Impact of a Weight-Loss Rehabilitation Program on Sleep Apnea Risk and Subjective Sleepiness in Patients with Overweight/Obesity: The DietSleep Study
Abstract
1. Introduction
2. Materials and Methods
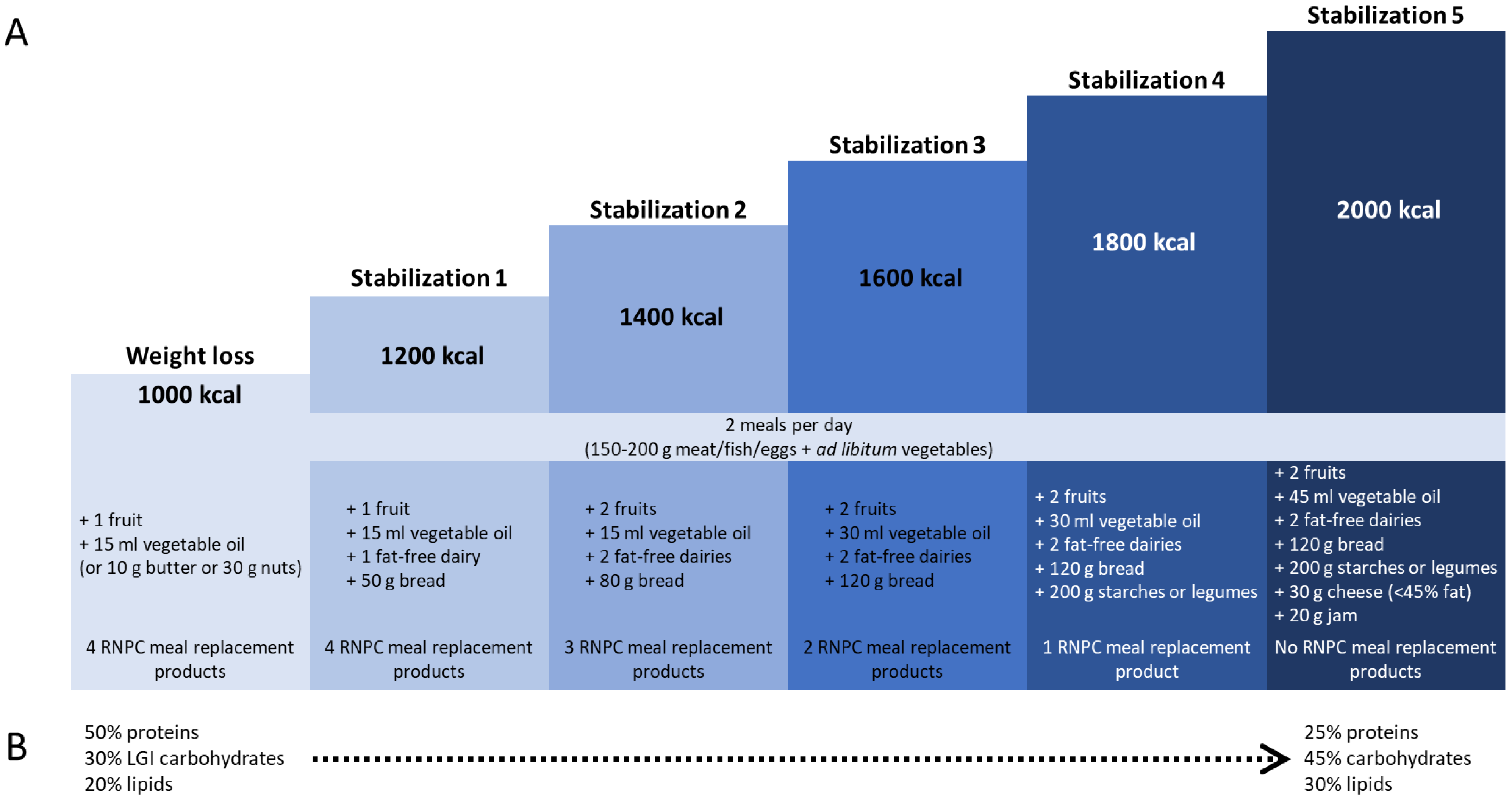
2.1. Study Design
2.2. Study Objectives
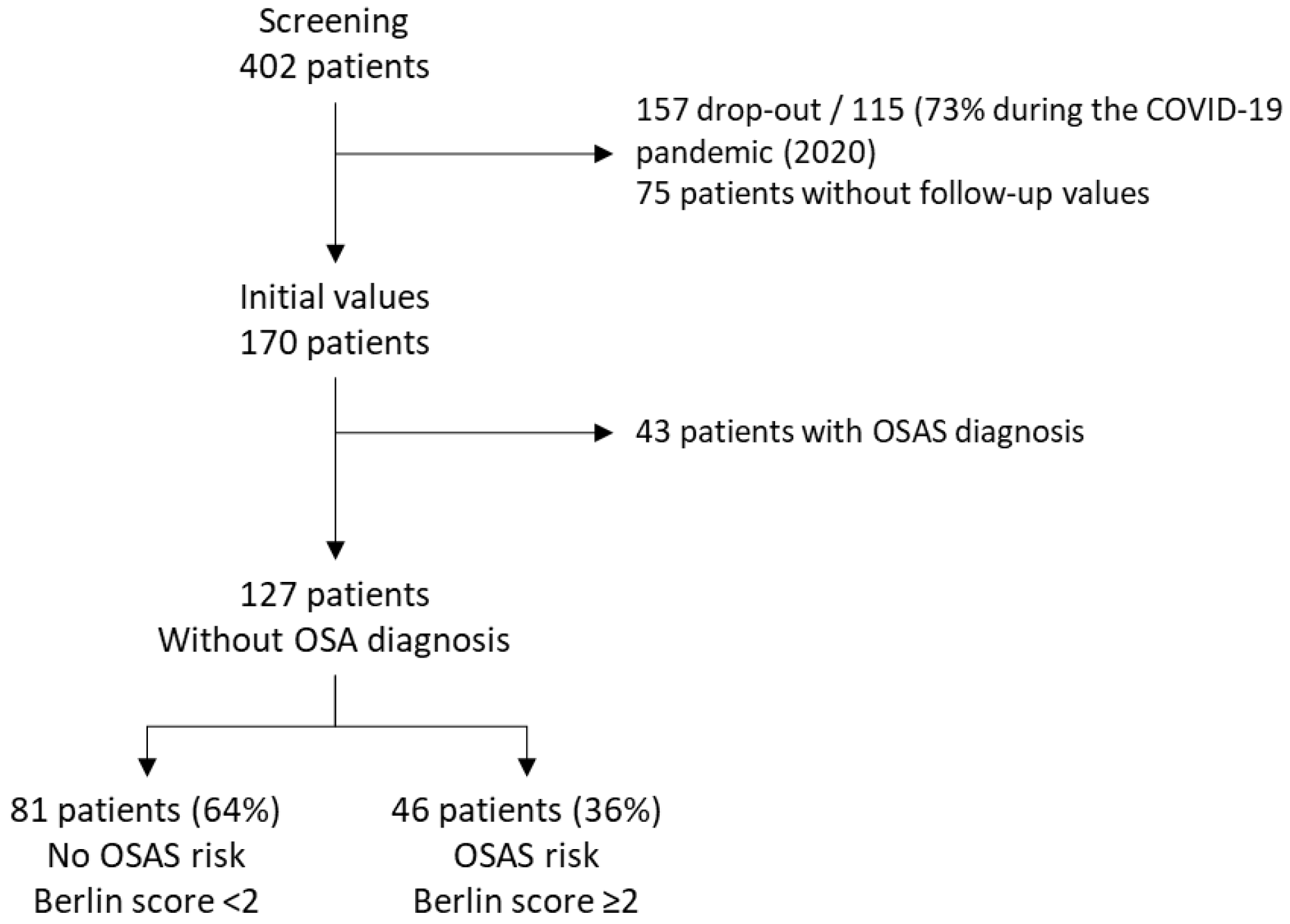
2.3. Study Population
2.4. Data Collection and Measures
2.5. Statistical Analyses
3. Results
3.1. Population
3.2. Prevalence of Patients at Risk of OSA and Sleepy at Baseline
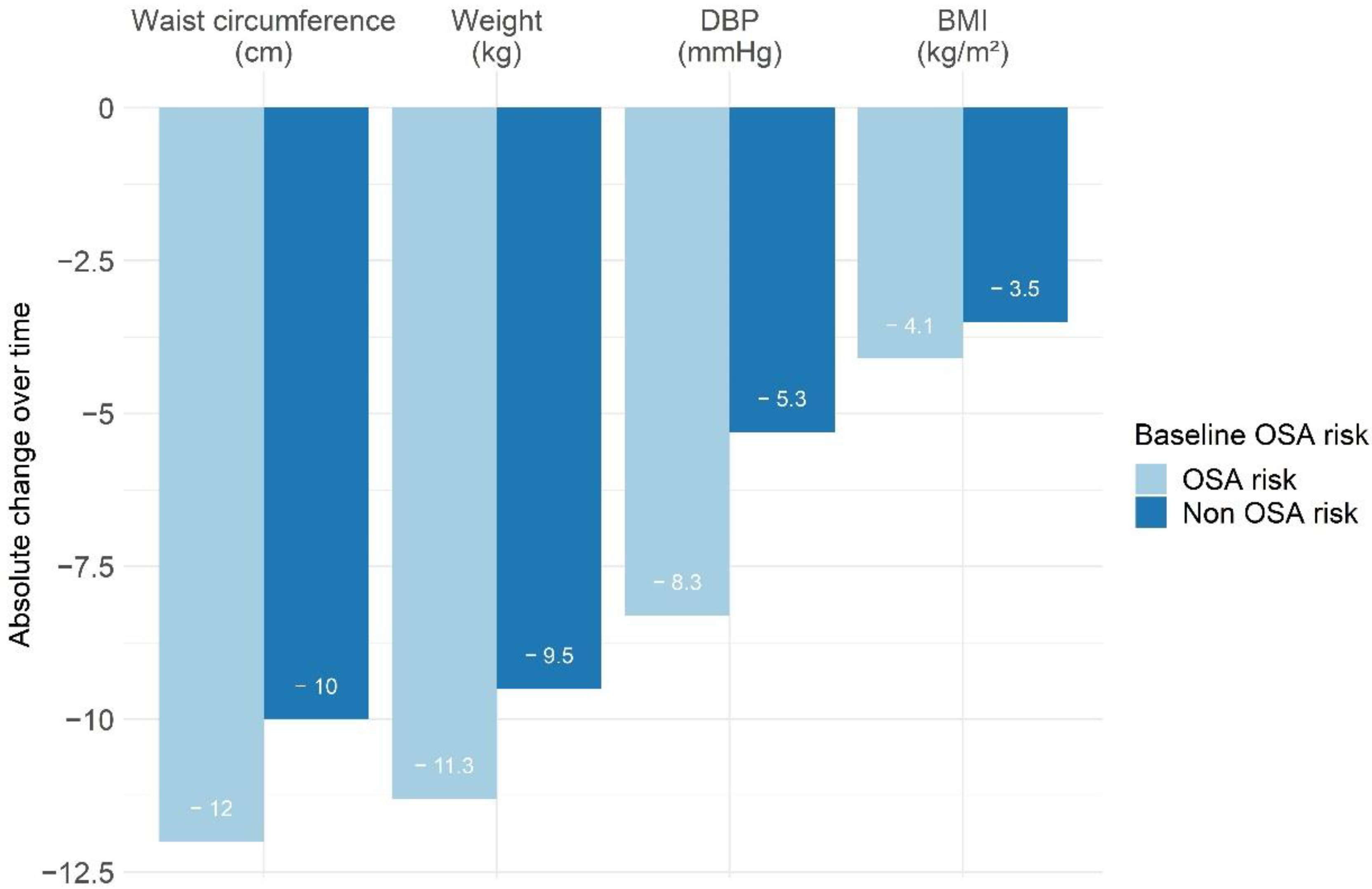
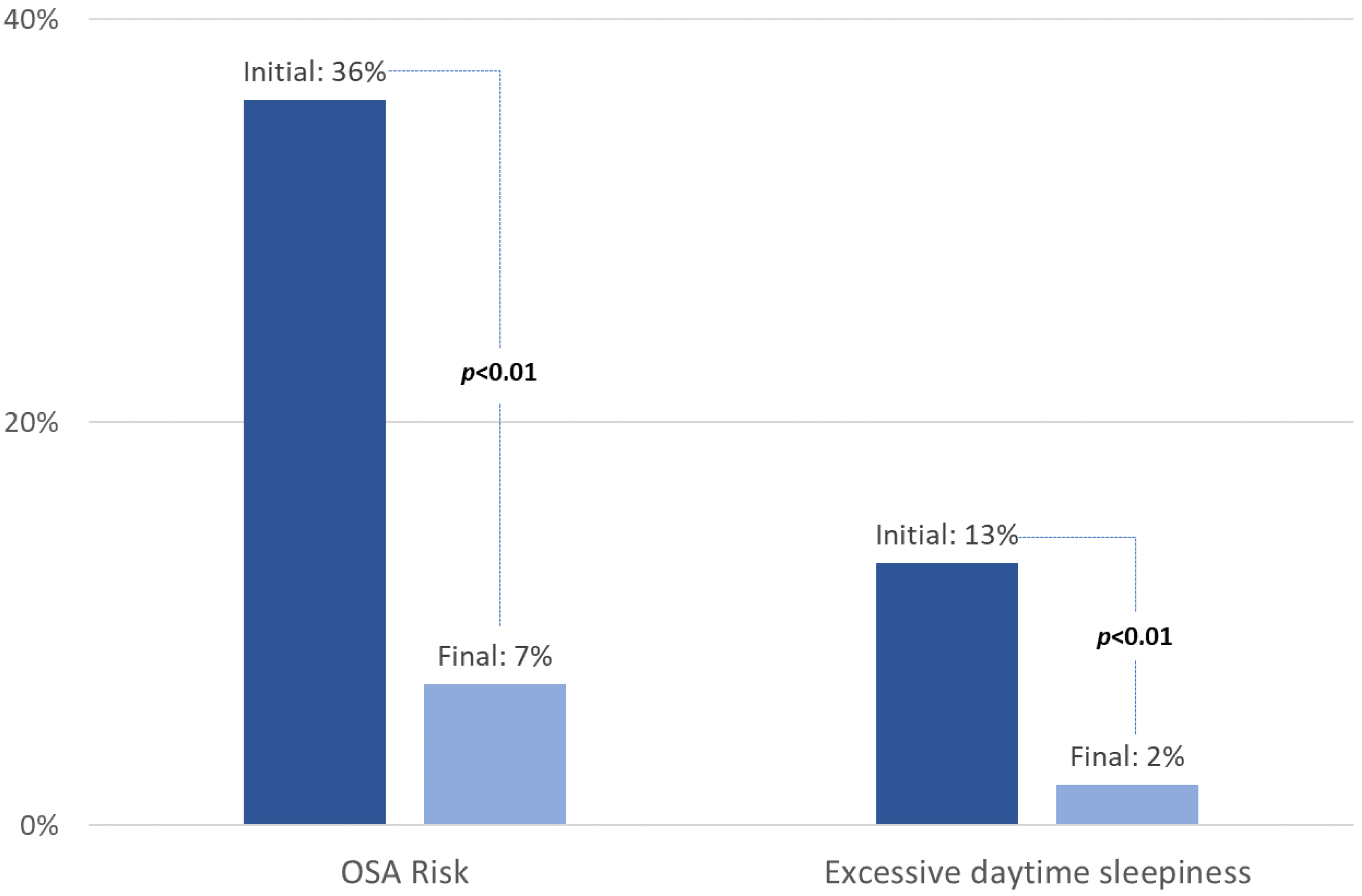
3.3. Changes in Anthropometric Measures, OSA Risk, and Excessive Daytime Sleepiness between Initiation and End of the Program according to Initial OSA Risk
3.4. Factor Associated with a Decrease in Berlin Score for Initial High Risk of OSA Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pepin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Levy, P.; Kohler, M.; McNicholas, W.T.; Barbe, F.; McEvoy, R.D.; Somers, V.K.; Lavie, L.; Pepin, J.L. Obstructive sleep apnoea syndrome. Nat. Rev. Dis. Primers 2015, 1, 15015. [Google Scholar] [CrossRef]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e56–e67. [Google Scholar] [CrossRef]
- Ryan, S.; Cummins, E.P.; Farre, R.; Gileles-Hillel, A.; Jun, J.C.; Oster, H.; Pepin, J.L.; Ray, D.W.; Reutrakul, S.; Sanchez-de-la-Torre, M.; et al. Understanding the pathophysiological mechanisms of cardiometabolic complications in obstructive sleep apnoea: Towards personalised treatment approaches. Eur. Respir. J. 2020, 56, 1902295. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Palta, M.; Dempsey, J.; Skatrud, J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000, 284, 3015–3021. [Google Scholar] [CrossRef]
- Wolk, R.; Shamsuzzaman, A.S.; Somers, V.K. Obesity, sleep apnea, and hypertension. Hypertension 2003, 42, 1067–1074. [Google Scholar] [CrossRef]
- Romero-Corral, A.; Caples, S.M.; Lopez-Jimenez, F.; Somers, V.K. Interactions between obesity and obstructive sleep apnea: Implications for treatment. Chest 2010, 137, 711–719. [Google Scholar] [CrossRef]
- Wu, Y.; Zhai, L.; Zhang, D. Sleep duration and obesity among adults: A meta-analysis of prospective studies. Sleep Med. 2014, 15, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Sperry, S.D.; Scully, I.D.; Gramzow, R.H.; Jorgensen, R.S. Sleep Duration and Waist Circumference in Adults: A Meta-Analysis. Sleep 2015, 38, 1269–1276. [Google Scholar] [CrossRef]
- Newman, A.B.; Foster, G.; Givelber, R.; Nieto, F.J.; Redline, S.; Young, T. Progression and regression of sleep-disordered breathing with changes in weight: The Sleep Heart Health Study. Arch. Intern. Med. 2005, 165, 2408–2413. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Keenan, B.T.; Wiemken, A.; Zang, Y.; Staley, B.; Sarwer, D.B.; Torigian, D.A.; Williams, N.; Pack, A.I.; Schwab, R.J. Effect of Weight Loss on Upper Airway Anatomy and the Apnea-Hypopnea Index. The Importance of Tongue Fat. Am. J. Respir. Crit. Care Med. 2020, 201, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Pepin, J.L.; Bailly, S.; Rinder, P.; Adler, D.; Szeftel, D.; Malhotra, A.; Cistulli, P.A.; Benjafield, A.; Lavergne, F.; Josseran, A.; et al. CPAP Therapy Termination Rates by OSA Phenotype: A French Nationwide Database Analysis. J. Clin. Med. 2021, 10, 936. [Google Scholar] [CrossRef]
- Pepin, J.L.; Bailly, S.; Rinder, P.; Adler, D.; Benjafield, A.V.; Lavergne, F.; Josseran, A.; Sinel-Boucher, P.; Tamisier, R.; Cistulli, P.A.; et al. Relationship Between CPAP Termination and All-Cause Mortality: A French Nationwide Database Analysis. Chest 2022, 161, 1657–1665. [Google Scholar] [CrossRef]
- Jain, S.; Gurubhagavatula, I.; Townsend, R.; Kuna, S.T.; Teff, K.; Wadden, T.A.; Chittams, J.; Hanlon, A.L.; Maislin, G.; Saif, H.; et al. Effect of CPAP, Weight Loss, or CPAP Plus Weight Loss on Central Hemodynamics and Arterial Stiffness. Hypertension 2017, 70, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Alruwaily, A.; Alruwaili, H.; Garvey, J.; le Roux, C.W. Can Weight Loss Improve the Cardiovascular Outcomes of Patients with Obesity and Obstructive Sleep Apnea? Hearts 2022, 3, 54–65. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Gurubhagavatula, I.; Teff, K.; Rader, D.J.; Wadden, T.A.; Townsend, R.; Foster, G.D.; Maislin, G.; Saif, H.; Broderick, P.; et al. CPAP, weight loss, or both for obstructive sleep apnea. N. Engl. J. Med. 2014, 370, 2265–2275. [Google Scholar] [CrossRef]
- Hudgel, D.W.; Patel, S.R.; Ahasic, A.M.; Bartlett, S.J.; Bessesen, D.H.; Coaker, M.A.; Fiander, P.M.; Grunstein, R.R.; Gurubhagavatula, I.; Kapur, V.K.; et al. The Role of Weight Management in the Treatment of Adult Obstructive Sleep Apnea. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e70–e87. [Google Scholar] [CrossRef]
- Randerath, W.; Verbraecken, J.; de Raaff, C.A.L.; Hedner, J.; Herkenrath, S.; Hohenhorst, W.; Jakob, T.; Marrone, O.; Marklund, M.; McNicholas, W.T.; et al. European Respiratory Society guideline on non-CPAP therapies for obstructive sleep apnoea. Eur. Respir. Rev. 2021, 30, 210200. [Google Scholar] [CrossRef]
- Amra, B.; Rahmati, B.; Soltaninejad, F.; Feizi, A. Screening Questionnaires for Obstructive Sleep Apnea: An Updated Systematic Review. Oman Med. J. 2018, 33, 184–192. [Google Scholar] [CrossRef]
- Senaratna, C.V.; Perret, J.L.; Matheson, M.C.; Lodge, C.J.; Lowe, A.J.; Cassim, R.; Russell, M.A.; Burgess, J.A.; Hamilton, G.S.; Dharmage, S.C. Validity of the Berlin questionnaire in detecting obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med. Rev. 2017, 36, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.S.; Tam, W.; Chan, T.O.; To, K.W.; Ngai, J.; Chan, K.K.P.; Yip, W.H.; Lo, R.L.; Yiu, K.; Ko, F.W.; et al. Use of Berlin questionnaire in comparison to polysomnography and home sleep study in patients with obstructive sleep apnea. Respir. Res. 2019, 20, 40. [Google Scholar] [CrossRef]
- Tan, A.; Yin, J.D.; Tan, L.W.; van Dam, R.M.; Cheung, Y.Y.; Lee, C.H. Using the Berlin Questionnaire to Predict Obstructive Sleep Apnea in the General Population. J. Clin. Sleep Med. 2017, 13, 427–432. [Google Scholar] [CrossRef]
- Thorning, T.K.; Fabre, O.; Legrand, R.; Astrup, A.; Hjorth, M.F. Weight loss and weight loss maintenance efficacy of a novel weight loss program: The retrospective RNPC® cohort. Obes. Med. 2018, 10, 16–23. [Google Scholar] [CrossRef]
- Bailly, S.; Fabre, O.; Legrand, R.; Pantagis, L.; Mendelson, M.; Terrail, R.; Tamisier, R.; Astrup, A.; Clement, K.; Pepin, J.L. The Impact of the COVID-19 Lockdown on Weight Loss and Body Composition in Subjects with Overweight and Obesity Participating in a Nationwide Weight-Loss Program: Impact of a Remote Consultation Follow-Up-The CO-RNPC Study. Nutrients 2021, 13, 2152. [Google Scholar] [CrossRef] [PubMed]
- Truche, A.S.; Bailly, S.; Fabre, O.; Legrand, R.; Zaoui, P. A Specific High-Protein Weight Loss Program Does Not Impair Renal Function in Patients Who Are Overweight/Obese. Nutrients 2022, 14, 384. [Google Scholar] [CrossRef] [PubMed]
- Netzer, N.C.; Stoohs, R.A.; Netzer, C.M.; Clark, K.; Strohl, K.P. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann. Intern. Med. 1999, 131, 485–491. [Google Scholar] [CrossRef]
- Carneiro-Barrera, A.; Amaro-Gahete, F.J.; Guillen-Riquelme, A.; Jurado-Fasoli, L.; Saez-Roca, G.; Martin-Carrasco, C.; Buela-Casal, G.; Ruiz, J.R. Effect of an Interdisciplinary Weight Loss and Lifestyle Intervention on Obstructive Sleep Apnea Severity: The INTERAPNEA Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e228212. [Google Scholar] [CrossRef]
- Kline, C.E.; Burke, L.E.; Sereika, S.M.; Imes, C.C.; Rockette-Wagner, B.; Mendez, D.D.; Strollo, P.J.; Zheng, Y.; Rathbun, S.L.; Chasens, E.R. Bidirectional Relationships Between Weight Change and Sleep Apnea in a Behavioral Weight Loss Intervention. Mayo. Clin. Proc. 2018, 93, 1290–1298. [Google Scholar] [CrossRef]
- Janney, C.A.; Kilbourne, A.M.; Germain, A.; Lai, Z.; Hoerster, K.D.; Goodrich, D.E.; Klingaman, E.A.; Verchinina, L.; Richardson, C.R. The Influence of Sleep Disordered Breathing on Weight Loss in a National Weight Management Program. Sleep 2016, 39, 59–65. [Google Scholar] [CrossRef]
- Borel, A.L.; Leblanc, X.; Almeras, N.; Tremblay, A.; Bergeron, J.; Poirier, P.; Despres, J.P.; Series, F. Sleep apnoea attenuates the effects of a lifestyle intervention programme in men with visceral obesity. Thorax 2012, 67, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Gaborit, B.; Jacquier, A.; Kober, F.; Abdesselam, I.; Cuisset, T.; Boullu-Ciocca, S.; Emungania, O.; Alessi, M.C.; Clement, K.; Bernard, M.; et al. Effects of bariatric surgery on cardiac ectopic fat: Lesser decrease in epicardial fat compared to visceral fat loss and no change in myocardial triglyceride content. J. Am. Coll. Cardiol. 2012, 60, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Baguet, J.P.; Hammer, L.; Levy, P.; Pierre, H.; Rossini, E.; Mouret, S.; Ormezzano, O.; Mallion, J.M.; Pepin, J.L. Night-time and diastolic hypertension are common and underestimated conditions in newly diagnosed apnoeic patients. J. Hypertens. 2005, 23, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Le-Dong, N.N.; Martinot, J.B.; Coumans, N.; Cuthbert, V.; Tamisier, R.; Bailly, S.; Pepin, J.L. Machine Learning-based Sleep Staging in Patients with Sleep Apnea Using a Single Mandibular Movement Signal. Am. J. Respir. Crit. Care Med. 2021, 204, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Pepin, J.L.; Letesson, C.; Le-Dong, N.N.; Dedave, A.; Denison, S.; Cuthbert, V.; Martinot, J.B.; Gozal, D. Assessment of Mandibular Movement Monitoring With Machine Learning Analysis for the Diagnosis of Obstructive Sleep Apnea. JAMA Netw. Open 2020, 3, e1919657. [Google Scholar] [CrossRef] [PubMed]
- Verhaert, D.V.M.; Betz, K.; Gawalko, M.; Hermans, A.N.L.; Pluymaekers, N.; van der Velden, R.M.J.; Philippens, S.; Vorstermans, B.; Simons, S.O.; den Uijl, D.W.; et al. A VIRTUAL Sleep Apnoea management pathway For the work-up of Atrial fibrillation patients in a digital Remote Infrastructure: VIRTUAL-SAFARI. Europace 2022, 24, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Johansson, K.; Hemmingsson, E.; Harlid, R.; Trolle Lagerros, Y.; Granath, F.; Rossner, S.; Neovius, M. Longer term effects of very low energy diet on obstructive sleep apnoea in cohort derived from randomised controlled trial: Prospective observational follow-up study. BMJ 2011, 342, d3017. [Google Scholar] [CrossRef]
- Jullian-Desayes, I.; Joyeux-Faure, M.; Tamisier, R.; Launois, S.; Borel, A.L.; Levy, P.; Pepin, J.L. Impact of obstructive sleep apnea treatment by continuous positive airway pressure on cardiometabolic biomarkers: A systematic review from sham CPAP randomized controlled trials. Sleep Med. Rev. 2015, 21, 23–38. [Google Scholar] [CrossRef]
- Kaikkonen, K.M.; Korpelainen, R.; Vanhala, M.L.; Keinanen-Kiukaanniemi, S.M.; Korpelainen, J.T. Long-term Effects on Weight Loss and Maintenance by Intensive Start with Diet and Exercise. Scand. J. Med. Sci. Sport. 2022, 1–11. [Google Scholar] [CrossRef]
| All Population N = 127 | Patients without Baseline OSA Risk N = 81 (55%) | Patients with Baseline OSA Risk N = 46 (45%) | p-Value | |
|---|---|---|---|---|
| Age (years) | 52 (44; 61) | 53 (42; 62) | 52 (45; 59) | 0.29 |
| Sex (female) | 109 (85.8) | 73 (90.1) | 36 (78.3) | 0.07 |
| Tobacco consumption/yes | 12 (9.4) | 7 (8.6) | 5 (10.9) | 0.68 |
| Arthrosis | 29 (22.8) | 15 (18.5) | 14 (30.4) | 0.12 |
| Hypertension * | 24 (18.9) | 11 (13.6) | 13 (28.3) | 0.04 |
| Diabetes * | 5 (3.9) | 1 (1.2) | 4 (8.7) | 0.04 |
| Depression * | 8 (6.3) | 4 (4.9) | 4 (8.7) | 0.40 |
| Anthropometric measures | ||||
| Weight (kg) | 81.2 (74.2; 91.3) | 78.8 (73.3; 84.4) | 90.5 (79.7; 107) | <0.01 |
| Height (cm) | 165 (160; 170) | 164 (161; 170) | 165 (160; 171) | 0.92 |
| Body mass index (kg/m2) | 29.7 (27.6; 34) | 28.4 (27.3; 31.2) | 33.2 (29.6; 36) | <0.01 |
| Waist circumference (cm) | 94 (89; 105) | 92 (88; 100) | 100 (91; 116) | <0.01 |
| Fat mass (% of body weight) | 41 (37.5; 43.4) | 40.7 (37.1; 42.9) | 42.4 (39.4; 45.2) | 0.01 |
| Muscle mass (% of body weight) | 28.5 (26.4; 31.1) | 28.9 (26.5; 31.1) | 28.5 (27.2; 32) | 0.50 |
| Body water (% of body weight) | 42.7 (40.4; 45) | 43.3 (41.7; 45.9) | 42 (40; 44.1) | 0.01 |
| Systolic blood pressure (mmHg) * | 129.2 (117.8; 141.5) | 126.7 (117.3; 136.3) | 131 (121; 142.3) | 0.12 |
| Diastolic blood pressure (mmHg) * | 85.3 (78; 93) | 83.7 (77.3; 89.7) | 86.2 (81.3; 93) | 0.05 |
| All Population with Baseline Values N = 127 | Patients without Baseline OSA Risk N = 81 (55%) | Patients with Baseline OSA Risk N = 46 (45%) | p-Value | |
|---|---|---|---|---|
| Berlin score | ||||
| Snoring N,(%) | 51 (40.2) | 17 (21) | 34 (73.9) | |
| Sleepiness N,(%) | 27 (21.3) | 7 (8.6) | 20 (43.5) | |
| Comorbidities N,(%) | 66 (52) | 24 (29.6) | 42 (91.3) | |
| Total Berlin score | 1 (1; 2) | 1 (0; 1) | 2 (2; 2) | |
| Epworth Sleepiness Scale | 6 (4; 9) | 6 (3; 8) | 7 (5; 10) | <0.01 |
| Excessive daytime sleepiness (ESS ≥ 11) N,(%) | 17 (13.4) | 8 (9.9) | 9 (19.6) | 0.12 |
| Patients without Baseline OSA Risk N = 81 (64%) | Patients with Baseline OSA Risk N = 46 (36%) | p-Value $ | Adjusted p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Initial Measure | Final Measure | Delta (Final-Initial) | Initial Measure | Final Measure | Delta (Final-Initial) | |||
| Duration of follow-up (months) | 5.1 (3.7; 8) | 5.7 (3.9; 9.2) | 0.50 | |||||
| Delta anthropometric measures | ||||||||
| Weight (kg) | 79 (73.4; 84.7) | 69.6 (64.1; 75.6) | −9.5 (−13.2; −6.8) * | 89.6 (77.5; 107) | 74.6 (65.5; 94.4) | −11.3 (−15.3; −8.5) * | 0.02 | 0.69 |
| Weight (% change) | −12.3 (−15.5; −8.8) | −11.9 (−17.1; 10.0) | 0.37 | |||||
| Body mass index (kg/m2) | 28.6 (27.3; 31.3) | 25.3 (23.6; 27.6) | −3.5 (−4.8; −2.6) * | 33.2 (29.5; 36) | 28.8 (25.4; 31.1) | −4.1 (−5.7; −3.3) * | 0.02 | 0.98 |
| Waist circumference (cm) | 92.5 (88; 100) | 81 (77; 86) | −10 (−16; −8) * | 100 (90; 116) | 87 (79; 99) | −12 (−19; −10) * | 0.06 | 0.23 |
| Fat mass (%) | 40.7 (37; 43) | 36.4 (32.4; 39.2) | −4.3 (−5.6; −3) * | 42.3 (39.4; 45.2) | 37.4 (35.1; 42) | −3.7 (−5.4; −2.4) * | 0.42 | 0.42 |
| Muscle mass (%) | 28.9 (26.5; 31.2) | 30.7 (27.7; 32.2) | 1.3 (0.9; 1.9) * | 28.5 (27.2; 32) | 29.8 (28.7; 32.6) | 1.5 (0.8; 2.2) * | 0.69 | <0.01 * |
| Body water (%) | 43.4 (41.7; 46) | 46.4 (44.5; 49.6) | 3.1 (2.2; 4.1) * | 42.1 (40; 44.1) | 45.8 (42.3; 47.4) | 2.8 (2; 3.9) * | 0.61 | 0.64 |
| Systolic blood pressure | 125.8 (117; 136) | 118.3 (108; 125) | −10.3 (−17.3; −3) * | 131 (121; 145) | 119.7 (110; 128) | −13.3 (−22.7; −4.7) * | 0.22 | 0.46 |
| Diastolic blood pressure | 83.5 (77; 89) | 78.3 (71; 84) | −5.3 (−10.3; −0.3) * | 86.3 (81; 93) | 78 (73; 84) | −8.3 (−13; −1.7) * | 0.05 | 0.18 |
| Berlin score | 1 (0; 1) | 0 (0; 0) | 0 (−1; 0) * | 2 (2; 2) | 1 (0; 1) | −1 (−2; −1) * | <0.01 | |
| Epworth Sleepiness Scale | 6 (3; 8) | 4 (2; 5.5) | −1 (−3; 0) * | 7 (5; 10) | 4 (2; 6) | −2 (−6; −1) * | 0.02 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailly, S.; Fabre, O.; Cals-Maurette, M.; Pantagis, L.; Terrail, R.; Legrand, R.; Astrup, A.; Pépin, J.-L. Impact of a Weight-Loss Rehabilitation Program on Sleep Apnea Risk and Subjective Sleepiness in Patients with Overweight/Obesity: The DietSleep Study. J. Clin. Med. 2022, 11, 6890. https://doi.org/10.3390/jcm11236890
Bailly S, Fabre O, Cals-Maurette M, Pantagis L, Terrail R, Legrand R, Astrup A, Pépin J-L. Impact of a Weight-Loss Rehabilitation Program on Sleep Apnea Risk and Subjective Sleepiness in Patients with Overweight/Obesity: The DietSleep Study. Journal of Clinical Medicine. 2022; 11(23):6890. https://doi.org/10.3390/jcm11236890
Chicago/Turabian StyleBailly, Sébastien, Odile Fabre, Mallory Cals-Maurette, Laurent Pantagis, Robin Terrail, Rémy Legrand, Arne Astrup, and Jean-Louis Pépin. 2022. "Impact of a Weight-Loss Rehabilitation Program on Sleep Apnea Risk and Subjective Sleepiness in Patients with Overweight/Obesity: The DietSleep Study" Journal of Clinical Medicine 11, no. 23: 6890. https://doi.org/10.3390/jcm11236890
APA StyleBailly, S., Fabre, O., Cals-Maurette, M., Pantagis, L., Terrail, R., Legrand, R., Astrup, A., & Pépin, J.-L. (2022). Impact of a Weight-Loss Rehabilitation Program on Sleep Apnea Risk and Subjective Sleepiness in Patients with Overweight/Obesity: The DietSleep Study. Journal of Clinical Medicine, 11(23), 6890. https://doi.org/10.3390/jcm11236890







