeau, V.L. New insights into granin-derived peptides: Evolution and endocrine roles. Gen. Comp. Endocrinol. 2009, 164, 161–174. [Google Scholar] [CrossRef]
- Ischia, R.; Gasser, R.W.; Fischer-Colbrie, R.; Eder, U.; Pagani, A.; Cubeddu, L.X.; Lovisetti-Scamihorn, P.; Finkenstedt, G.; Laslop, A.; Winkler, H. Levels and molecular properties of secretoneurin-immunoreactivity in the serum and urine of control and neuroendocrine tumor patients. J. Clin. Endocrinol. Metab. 2000, 85, 355–360. [Google Scholar] [CrossRef]
- Stridsberg, M.; Eriksson, B.; Janson, E.T. Measurement of secretogranins II, III, V and proconvertases 1/3 and 2 in plasma from patients with neuroendocrine tumours. Regul. Pept. 2008, 148, 95–98. [Google Scholar] [CrossRef]
- Taupenot, L.; Harper, K.L.; O’Connor, D.T. The chromogranin-secretogranin family. N. Engl. J. Med. 2003, 348, 1134–1149. [Google Scholar] [CrossRef]
- Myhre, P.L.; Ottesen, A.H.; Faaren, A.L.; Tveit, S.H.; Springett, J.; Pyylampi, J.; Stridsberg, M.; Christensen, G.; Høiseth, A.D.; Omland, T.; et al. Performance of a novel research-use-only secretoneurin ELISA in patients with suspected acute coronary syndrome: Comparison with an established secretoneurin radioimmunoassay. Cardiology 2021, 146, 566–574. [Google Scholar] [CrossRef]
- Yuan, G.; Chen, H.; Xia, C.; Gao, L.; Yu, C. Ultrasensitive electrochemical detection of secretoneurin based on Pb(2+)-decorated reduced graphene oxide-tetraethylene pentamine as a label. Biosens. Bioelectron. 2015, 69, 95–99. [Google Scholar] [CrossRef]
- Aakre, K.M.; Ottesen, A.H.; Strand, H.; Faaren, A.L.; Alaour, B.; Torsvik, J.; Sylte, M.S.; Marber, M.; Christensen, G.; Røsjø, H.; et al. Biologial variation of secretoneurin;a novel cardiovascular biomarker implicated in arrhythmogenesis. Clin. Biochem. 2021, 98, 74–77. [Google Scholar] [CrossRef]
- Kujala, K.; Paavola, J.; Lahti, A.; Larsson, K.; Pekkanen-Mattila, M.; Viitasalo, M.; Lahtinen, A.M.; Toivonen, L.; Kontula, K.; Swan, H.; et al. Cell model of catecholaminergic polymorphic ventricular tachycardia reveals early and delayed afterdepolarizations. PLoS ONE 2012, 7, e44660. [Google Scholar] [CrossRef]
- Brynildsen, J.; Petäjä, L.; Myhre, P.L.; Lyngbakken, M.N.; Nygård, S.; Stridsberg, M.; Christensen, G.; Ottesen, A.H.; Pettilä, V.; Omland, T.; et al. Circulating Secretoneurin concentrations after cardiac surgery: Data from the FINNish Acute Kidney injury Heart study. Crit. Care Med. 2019, 47, e412–e419. [Google Scholar] [CrossRef]
- Christensen, G.; Pettilä, V.Y.O.; Linko, R.; Okkonen, M.; Omland, T.; Nygård, S.; Røsjø, H.; Ottesen, A.H.; Myhre, P.L.; Stridsberg, M. Prognostic value of secretoneurin in patients with acute respiratory failure: Data from the FINNALI study. Clin. Chem. 2016, 62, 1380–1389. [Google Scholar]
- Linko, R.; Omland, T.; Ottesen, A.H.; Karlsson, S.; Christensen, G.; Røsjø, H.; Varpula, T.; Stridsberg, M.; Pettilä, V.Y.O.; Nygård, S.; et al. Prognostic value of secretoneurin in critically ill patients with infections. Crit. Care 2016, 44, 1882–1890. [Google Scholar]
- Brynildsen, J.; Myhre, P.L.; Lyngbakken, M.N.; Klaeboe, L.G.; Stridsberg, M.; Christensen, G.; Edvardsen, T.; Omland, T.; Røsjø, H. Circulating secretoneurin concentrations in patients with moderate to severe aortic stenosis. Clin. Biochem. 2019, 71, 17–23. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plášek, J.; Lazárová, M.; Dodulík, J.; Šulc, P.; Stejskal, D.; Švagera, Z.; Všianský, F.; Václavík, J. Secretoneurin as a Novel Biomarker of Cardiovascular Episodes: Are We There Yet? A Narrative Review. J. Clin. Med. 2022, 11, 7191. https://doi.org/10.3390/jcm11237191
Plášek J, Lazárová M, Dodulík J, Šulc P, Stejskal D, Švagera Z, Všianský F, Václavík J. Secretoneurin as a Novel Biomarker of Cardiovascular Episodes: Are We There Yet? A Narrative Review. Journal of Clinical Medicine. 2022; 11(23):7191. https://doi.org/10.3390/jcm11237191
Chicago/Turabian StylePlášek, Jiří, Marie Lazárová, Jozef Dodulík, Patrik Šulc, David Stejskal, Zdeněk Švagera, František Všianský, and Jan Václavík. 2022. "Secretoneurin as a Novel Biomarker of Cardiovascular Episodes: Are We There Yet? A Narrative Review" Journal of Clinical Medicine 11, no. 23: 7191. https://doi.org/10.3390/jcm11237191
APA StylePlášek, J., Lazárová, M., Dodulík, J., Šulc, P., Stejskal, D., Švagera, Z., Všianský, F., & Václavík, J. (2022). Secretoneurin as a Novel Biomarker of Cardiovascular Episodes: Are We There Yet? A Narrative Review. Journal of Clinical Medicine, 11(23), 7191. https://doi.org/10.3390/jcm11237191





