Risk Predictors of 3-Month and 1-Year Outcomes in Heart Failure Patients with Prior Ischemic Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Diagnosis of Prior Ischemic Stroke
2.3. Data Collection
2.4. Study Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics in the Entire Cohort
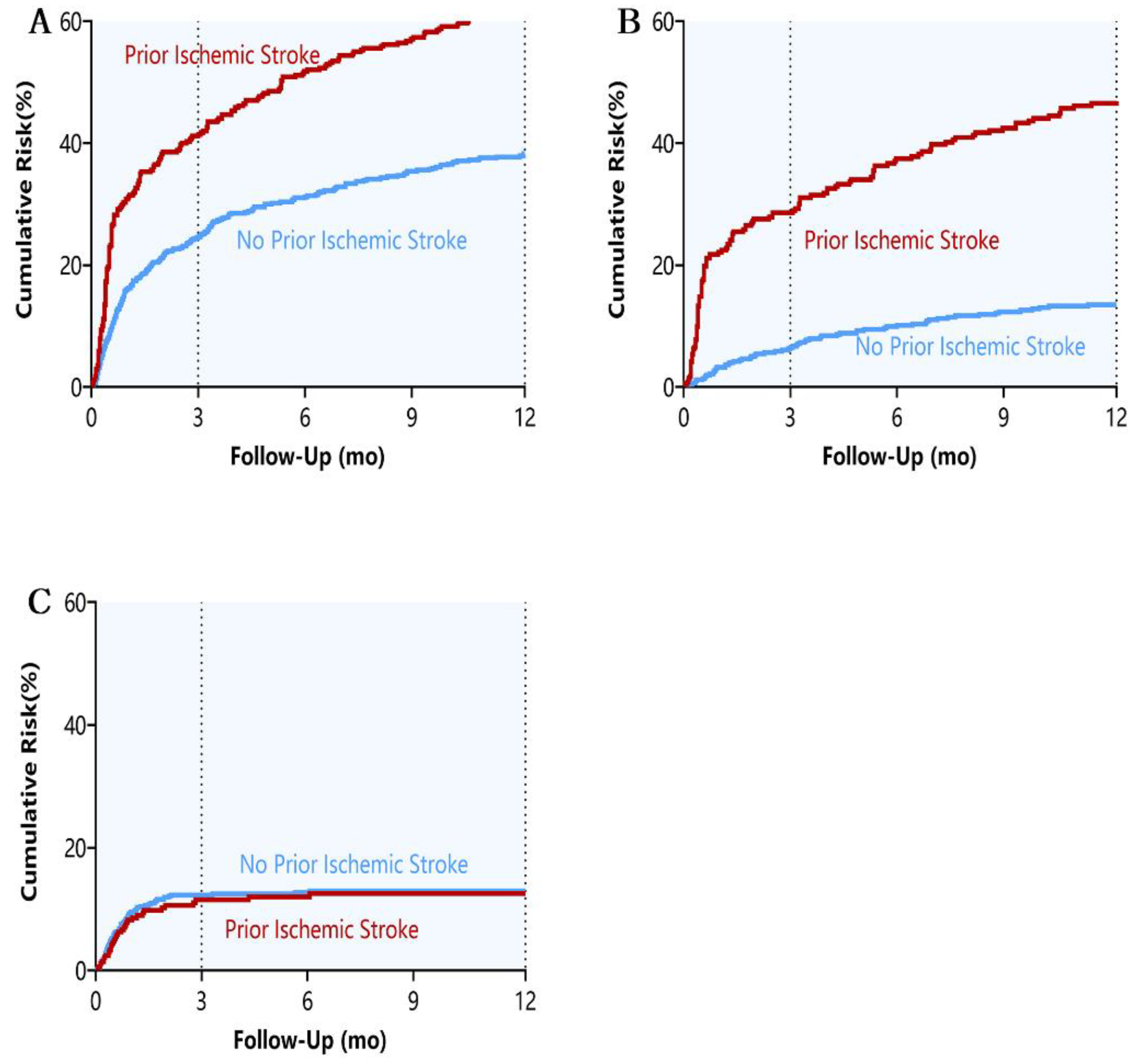
3.2. Month Outcomes
3.3. Year Outcomes
3.4. Multivariable Analysis of Factors for 3-Month All-Cause Readmission or Death in HF Patients with Prior Ischemic Stroke
3.5. Multivariable Analysis of Factors for 1-Year All-Cause Readmission or Death in HF Patients with Prior Ischemic Stroke
4. Discussion
4.1. Antiplatelet Therapy
4.2. Thyroid Disease
4.3. DBP
4.4. Beta-Blockers
4.5. Hypoalbuminemia and Hyperglycosemia
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ziaeian, B.; Fonarow, B.Z.G.C. Epidemiology and aetiology of heart failure. Nat. Rev. Cardiol. 2016, 13, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Wang, X.; Chen, Z.; Zhang, L.; Zhang, Y.; Wei, B.; Zheng, C.; Kang, Y.; Jiang, L.; Zhu, Z.; et al. Prevalence of heart failure and left ventricular dysfunction in China: The China Hypertension Survey, 2012–2015. Eur. J. Heart Fail. 2019, 21, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.J.; Fonarow, G.; Vaduganathan, M.; Khan, S.; Butler, J.; Gheorghiade, M. The vulnerable phase after hospitalization for heart failure. Nat. Rev. Cardiol. 2015, 12, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Vaduganathan, M.; Fonarow, G.C.; Bonow, R.O. Rehospitalization for Heart Failure. J. Am. Coll. Cardiol. 2013, 61, 391–403. [Google Scholar] [CrossRef]
- The GBD 2016 Lifetime Risk of Stroke Collaborators; Feigin, V.L.; Nguyen, G.; Cercy, K.; Johnson, C.O.; Alam, T.; Parmar, P.G.; Abajobir, A.A.; Abate, K.H.; Abd-Allah, F.; et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N. Engl. J. Med. 2018, 379, 2429–2437. [Google Scholar] [CrossRef]
- Doehner, W.; Ural, D.; Haeusler, K.G.; Čelutkienė, J.; Bestetti, R.; Cavusoglu, Y.; Peña-Duque, M.A.; Glavas, D.; Iacoviello, M.; Laufs, U.; et al. Heart and brain interaction in patients with heart failure: Overview and proposal for a taxonomy. A position paper from the Study Group on Heart and Brain Interaction of the Heart Failure Association. Eur. J. Heart Fail. 2018, 20, 199–215. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.-X.; Tu, Q.; Tao, L.-Y.; Liu, G.; An, H.; Zhang, H.; Jin, J.-L.; Fan, J.-S.; Du, Y.-F.; et al. Impact of Prior Ischemic Stroke on Outcomes in Patients with Heart Failure―A Propensity-Matched Study. Circ. J. 2020, 84, 1797–1806. [Google Scholar] [CrossRef]
- Chinese Society of Cardiology of Chinese Medical Association, Editorial Board of Chinese Journal of Cardiology. Chinese guidelines for the diagnosis and treatment of heart failure 2014. Zhonghua Xin Xue Guan Bing Za Zhi 2014, 42, 98–122. [Google Scholar]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef]
- Kernan, W.N.; Ovbiagele, B.; Black, H.R.; Bravata, D.M.; Chimowitz, M.I.; Ezekowitz, M.D.; Fang, M.C.; Fisher, M.; Furie, K.L.; Heck, D.V.; et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke 2014, 45, 2160–2236. [Google Scholar] [CrossRef]
- Metra, M.; Gheorghiade, M.; Bonow, R.O.; Cas, L.D. Postdischarge Assessment After a Heart Failure Hospitalization. Circulation 2010, 122, 1782–1785. [Google Scholar] [CrossRef]
- Zile, M.R.; Bennett, T.D.; Sutton, M.S.J.; Cho, Y.K.; Adamson, P.B.; Aaron, M.F.; Aranda, J.J.M.; Abraham, W.T.; Smart, F.W.; Stevenson, L.W.; et al. Transition from Chronic Compensated to Acute Decompensated Heart Failure. Circulation 2008, 118, 1433–1441. [Google Scholar] [CrossRef]
- Adamson, P.B.; Magalski, A.; Braunschweig, F.; Böhm, M.; Reynolds, D.; Steinhaus, D.; Luby, A.; Linde, C.; Ryden, L.; Cremers, B.; et al. Ongoing right ventricular hemodynamics in heart failure: Clinical value of measurements derived from an implantable monitoring system. J. Am. Coll. Cardiol. 2003, 41, 565–571. [Google Scholar] [CrossRef]
- Mohr, J.; Thompson, J.; Lazar, R.; Levin, B.; Sacco, R.; Furie, K.; Kistler, J.; Albers, G.; Pettigrew, L.; Adams, H.; et al. A Comparison of Warfarin and Aspirin for the Prevention of Recurrent Ischemic Stroke. N. Engl. J. Med. 2001, 345, 1444–1451. [Google Scholar] [CrossRef]
- Lin, H.-J.; Chang, W.-L.; Tseng, M.-C. Readmission after stroke in a hospital-based registry: Risk, etiologies, and risk factors. Neurology 2011, 76, 438–443. [Google Scholar] [CrossRef]
- Chaker, L.; Berg, M.E.V.D.; Niemeijer, M.N.; Franco, O.H.; Dehghan, A.; Hofman, A.; Rijnbeek, P.R.; Deckers, J.W.; Eijgelsheim, M.; Stricker, B.H.; et al. Thyroid Function and Sudden Cardiac Death. Circulation 2016, 134, 713–722. [Google Scholar] [CrossRef]
- Cooper, D.S.; Biondi, B. Subclinical thyroid disease. Lancet 2012, 379, 1142–1154. [Google Scholar] [CrossRef]
- Rodondi, N.; Bauer, D.C.; Cappola, A.R.; Cornuz, J.; Robbins, J.; Fried, L.P.; Ladenson, P.W.; Vittinghoff, E.; Gottdiener, J.S.; Newman, A.B. Subclinical Thyroid Dysfunction, Cardiac Function, and the Risk of Heart Failure: The Cardiovascular Health Study. J. Am. Coll. Cardiol. 2008, 52, 1152–1159. [Google Scholar] [CrossRef]
- Leonards, C.O.; Schneider, H.J.; Liman, T.G.; Fiebach, J.B.; Endres, M.; Ebinger, M. Thyroid-Stimulating Hormone, White Matter Hyperintensities, and Functional Outcome in Acute Ischemic Stroke Patients. Cerebrovasc. Dis. Extra 2014, 4, 61–68. [Google Scholar] [CrossRef]
- O’Keefe, L.M.; Conway, S.E.; Czap, A.; Malchoff, C.D.; Benashski, S.; Fortunato, G.; Staff, I.; McCullough, L.D. Thyroid hormones and functional outcomes after ischemic stroke. Thyroid Res. 2015, 8, 9. [Google Scholar] [CrossRef]
- Davis, P.J.; Glinsky, G.V.; Lin, H.-Y.; Mousa, S.A. Actions of Thyroid Hormone Analogues on Chemokines. J. Immunol. Res. 2016, 2016, 3147671. [Google Scholar] [CrossRef]
- Messerli, F.; Mancia, G.; Conti, C.; Hewkin, A.; Kupfer, S.; Champion, A.; Kolloch, R.; Benetos, A.; Pepine, C. Lowering of Blood Pressure—The Lower, the Better? J. Am. Soc. Nephrol. 2006, 17, 2345–2352. [Google Scholar] [CrossRef][Green Version]
- Bangalore, S.; Messerli, F.H.; Wun, C.-C.; Zuckerman, A.L.; DeMicco, D.; Kostis, J.B.; LaRosa, J.C.; for the Treating to New Targets Steering Committee and Investigators. J-curve revisited: An analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) Trial. Eur. Heart J. 2010, 31, 2897–2908. [Google Scholar] [CrossRef]
- McEvoy, J.W.; Chen, Y.; Rawlings, A.; Hoogeveen, R.C.; Ballantyne, C.M.; Blumenthal, R.S.; Coresh, J.; Selvin, E. Diastolic Blood Pressure, Subclinical Myocardial Damage, and Cardiac Events. J. Am. Coll. Cardiol. 2016, 68, 1713–1722. [Google Scholar] [CrossRef]
- Zhao, M.; Li, X.; Zheng, L.; Zhang, J.; Zhang, L.; Nguyen, T.; Sun, Y.; Li, J. Optimal Blood Pressure in Patients after Stroke in Rural Areas of China. J. Stroke Cerebrovasc. Dis. 2015, 25, 270–280. [Google Scholar] [CrossRef]
- Kjekshus, J.; Apetrei, E.; Barrios, V.; Boehm, M.; Cleland, J.G.F.; Cornel, J.H.; Dunselman, P.; Fonseca, C.; Goudev, A.; Grande, P.; et al. Rosuvastatin in Older Patients with Systolic Heart Failure. N. Engl. J. Med. 2007, 357, 2248–2261. [Google Scholar] [CrossRef]
- Sykora, M.; Siarnik, P.; Diedler, J.; Lees, K.R.; Alexandrov, A.; Bath, P.M.; Bluhmki, E.; Bornstein, N.; Claesson, L.; Davis, S.M.; et al. β-Blockers, Pneumonia, and Outcome After Ischemic Stroke. Stroke 2015, 46, 1269–1274. [Google Scholar] [CrossRef]
- Barron, S.A.; Rogovski, Z.; Hemli, J. Autonomic consequences of cerebral hemisphere infarction. Stroke 1994, 25, 113–116. [Google Scholar] [CrossRef]
- Makikallio, A.M.; Makikallio, T.H.; Korpelainen, J.T.; Sotaniemi, K.A.; Huikuri, H.V.; Myllyla, V.V. Heart rate dynamics predict poststroke mortality. Neurology 2004, 62, 1822–1826. [Google Scholar] [CrossRef]
- Sposato, L.A.; Hilz, M.J.; Aspberg, S.; Murthy, S.B.; Bahit, M.C.; Hsieh, C.-Y.; Sheppard, M.N.; Scheitz, J.F. Post-Stroke Cardiovascular Complications and Neurogenic Cardiac Injury. J. Am. Coll. Cardiol. 2020, 76, 2768–2785. [Google Scholar] [CrossRef]
- Sykora, M.; Diedler, J.; Turcani, P.; Hacke, W.; Steiner, T. Baroreflex: A New Therapeutic Target in Human Stroke? Stroke 2009, 40, e678–e682. [Google Scholar] [CrossRef] [PubMed]
- Ancion, A.; Allepaerts, S.; Oury, C.; Gori, A.-S.; Piérard, L.A.; Lancellotti, P. Serum albumin level and hospital mortality in acute non-ischemic heart failure. ESC Heart Fail. 2017, 4, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.P.; Lourenço, P.; Goncalves, F.; Ferreira, A.; Bettencourt, P. Nutritional markers and prognosis in cardiac cachexia. Int. J. Cardiol. 2011, 146, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Arques, S.; Ambrosi, P. Human Serum Albumin in the Clinical Syndrome of Heart Failure. J. Card. Fail. 2011, 17, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Dongaonkar, R.M.; Stewart, R.H.; Geissler, H.J.; Laine, G.A. Myocardial microvascular permeability, interstitial oedema, and compromised cardiac function. Cardiovasc. Res. 2010, 87, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Hankey, G.J. Nutrition and the risk of stroke. Lancet Neurol. 2012, 11, 66–81. [Google Scholar] [CrossRef]
- Leszczak, J.; Czenczek-Lewandowska, E.; Przysada, G.; Wyszyńska, J.; Weres, A.; Baran, J.; Kwolek, A.; Mazur, A. Diet after Stroke and Its Impact on the Components of Body Mass and Functional Fitness—A 4-Month Observation. Nutrients 2019, 11, 1227. [Google Scholar] [CrossRef]
- Didangelos, T.; Kantartzis, K. Diabetes and Heart Failure: Is it Hyperglycemia or Hyperinsulinemia? Curr. Vasc. Pharmacol. 2019, 17, 1–10. [Google Scholar] [CrossRef]

| Variable | Total Cohort | ||
|---|---|---|---|
| No Prior Ischemic Stroke (n = 611) | Prior Ischemic Stroke (n = 340) | p Value | |
| Age, years | 76.1 ± 6.4 | 78.6 ± 6.5 | 0.680 |
| Man | 313 (51.2) | 192 (56.5) | 0.120 |
| SBP, mmHg | 130.1 ± 24.0 | 130.2 ± 26.1 | 0.880 |
| DBP, mmHg | 73.3 ± 12.7 | 74.1 ± 15.4 | 0.041 |
| Heart rate, bpm | 78.6 ± 17.2 | 77.5 ± 15.8 | 0.331 |
| BMI, kg/m2 | 24.3 ± 4.2 | 24.8 ± 3.8 | 0.790 |
| History of smoking | 246 (40.9) | 136 (30.5) | 0.892 |
| Co-morbidities | |||
| Coronary heart disease | 378 (61.9) | 258 (75.9) | <0.001 |
| Hypertension | 453 (74.1) | 287 (84.4) | <0.001 |
| Dyslipidemia | 296 (48.4) | 187 (55.0) | 0.052 |
| Diabetes mellitus | 225 (36.8) | 147 (43.2) | 0.053 |
| Atrial fibrillation | 195 (31.9) | 128 (37.6) | 0.075 |
| Anemia | 213 (34.9) | 135 (39.7) | 0.138 |
| CKD | 71 (11.6) | 75 (22.1) | <0.001 |
| Well control Cancer | 125 (20.5) | 85 (25.0) | 0.108 |
| COPD | 111 (18.2) | 45 (13.2) | 0.046 |
| Thyroid dysfunction | 33 (5.5) | 27 (7.9) | 0.145 |
| Laboratory findings | |||
| White cell, 109/L * | 7.6 ± 3.4 | 7.6 ± 3.2 | 0.773 |
| Neutrophil ratio, % * | 70.3 ± 12.5 | 70.7 ± 11.2 | 0.017 |
| Hemoglobin, g/L | 118.2 ± 22.4 | 117.9 ± 20.6 | 0.164 |
| Serum albumin, g/L * | 36.6 ± 4.6 | 36.5 ± 4.8 | 0.197 |
| Glucose, mmol/L | 7.3 ± 3.3 | 7.7 ± 3.6 | 0.805 |
| HbA1c, % | 6.8 ± 1.4 | 6.6 ± 1.2 | 0.359 |
| Serum sodium, mmol/L * | 139.8 ± 4.5 | 138.7 ± 5.3 | 0.110 |
| Serum potassium, mmol/L * | 4.1 ± 0.6 | 4.2 ± 0.6 | 0.044 |
| Serum calcium, mmol/L | 2.1 ± 0.3 | 2.1 ± 0.3 | 0.988 |
| Total cholesterol, mmol/L | 3.9 ± 1.0 | 3.9 ± 1.1 | 0.056 |
| Triglycerides, mmol/L | 1.2 ± 0.8 | 1.3 ± 1.0 | 0.191 |
| LDL-C, mmol/L | 2.3 ± 0.8 | 2.4 ± 1.0 | 0.028 |
| HDL-C, mmol/L | 1.0 ± 0.3 | 0.9 ± 0.3 | 0.131 |
| Total bilirubin | 15.4 ± 12.3 | 14.8 ± 9.6 | 0.785 |
| Direct bilirubin | 5.8 ± 8.2 | 5.4 ± 5.5 | 0.682 |
| Serum creatinine, μmol/L | 110.3 ± 104.7 | 107.3 ± 80.8 | 0.049 |
| eGFR, ml/min/1.73 m2 | 68.7 ± 26.3 | 64.5 ± 25.8 | 0.790 |
| ≥60 | 380 (65.4) | 182 (57.8) | 0.025 |
| <60 | 201 (34.6) | 133 (42.2) | |
| ≥30 | 553 (90.5) | 308 (90.6) | 0.967 |
| <30 | 58 (9.5) | 32 (9.4) | |
| CRP * | 46.0 ± 65.0 | 48.0 ± 56.6 | 0.955 |
| NT-proBNP, pg/mL * | 1946.0 (734.1, 5284.1) | 1495.0 (466.5, 3477.75) | 0.003 |
| TSH, μIU/mL * | 4.0 ± 12.2 | 3.0 ± 5.3 | 0.046 |
| T3, ng/dL * | 83.6 ± 38.1 | 82.1 ± 25.0 | 0.204 |
| T4, ug/dL | 7.9 ± 2.3 | 7.7 ± 2.1 | 0.724 |
| FT3, pmol/L * | 3.7 ± 1.1 | 3.7 ± 0.7 | 0.039 |
| FT4, pmol/L * | 7.9 ± 2.3 | 7.7 ± 2.1 | 0.329 |
| LVEF, % | 56.7± 13.2 | 60.2 ± 11.6 | <0.001 |
| HFrEF | 58 (13.3) | 15 (7.7) | |
| HfmrEF | 66 (15.2) | 24 (12.4) | 0.018 |
| HfpEF | 311 (71.5) | 155 (79.9) | |
| Medications at discharge | |||
| ACEIs/ARBs/ARNI | 354 (57.9) | 210 (61.8) | 0.249 |
| β-blockers | 365 (59.7) | 202 (59.4) | 0.922 |
| MRAs | 233 (38.1) | 111 (32.6) | 0.090 |
| Statins | 265 (43.4) | 151 (44.4) | 0.757 |
| Loop diuretics | 440 (72.0) | 283 (83.2) | <0.001 |
| Nitrates | 274 (44.8) | 185 (54.4) | 0.005 |
| Digoxin | 145 (23.7) | 62 (18.2) | 0.047 |
| Antiplatelet drugs | 284 (46.5) | 202 (59.4) | <0.001 |
| Anticoagulants | 128 (20.9) | 38 (11.2) | <0.001 |
| Calcium channel blocker | 155 (25.4) | 122 (35.9) | 0.001 |
| Length of stay, days * | 19.9± 18.0 | 22.0 ± 18.6 | 0.733 |
| Events (%) | HR (95% CI) | p Value | ||
|---|---|---|---|---|
| No Prior Ischemic Stroke (n = 611) | Prior Ischemic Stroke (n = 340) | |||
| 3-month outcomes | ||||
| All-cause readmission | 97 (15.8) | 113 (33.2) | 2.33 (1.76–3.06) | <0.001 |
| HF readmission | 56 (9.2) | 22 (6.5) | 0.80 (0.49–1.31) | 0.378 |
| Non-HF readmission | 40 (6.5) | 91 (26.8) | 4.48 (3.09–6.51) | <0.001 |
| All-cause death | 70 (11.5) | 32 (9.4) | 0.82 (0.54–1.25) | 0.363 |
| All-cause death or readmission | 199 (32.6) | 141 (41.5) | 1.75 (1.39–2.19) | <0.001 |
| 1-year outcomes | ||||
| All-cause readmission | 175 (32.2) | 181 (58.4) | 2.27 (1.84–2.79) | <0.001 |
| HF readmission | 102 (16.7) | 42 (12.4) | 0.92 (0.64–1.32) | 0.653 |
| Non-HF readmission | 73 (13.4) | 139 (44.8) | 4.12 (3.10–5.47) | <0.001 |
| All-cause death | 73 (11.9) | 35 (10.3) | 0.80 (0.53–1.20) | 0.797 |
| All-cause death or readmission | 243 (39.8) | 211 (62.1) | 1.87 (1.55–2.25) | <0.001 |
| Univariate Anslysis | Multivariate Analysis | |||||
| Variable | without 3-Month Readmission or Death n = 199 (58.5) | with 3-Month Readmission or Death n = 141 (41.5) | HR (95%CI) | p Value | HR (95%CI) | p Value |
| Age, years | 77.51 ± 6.11 | 80.06 ± 6.80 | 1.09 (0.91–1.30) | 0.220 | ||
| Man, n (%) | 116 (58.3) | 76 (53.9) | 1.61 (0.84–3.05) | 0.421 | ||
| SBP, mmHg | 133.35 ± 20.48 | 125.67 ± 32.06 | 1.49 (1.11–2.00) | 0.006 | 1.00 (0.99–1.01) | 0.447 |
| DBP, mmHg | 75.05 ± 12.49 | 72.87 ± 18.65 | 0.96 (0.94–0.98) | 0.026 | 0.99 (0.97–1.00) | 0.045 |
| Length of stay, days | 19.01 ± 15.58 | 26.16 ± 21.48 | 1.00 (0.99–1.01) | <0.001 | 1.00 (0.99–1.01) | 0.837 |
| eGFR, mL/min/1.73 m2 | 66.57 ± 23.83 | 61.00 ± 28.61 | 1.55 (1.28–1.87) | 0.006 | 1.00 (0.99–1.01) | 0.742 |
| eGFR ≥ 60 | 120 (61.2) | 62 (52.1) | ||||
| Infection | 22 (11.1) | 31 (22.3) | 1.87 (1.68–2.08) | 0.006 | 1.68 (1.00–2.82) | 0.050 |
| Serum albumin, g/L | 37.78 ± 4.27 | 34.69 ± 5.00 | 0.90 (0.87–0.93) | 0.018 | 0.95 (0.90–0.99) | 0.025 |
| Serum sodium, mmol/L | 139.47 ± 4.31 | 137.71 ± 6.37 | 0.95 (0.93–0.97) | 0.010 | 0.98 (0.95–1.02) | 0.336 |
| Serum potassium, mmol/L | 4.08 ± 0.54 | 4.28 ± 0.65 | 1.14 (0.81–1.60) | 0.068 | 1.08 (0.76–1.54) | 0.676 |
| Serum creatinine, μmol/L | 97.44 ± 53.31 | 121.39 ± 107.26 | 1.45 (1.24–1.70) | <0.001 | 1.16(0.87–1.55) | 0.341 |
| TSH, μIU/mL | 2.61 ± 3.17 | 3.60 ± 7.68 | 1.06 (0.99–1.13) | 0.033 | 1.04 (1.01–1.08) | 0.017 |
| Coronary heart disease | 139 (70.2) | 112 (79.4) | 0.84 (0.71–0.99) | 0.041 | 0.87 (0.51–1.46) | 0.588 |
| Cardiomyopathy | 1 (0.5) | 9 (6.4) | 4.10 (1.30–12.9) | 0.001 | 3.84 (1.52–9.72) | 0.005 |
| Valvular disorders | 14 (7.4) | 1 (0.7) | 0.25 (0.08–0.78) | 0.001 | 0.13 (0.02–1.01) | 0.051 |
| Anemia | 67 (33.7) | 68 (48.2) | 0.86 (0.78–0.94) | 0.007 | 0.69 (0.43–1.09) | 0.113 |
| Cancer | 43 (21.6) | 42 (29.8) | 0.80 (0.59–1.09) | 0.088 | 0.78 (0.50–1.23) | 0.290 |
| COPD | 20 (10.1) | 25 (17.7) | 0.78 (0.64–0.94) | 0.041 | 0.85 (0.48–1.50) | 0.566 |
| ACE-inhibitors/ARBs/ARNI | 150 (75.4) | 60 (42.6) | 0.59 (0.37–0.93) | <0.001 | 0.59 (0.37–0.93) | 0.024 |
| β-Blockers | 142 (71.4) | 60 (42.6) | 0.68 (0.47–0.97) | <0.001 | 0.62 (0.40–0.95) | 0.029 |
| MRAs | 76 (38.2) | 35 (24.8) | 0.88 (0.80–0.97) | 0.009 | 0.85 (0.53–1.38) | 0.513 |
| Antiplatelet drugs | 148 (74.4) | 37 (26.2) | 0.51 (0.32–0.82) | <0.001 | 0.51 (0.32–0.82) | 0.005 |
| Statins | 114 (57.3) | 37 (26.2) | 0.69 (0.41–1.16) | <0.001 | 0.69 (0.41–1.16) | 0.159 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | without 1 Year Readmission Or Death n = 129 (37.9) | with 1 Year Readmission Or Death n = 211 (62.1) | HR (95%CI) | p Value | HR (95%CI) | p Value |
| Age, years | 76.53 ± 5.75 | 79.82 ± 6.66 | 1.34(0.91–1.97) | 0.147 | ||
| Man, n (%) | 76 (58.9) | 116 (55.0) | 1.85(0.87–3.93) | 0.477 | ||
| SBP, mmHg | 133.44 ± 20.50 | 128.17 ± 28.93 | 1.37(0.97–1.09) | 0.086 | 1.00 (0.99–1.01) | 0.799 |
| DBP, mmHg | 75.30 ± 11.53 | 73.43 ± 17.28 | 0.87 (0.78–0.97) | 0.010 | 0.99 (0.98–1.00) | 0.164 |
| Heart rate, bpm | 78.29 ± 18.08 | 77.09 ± 14.18 | 0.89 (0.76–1.04) | 0.058 | 0.99 (0.97–1.00) | 0.040 |
| Length of stay, days | 17.91 ± 10.86 | 24.45 ± 21.64 | 1.17 (0.97–1.41) | 0.010 | 1.00 (0.99–1.01) | 0.684 |
| Hemoglobin, g/L | 122.23 ± 18.53 | 115.25 ± 21.32 | 1.17 (1.08–1.26) | 0.034 | 1.00 (0.99–1.02) | 0.401 |
| eGFR, mL/min/1.73 m2 | 68.08 ± 24.72 | 61.96 ± 26.35 | 1.03 (0.98–1.08) | 0.124 | ||
| eGFR ≥ 60 | 82 (63.6) | 100 (53.8) | 0.97 (0.64–1.47) | 0.082 | 0.97 (0.64–1.47) | 0.883 |
| Infection | 20 (15.5) | 33 (15.8) | 2.51 (1.48–4.26) | 0.944 | ||
| Serum albumin, g/L | 37.91 ± 4.18 | 35.64 ± 5.00 | 0.89 (0.81–0.97) | 0.013 | 0.93 (0.87–0.97) | 0.003 |
| Serum potassium, mmol/L | 4.06 ± 0.54 | 4.22 ± 0.62 | 1.55 (0.94–2.55) | 0.095 | 1.41 (0.98–2.03) | 0.063 |
| CRP | 22.63 ± 28.83 | 59.35 ± 62.25 | 1.35 (1.12–1.62) | 0.001 | 1.01 (1.00–1.02) | 0.028 |
| TSH, μIU/mL | 2.79 ± 3.71 | 3.10 ± 6.13 | 1.45 (0.872–2.42) | 0.442 | ||
| FT4, pmol/L | 15.77 ± 2.77 | 16.25 ± 3.54 | 1.15 (1.09–1.21) | 0.034 | 1.08 (1.01–1.15) | 0.018 |
| COPD | 11 (8.5) | 34 (16.1) | 0.92 (0.86–0.98) | 0.040 | 0.88 (0.44–1.77) | 0.727 |
| ACE-inhibitors/ARBs/ARNI | 105(81.4) | 105 (49.8) | 0.81 (0.69–0.94) | <0.001 | 0.68 (0.44–1.04) | 0.075 |
| β-Blockers | 102 (79.1) | 100 (47.4) | 0.61 (0.41–0.91) | <0.001 | 0.53 (0.35–0.80) | 0.003 |
| MRAs | 51 (39.5) | 60 (28.4) | 0.97 (0.67–1.40) | 0.035 | 1.14 (0.74–1.76) | 0.543 |
| Antiplatelet drugs | 96 (74.4) | 106 (50.2) | 0.58 (0.437–0.92) | <0.001 | 0.76 (0.48–1.19) | 0.232 |
| Statins | 79 (61.2) | 72 (34.1) | 0.62 (0.42–0.91) | <0.001 | 0.73 (0.47–1.16) | 0.182 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Wang, Y.; Ze, F.; Zhou, X.; Li, X.-B. Risk Predictors of 3-Month and 1-Year Outcomes in Heart Failure Patients with Prior Ischemic Stroke. J. Clin. Med. 2022, 11, 5922. https://doi.org/10.3390/jcm11195922
Li D, Wang Y, Ze F, Zhou X, Li X-B. Risk Predictors of 3-Month and 1-Year Outcomes in Heart Failure Patients with Prior Ischemic Stroke. Journal of Clinical Medicine. 2022; 11(19):5922. https://doi.org/10.3390/jcm11195922
Chicago/Turabian StyleLi, Ding, Yu Wang, Feng Ze, Xu Zhou, and Xue-Bin Li. 2022. "Risk Predictors of 3-Month and 1-Year Outcomes in Heart Failure Patients with Prior Ischemic Stroke" Journal of Clinical Medicine 11, no. 19: 5922. https://doi.org/10.3390/jcm11195922
APA StyleLi, D., Wang, Y., Ze, F., Zhou, X., & Li, X.-B. (2022). Risk Predictors of 3-Month and 1-Year Outcomes in Heart Failure Patients with Prior Ischemic Stroke. Journal of Clinical Medicine, 11(19), 5922. https://doi.org/10.3390/jcm11195922




