Abstract
Background: It is unclear whether and how COVID-19 vaccination may affect the outcome of patients with acute ischemic stroke (AIS). We investigated this potential association in a retrospective study by comparing previously vaccinated (VAX) versus unvaccinated (NoVAX) stroke patients. Methods: We collected clinical reports for all consecutive AIS patients admitted to our hospital and evaluated the outcome predictors in VAX and NoVAX groups. Adjustments were made for possible confounders in multivariable logistic regression analysis, and adjusted hazard ratios were calculated. Results: A total of 466 AIS patients (287 VAX and 179 NoVAX) were included in this study. The NIHSS score at discharge and mRS score at a 3-month follow-up visit were significantly lower in VAX patients compared to NoVAX patients (p < 0.001). Good outcomes (mRS 0–2) were significantly associated with COVID-19 vaccination before AIS (adjusted hazard ratio, 0.400 [95% CI = 0.216–0.741]). Conclusions: The observation that COVID-19 vaccination can influence the outcome of AIS provides support for further studies investigating the role of immunity in ischemic brain damage.
1. Introduction
Since the outbreak of the coronavirus disease 2019 (COVID-19) pandemic, the national healthcare system and economic markets have suffered a heavy backlash. The rapid growth of infected and deceased patients due to SARS-CoV-2 and the consequent efforts of pharmaceutical companies resulted in the rapid development of vaccines against SARS-CoV-2, such as Comirnaty (BNT162b2, Pfizer-BioNTech, Mainz, Germany), Spikevax mRNA-1273 (Moderna, Cambridge, MA, USA), Vaxzevria (ChAdOx1 nCov-19, AstraZeneca, Cambridge, UK) and a recombinant adenovirus type 26 vector encoding SARS-CoV-2 spike protein (Ad26.COV-2.S, Johnson & Johnson/Janssen, Beerse, Belgium), with more than 11 billion vaccines administered up until September 2022 [1]. On the other hand, the rapid development, authorization, distribution, and administration of COVID-19 vaccines aroused in some circles of public opinion, suspicions and doubts about their efficacy and safety. This was partly due to often unclear media communications and to the nature of the vaccine, considered a preventative measure that can lead to side effects and adverse reactions for healthy subjects. Although side effects such as vaccine-induced immune thrombotic thrombocytopenia (VITT), immune-mediated headache, cerebral venous sine thrombosis, anaphylactic shock, myocarditis, pericarditis, Guillain–Barrè syndrome and capillary leak syndrome have been described [2,3], phase-3 trials did not evidence an increase in cardiovascular or neurological events [4,5,6,7]. Some studies conducted in France [8], the US [9] and Israel [10] have analyzed the incidence of pulmonary embolism, myocardial infarction or cerebrovascular events without reporting a significant increase. In the UK, a study on the whole population found increased rates of intracranial venous thrombosis (ICVT) and thrombocytopenia in adults aged <70 years who received ChAdOx1-S vaccination compared to BNT162b2 [11]. Although the benefit for curbing the disease course and the side effects are clear, not enough research has investigated whether COVID-19 vaccines can have favorable effects on other pathological conditions, such as acute ischemic stroke (AIS). Therefore, the aim of this study was to evaluate the outcome for AIS patients in previously vaccinated versus unvaccinated stroke patients.
2. Materials and Methods
2.1. Study Design and Population
In this retrospective observational study, we included consecutive adult patients with a primary diagnosis of AIS who were admitted to our hospital between 1 January 2021 and 31 December 2021. We excluded patients with a diagnosis of intracranial hemorrhage or subdural hemorrhage, subarachnoid hemorrhage and patients with ongoing SARS-CoV-2 infection, and any patients aged <18 years. This study was approved by the local ethics committee (ID: 4895, Prot. No. 0016531/22-12/05/2022).
2.2. Clinical Evaluation
All patients underwent baseline neurological examination using the National Institutes of Health Stroke Scale (NIHSS) [12], laboratory tests, brain imaging and cardiological workout. A COVID-19 nasopharyngeal swab was performed at admission and every 5 days during hospitalization to exclude concomitant SARS-CoV-2 infection. Demographic data, pre-stroke medical history, cerebrovascular risk factors, previous SARS-CoV-2 infection, COVID-19 vaccination status, flu vaccinations within the previous 3 years, type of vaccine and vaccination dates were collected. Measures of outcome were the NIHSS score at discharge and the functional outcome at 3 months, which was assessed using the modified Rankin Scale (mRS) in an outpatient follow-up (cerebrovascular disease clinic) [13]. Details of the retrospective data collection are shown in the Supplementary Materials (Table S1).
2.3. Statistical Analysis
Continuous data were summarized using the mean and standard deviation (SD) and median and interquartile range (IQR); categorical data were summarized using counts and percentages. The distribution was studied using the Kolmogorov–Smirnov test. The Mann–Whitney U-test, with exact significance, was used for the non-normal distribution of data. An independent t-test was chosen for the analysis of continuous variables between two sets of normally distributed data. Dichotomous variables were compared using Fisher’s exact and chi-squared (χ2) tests. Ordinal variables were analyzed with the Wilcoxon signed-rank test, utilizing the exact test if necessary. All data obtained in this study were regularly registered. The study population was divided into two subgroups: COVID-19-vaccinated patients (VAX) and non-vaccinated patients (NoVAX). In the univariate analysis, all variables were compared between the subgroups VAX and NoVAX. Successively, to adjust the effect size for potential confounders, a multivariate analysis was performed. Variables used in the univariate analysis were entered into a multivariate logistic regression analysis to determine adjusted odds ratios. The multivariate model was built by selecting variables for their significance in the univariate comparison (e.g., age, COVID-19 vaccination status, hypertension, obesity, previous stroke, patent foramen ovale, NIHSS and hospitalization for COVID-19 at 3-month follow-up) and for their clinical relevance (e.g., treatments, coagulopathy and chronic obstructive pulmonary disease (COPD)). The level of significance was set at p < 0.05. All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS®) software version 22 (SPSS, Inc., Chicago, IL, USA).
3. Results
From 1 January 2021 to 31 May 2022, 1324 patients discharged from the stroke unit and the emergency neurology department with a diagnosis of AIS were assessed for study eligibility. According to the exclusion criteria, 275 patients were excluded due to hemorrhagic pathogenesis of stroke, 489 due to appropriate coding of acute stroke, 35 due to acute phase COVID-19 and 59 lost due to lack of response to the follow-up questionnaire three months after acute ischemic event. Hence, 466 AIS patients were included in this study: 179 NoVAX and 287 VAX. A flow diagram showing the enrolment process is represented in Figure 1.
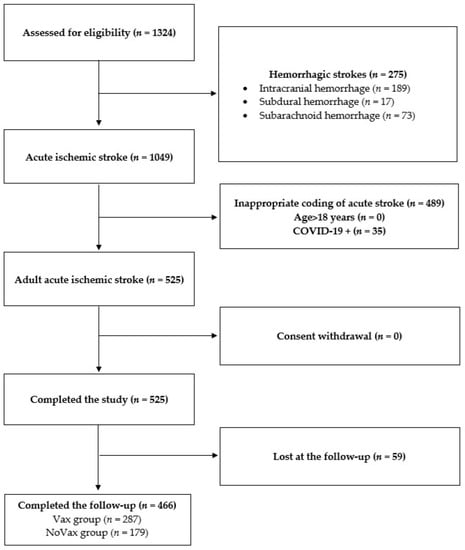
Figure 1.
Participant screening flowchart. In this study, 1324 patients with acute stroke admitted to Fondazione Policlinico Universitario Agostino Gemelli were screened for participation eligibility. A total of 858 patients failed screening for the following reasons: (1) 275 patients were affected by hemorrhagic stroke; (2) 489 patients received a wrong or inappropriate coding of acute stroke; (3) no patients were <18 years; (4) 35 patients were COVID-19-positive. Finally, 59 patients were lost at the follow-up visit since they were untraceable. No patient declined to participate.
As expected, VAX patients were slightly older than NoVAX patients, with a similar male/female ratio. Hypertension and COPD were significantly more frequent in the VAX than in the NoVAX group, while coagulopathy was slightly more frequent in the NoVAX group. Most patients in the VAX group had been vaccinated with BNT162b2 (Pfizer), followed by ChAdOx1-S (AstraZeneca), Spikevax (Moderna) and Ad26.COV-2.S (Johnson & Johnson/Janssen) vaccine. Most of the patients (156, 80%) experienced the ischemic event after the second administration of the COVID-19 vaccination, with a mean timelapse of 101 days (SD = 68).
Thirty-four patients (15%) had a stroke after the first dose of the COVID-19 vaccine, in a mean time of 43 days. Five patients (3%) experienced an ischemic event after the third dose of the vaccine, at 14 days after administration. Stroke severity on admission, assessed by NIHSS, was similar between the two groups.
Despite being older and with more comorbidities, VAX patients had significantly lower NIHSS scores at discharge (p < 0.001) and mRS scores at 3-month follow-up evaluations (p < 0.001). After mRS dichotomization, the number of patients with poor prognosis (mRS 3–6) in the NoVAX group was higher than in the VAX group (p = 0.007). Finally, the death rate was lower in the VAX group compared to the NoVAX group (p < 0.001). During the 3-month follow-up period, 14 (7%) of VAX patients and 11 (9%) of NoVAX patients were infected with SARS-CoV-2. As expected, the majority of NoVAX patients (54%) and a minority (14%) of VAX patients required hospitalization for COVID-19. No patients in either group died from COVID-19-related causes. The demographics, risk factors and procedural data of the VAX and NoVAX groups are detailed in Table 1.

Table 1.
Demographic and clinical features of VAX and NoVAX groups.
Among risk factors, hypertension was significantly more frequent in patients with poor prognoses than those without (p = 0.004). Patients who received a COVID-19 vaccination prior to the cerebral ischemic event had a better prognosis than unvaccinated stroke patients (p = 0.006). Finally, while the COVID-19 prevalence was similar, hospitalization for COVID-19 was significantly more prevalent in patients with a worse outcome than in functionally independent patients. The demographics, risk factors and procedural data of patients with functional independence (mRS 0–2) versus patients with a poor outcome (mRS 3–6) are detailed in Table 2.

Table 2.
Demographic and clinical features of stroke patients according to good (mRS 0–2) and poor (mRS 3–6) outcomes.
In the multivariate analysis, a good outcome was significantly associated with vaccination before AIS (OR = 0.400; 95% CI = 0.216–0.741). On the contrary, a poor outcome (mRS 3–6) was associated with age > 85 years (OR = 2.374; 95% CI = 1.085–5.193), NIHSS score > 4 after discharge (OR = 7.524; 95% CI = 3.552–15.938) or previous stroke (OR = 2.451; 95% CI = 1.056–5.689). Detailed results of the multivariate analysis are displayed in Supplementary Table S1, and a forest plot is reported in Figure 2.
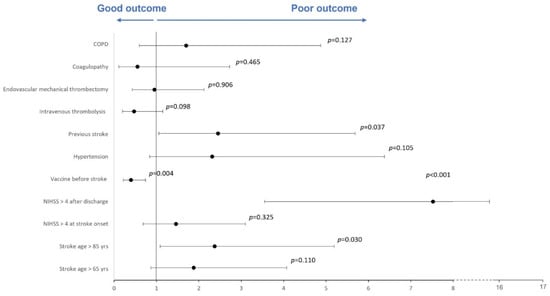
Figure 2.
Forest plot of multivariate regression analysis evaluating outcome predictors. A forest plot of multivariable regression analysis evaluating risk factors, active COVID-19 vaccination, NIHSS score and age as predictors of a good or poor outcome. The Hosmer–Lemeshow test was not significant (p = 0.878). Nagelkerke’s R2 was 0.524.
4. Discussion
In this observational study, we observed better short-term (NIHSS at discharge) and long-term outcomes (mRS at 3 months) for acute stroke patients vaccinated against COVID-19 before an ischemic event than a non-vaccinated stroke population. These data were even more significant considering the highest comorbidities, such as hypertension and COPD, in the VAX group. The old age of VAX patients is due to the COVID-19 vaccination campaigns, which prioritized the most fragile subjects, such as the elderly or patients with multiple comorbidities. In contrast, the mild prevalence of coagulopathies in NoVAX patients is justified by the initial exclusion of these patients from COVID-19 vaccination due to the increased risk of venous thrombosis or thromboembolic complications.
Considering the outcome predictors, we observed that an age above 85 years, NIHSS > 4 after discharge and previous stroke are significantly associated with a worse prognosis and a greater functional dependence of the patient. Conversely, the presence of an active vaccination against COVID-19 at the time of onset of an ischemic stroke constitutes the only predictor of a favorable prognosis and functional independence, regardless of SARS-CoV-2 infection or hospitalization for COVID-19 in the 3-month follow-up period.
While age and stroke severity are well-known outcome predictors [14], the same cannot be said for COVID-19 vaccination and, more generally, for vaccines.
COVID-19 RNA vaccines are able to induce balanced B- and T-cell responses, which are further increased by a second booster dose [15,16,17]. In addition to the expected stimulation of B cells, with a strong increase in B-cell percentage in peripheral blood and the production of specific antibodies, COVID-19 vaccination leads to the activation of T-cell-mediated immunity and to the differentiation of CD4+ T cells towards the Th1 response, which mediates proinflammatory functions aimed at the development of cell-mediated immune responses [18,19,20,21,22,23]. Limited information is available on the effect of the COVID-19 vaccine on regulatory lymphocytes, which play a critical role in immune homeostasis and immunological tolerance. However, although based on a few patients, it seems likely that CD4+ regulatory T (Treg) cells are increased following vaccination, performing an immunomodulatory function of the proinflammatory response [24].
Compelling evidence suggests a key role of the immune system, too, in the development, progression and, consequently, prognosis of ischemic stroke [25,26]. Brain ischemia triggers and, at the same time, influences from the local and systemic immune responses result in the induction of systemic immunosuppression, which increases the infectious risk [27], and an autoimmune response, which may further exacerbate brain injury [28]. In experimental models, ischemic stroke induces a Th1 polarization and promotes the production of proinflammatory cytokines, chemokines and reactive oxygen species and the disruption of the blood–brain barrier, inducing the evolution of ischemic brain injury [26]. On the contrary, high levels of Treg during the acute phase of ischemic stroke were independently associated with a good functional outcome at 3 months [29]. In our study, the administration of the SAR-CoV-2 vaccine and the consequent modification of the peripheral immune system could be the basis for a good outcome for VAX patients. On the basis of these observations, we could postulate a favorable effect on the prognosis of acute stroke patients with the COVID-19 vaccine thanks to its ability to induce a regulatory T response, which is able to suppress the damage related to the stroke proinflammatory response.
Another suggestion comes from the observation that in ischemic preconditioning, one or more short episodes of sublethal ischemia protect the brain against subsequent severe ischemic attacks [30]. These data were supported by the observation that ischemic preconditioning in a remote organ can lead to neuroprotection against brain ischemia due to a strong increase in circulating B cells, thereby reversing the reduction of the B-cell population after stroke [31].
As a result, the high percentage of circulating B cells observed in subjects undergoing COVID-19 vaccination may reveal the deleterious effects of the proinflammatory response caused by ischemic brain damage.
The main limitations of this study are the small sample size (at least partly due to the monocentric nature of this study), the impossibility of ruling out a selection bias and the duration of the follow-up having been limited to 3 months. Moreover, we did not evaluate previous SARS-CoV-2 infections before participants’ inclusion in the study.
5. Conclusions
Despite the small sample size and the short duration of the follow-up, the observation that the COVID-19 vaccination can influence the outcome of AIS provides support for further studies investigating the role of immunity in ischemic brain damage. In particular, it will be challenging to develop vaccines that can modulate the regulatory and B-mediated immune response to improve the prognosis for AIS patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11236878/s1, Table S1. Multivariate Logistic Regression Analysis for Predicting outcome in stroke patients.
Author Contributions
Conceptualization, G.F.; methodology, G.F. and G.D.M.; software, I.S.; validation, G.F. and G.D.M.; formal analysis, G.F., P.A.R., I.S., S.B., V.B., R.A. and G.D.M.; investigation, I.S. and S.B.; resources, F.C., F.V., B.M.A., R.D.I., M.M., P.C., A.B. and P.A.R.; data curation, S.B. and I.S.; writing—original draft preparation, S.B., I.S., R.D.I., M.M., M.L. and G.F.; writing —review and editing, G.F., V.B., S.B., I.S. and G.D.M.; visualization, G.F., V.B., S.B., I.S. and G.D.M.; supervision, G.F., R.D.I., M.M., P.C. and G.D.M.; project administration, G.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Fondazione Policlinico Universitario Agostino Gemelli, IRCCS, Rome (ID: 4895, Prot. No. 0016531/22, 12 May 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Johns Hopkins University & Medicine, Coronavirus Resource Center. COVID-19 Map. Available online: https://coronavirus.jhu.edu/map.html (accessed on 15 September 2022).
- Klok, F.A.; Pai, M.; Huisman, M.V.; Makris, M. Vaccine-induced immune thrombotic thrombocytopenia. Lancet Haematol. 2022, 9, e73–e80. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, M.; Waliszewska-Prosół, M.; Koutsokera, M.; Robotti, M.; Straburzyński, M.; Apostolakopoulou, L.; Capizzi, M.; Çibuku, O.; Ambat, F.D.F.; Frattale, I.; et al. Headache onset after vaccination against SARS-CoV-2: A systematic literature review and meta-analysis. J. Headache Pain 2022, 23, 41. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Jerald Sadoff, M.D.; Glenda Gray, M.B.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV-2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Jabagi, M.J.; Botton, J.; Bertrand, M.; Weill, A.; Farrington, P.; Zureik, M.; Dray-Spira, R. Myocardial Infarction, Stroke, and Pulmonary Embolism After BNT162b2 mRNA COVID-19 Vaccine in People Aged 75 Years or Older. JAMA 2022, 327, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.P.; Lewis, N.; Goddard, K.; Fireman, B.; Zerbo, O.; Hanson, K.E.; Donahue, J.G.; Kharbanda, E.O.; Naleway, A.; Nelson, J.C.; et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA 2021, 326, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Whiteley, W.N.; Ip, S.; Cooper, J.A.; Bolton, T.; Keene, S.; Walker, V.; Denholm, R.; Akbari, A.; Omigie, E.; Hollings, S.; et al. Association of COVID-19 vaccines ChAdOx1 and BNT162b2 with major venous, arterial, or thrombocytopenic events: A population-based cohort study of 46 million adults in England. PLoS Med. 2022, 19, e1003926. [Google Scholar] [CrossRef]
- Brott, T.; Adams, H.P., Jr.; Olinger, C.P.; Marler, J.R.; Barsan, W.G.; Biller, J.; Spilker, J.; Holleran, R.; Eberle, R.; Hertzberg, V.; et al. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 1989, 20, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Banks, J.L.; Marotta, C.A. Outcomes validity and reliability of the modified Rankin scale: Implications for stroke clinical trials: A literature review and synthesis. Stroke 2007, 38, 1091–1096. [Google Scholar] [CrossRef]
- Appelros, P.; Nydevik, I.; Viitanen, M. Poor outcome after first-ever stroke: Predictors for death, dependency, and recurrent stroke within the first year. Stroke 2003, 34, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef]
- Ewer, K.J.; Barrett, J.R.; Belij-Rammerstorfer, S.; Sharpe, H.; Makinson, R.; Morter, R.; Flaxman, A.; Wright, D.; Bellamy, D.; Bittaye, M.; et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat. Med. 2021, 27, 270–278. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, P.S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H.; et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Stephenson, K.E.; Le Gars, M.; Sadoff, J.; de Groot, A.M.; Heerwegh, D.; Truyers, C.; Atyeo, C.; Loos, C.; Chandrashekar, A.; McMahan, K.; et al. Immunogenicity of the Ad26.COV-2.S vaccine for COVID-19. JAMA 2021, 325, 1535–1544. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, P.G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Volzke, J.; Subin, B.; Müller, S.; Sombetzki, M.; Reisinger, E.C.; Müller-Hilke, B. Single-dose SARS-CoV-2 vaccinations with either BNT162b2 or AZD1222 induce disparate Th1 responses and IgA production. BMC Med. 2022, 20, 29. [Google Scholar] [CrossRef]
- Gupta, S.; Su, H.; Agrawal, S. Immune Response to SARS-CoV-2 Vaccine in 2 Men. Int. Arch. Allergy Immunol. 2022, 183, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, A.; Meisel, A.; Planas, A.M.; Urra, X.; van de Beek, D.; Veltkamp, R. The immunology of acute stroke. Nat. Rev. Neurol. 2012, 8, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Akter, F.; Qin, L.; Cheng, J.; Guo, M.; Yao, S.; Jian, Z.; Liu, R.; Wu, S. Adaptive Immunity Regulation and Cerebral Ischemia. Front. Immunol. 2020, 11, 689. [Google Scholar] [CrossRef]
- Vogelgesang, A.; Dressel, A. Immunological consequences of ischemic stroke: Immunosuppression and autoimmunity. J. Neuroimmunol. 2011, 231, 105–110. [Google Scholar] [CrossRef]
- Whiteley, W.; Chong, W.L.; Sengupta, A.; Sandercock, P. Blood markers for the prognosis of ischemic stroke: A systematic review. Stroke 2009, 40, e380–e389. [Google Scholar] [CrossRef]
- Santamaría-Cadavid, M.; Rodríguez-Castro, E.; Rodríguez-Yáñez, M.; Arias-Rivas, S.; López-Dequidt, I.; Pérez-Mato, M.; Rodríguez-Pérez, M.; López-Loureiro, I.; Hervella, P.; Campos, F.; et al. Regulatory T cells participate in the recovery of ischemic stroke patients. BMC Neurol. 2020, 20, 68. [Google Scholar] [CrossRef]
- Chen, F.; Qi, Z.; Luo, Y.; Hinchliffe, T.; Ding, G.; Xia, Y.; Ji, X. Non-pharmaceutical therapies for stroke: Mechanisms and clinical implications. Prog. Neurobiol. 2014, 115, 246–269. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Chen, C.; Li, X.-R.; Ran, Y.-Y.; Xu, T.; Zhang, Y.; Geng, X.-K.; Zhang, Y.; Du, H.-S.; Leak, R.K.; et al. Remote Ischemic Preconditioning-Mediated Neuroprotection against Stroke is Associated with Significant Alterations in Peripheral Immune Responses. CNS Neurosci. Ther. 2016, 22, 43–52. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).