Angina in 2022: Current Perspectives
Abstract
1. Introduction
2. Pathophysiology of Angina Pectoris
3. Lifestyle Modifications
4. Percutaneous Coronary Intervention and Coronary Artery Bypass Graft
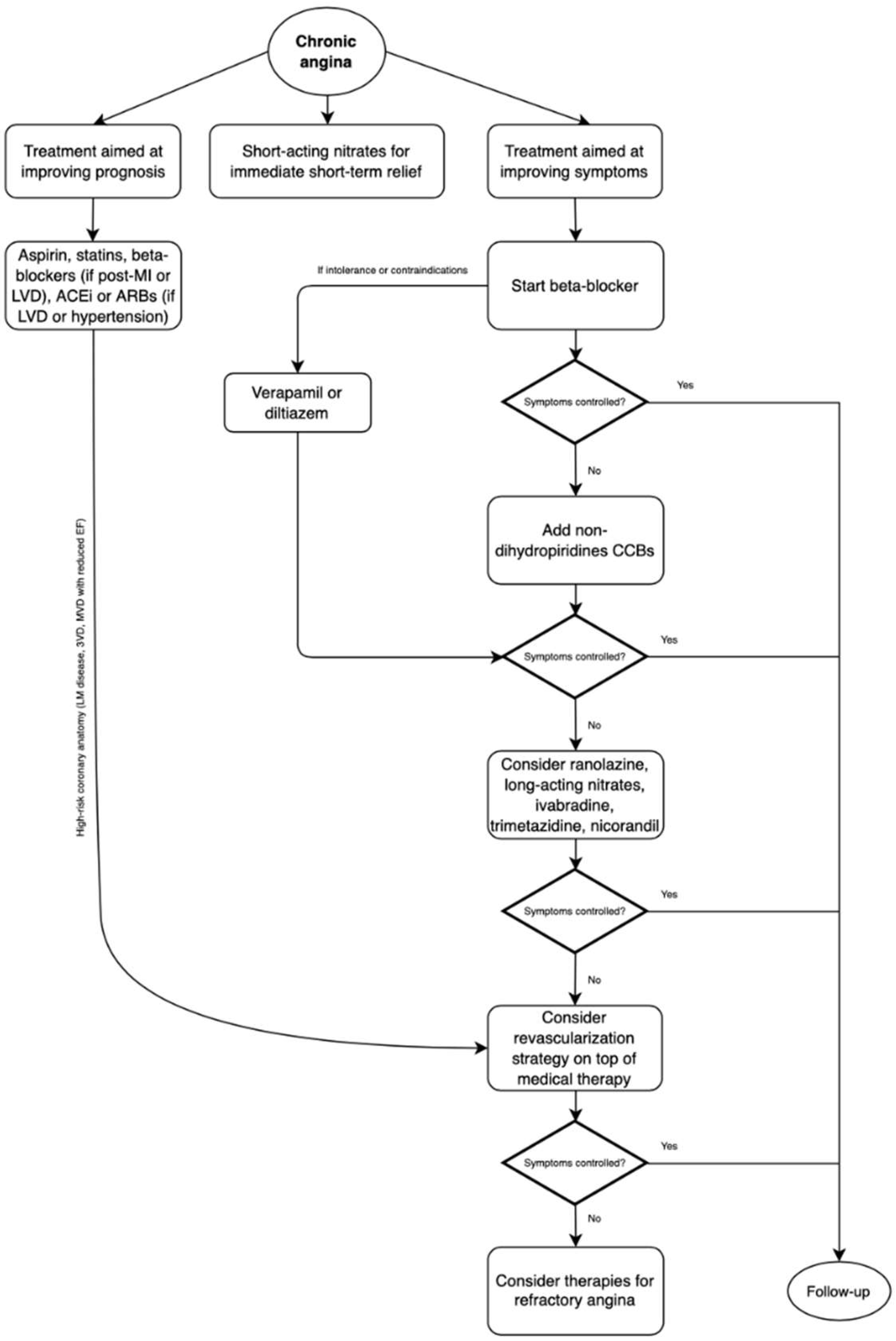
5. “Event-Reducing” Drugs
6. Symptomatic Drugs Not Conditioning Outcomes
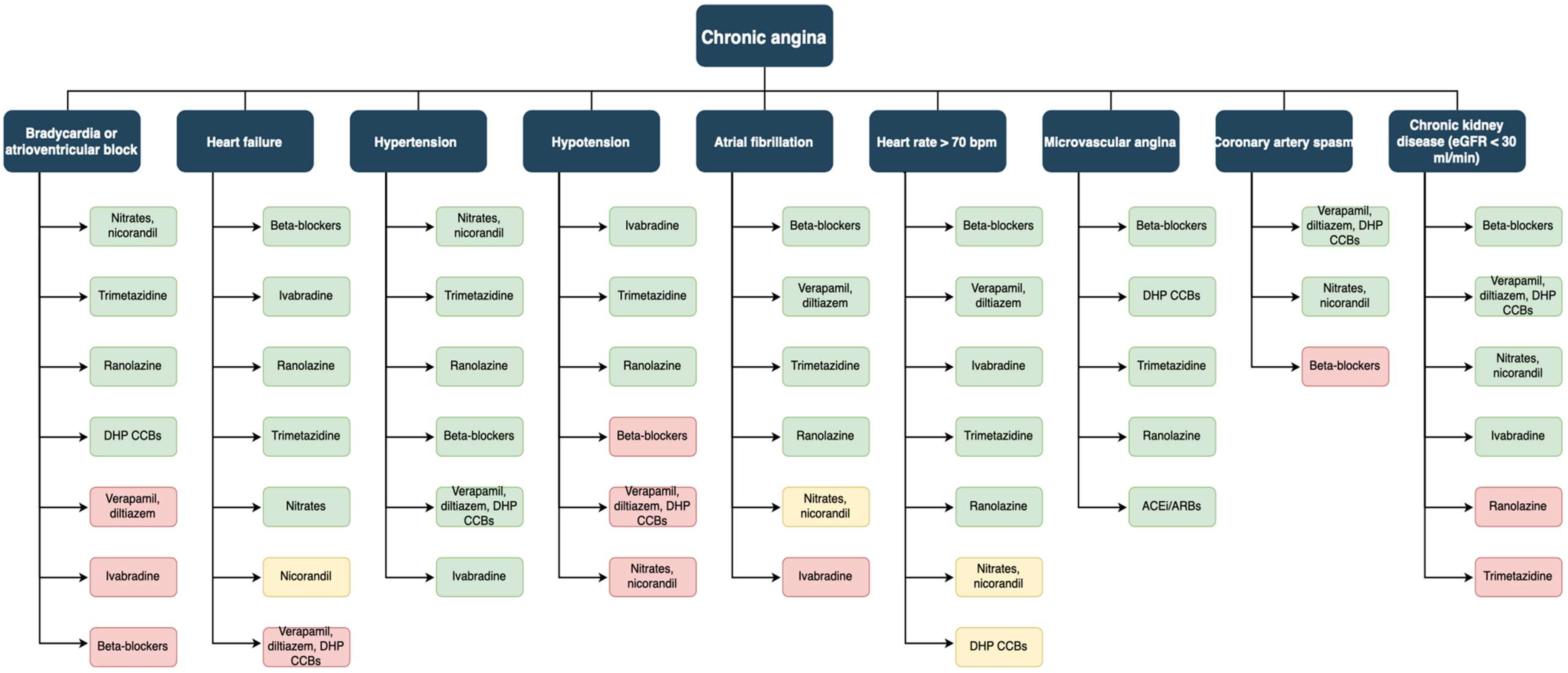
6.1. Nitrovasodilators
6.2. β-Blockers
6.3. Calcium Channel Blockers
6.4. Ranolazine
6.5. Ivabradine
6.6. Trimetazidine
7. Angina with Non-Obstructed Coronary Arteries
8. Refractory Angina
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Saglietto, A.; Manfredi, R.; Elia, E.; D’Ascenzo, F.; De Ferrari, G.M.; Biondi-Zoccai, G.; Munzel, T. Cardiovascular Disease Burden: Italian and Global Perspectives. Minerva Cardiol. Angiol. 2021, 69, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Bergmark, B.A.; Mathenge, N.; Merlini, P.A.; Lawrence-Wright, M.B.; Giugliano, R.P. Acute Coronary Syndromes. Lancet 2022, 399, 1347–1358. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sechtem, U.; Banning, A.P.; Bonaros, N.; Bueno, H.; Bugiardini, R.; Chieffo, A.; Crea, F.; Czerny, M.; Delgado, V.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Chronic Coronary Syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar]
- Joshi, P.H.; de Lemos, J.A. Diagnosis and Management of Stable Angina: A Review. J. Am. Med. Assoc. 2021, 325, 1765–1778. [Google Scholar] [CrossRef] [PubMed]
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.H.E.M.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur. Heart J. 2020, 41, 3504–3520. [Google Scholar] [CrossRef]
- Perera, D.; Berry, C.; Hoole, S.P.; Sinha, A.; Rahman, H.; Morris, P.D.; Kharbanda, R.K.; Petraco, R.; Channon, K. Invasive Coronary Physiology in Patients with Angina and Non-Obstructive Coronary Artery Disease: A Consensus Document from the Coronary Microvascular Dysfunction Workstream of the British Heart Foundation/National Institute for Health Research Partnership. Heart 2022. [Google Scholar] [CrossRef]
- Arbab-Zadeh, A.; Nakano, M.; Virmani, R.; Fuster, V. Acute Coronary Events. Circulation 2012, 125, 1147–1156. [Google Scholar] [CrossRef]
- Doll, R.; Peto, R.; Boreham, J.; Sutherland, I. Mortality in Relation to Smoking: 50 Years’ Observations on Male British Doctors. BMJ 2004, 328, 1519. [Google Scholar] [CrossRef]
- Critchley, J.A.; Capewell, S. Mortality Risk Reduction Associated With Smoking Cessation in Patients With Coronary Heart Disease. JAMA 2003, 290, 86. [Google Scholar] [CrossRef]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing Evidence on Benefits of Adherence to the Mediterranean Diet on Health: An Updated Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.S.; Ning, H.; Wilkins, J.T.; Allen, N.; Carnethon, M.; Berry, J.D.; Sweis, R.N.; Lloyd-Jones, D.M. Association of Body Mass Index With Lifetime Risk of Cardiovascular Disease and Compression of Morbidity. JAMA Cardiol. 2018, 3, 280. [Google Scholar] [CrossRef] [PubMed]
- Moholdt, T.; Lavie, C.J.; Nauman, J. Sustained Physical Activity, Not Weight Loss, Associated With Improved Survival in Coronary Heart Disease. J. Am. Coll. Cardiol. 2018, 71, 1094–1101. [Google Scholar] [CrossRef]
- Velazquez, E.J.; Lee, K.L.; Deja, M.A.; Jain, A.; Sopko, G.; Marchenko, A.; Ali, I.S.; Pohost, G.; Gradinac, S.; Abraham, W.T.; et al. Coronary-Artery Bypass Surgery in Patients with Left Ventricular Dysfunction. N. Engl. J. Med. 2011, 364, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, E.J.; Lee, K.L.; Jones, R.H.; Al-Khalidi, H.R.; Hill, J.A.; Panza, J.A.; Michler, R.E.; Bonow, R.O.; Doenst, T.; Petrie, M.C.; et al. Coronary-Artery Bypass Surgery in Patients with Ischemic Cardiomyopathy. N. Engl. J. Med. 2016, 374, 1511–1520. [Google Scholar] [CrossRef]
- The Veterans Administration Coronary Artery Bypass Surgery Cooperative Study Group Eleven-Year Survival in the Veterans Administration Randomized Trial of Coronary Bypass Surgery for Stable Angina. N. Engl. J. Med. 1984, 311, 1333–1339. [CrossRef]
- Alderman, E.L.; Fisher, L.D.; Litwin, P.; Kaiser, G.C.; Myers, W.O.; Maynard, C.; Levine, F.; Schloss, M. Results of Coronary Artery Surgery in Patients with Poor Left Ventricular Function (CASS). Circulation 1983, 68, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Zucker, D.; Passamani, E.; Peduzzi, P.; Takaro, T.; Fisher, L.D.; Kennedy, J.W.; Davis, K.; Killip, T.; Norris, R.; et al. Effect of Coronary Artery Bypass Graft Surgery on Survival: Overview of 10-Year Results from Randomised Trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet 1994, 344, 563–570. [Google Scholar] [CrossRef]
- Boden, W.E.; O’Rourke, R.A.; Teo, K.K.; Hartigan, P.M.; Maron, D.J.; Kostuk, W.J.; Knudtson, M.; Dada, M.; Casperson, P.; Harris, C.L.; et al. Optimal Medical Therapy with or without PCI for Stable Coronary Disease. N. Engl. J. Med. 2007, 356, 1503–1516. [Google Scholar] [CrossRef]
- The BARI 2D Study Group. A Randomized Trial of Therapies for Type 2 Diabetes and Coronary Artery Disease. N. Engl. J. Med. 2009, 360, 2503–2515. [Google Scholar] [CrossRef]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; López-Sendón, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- de Bruyne, B.; Pijls, N.H.J.; Kalesan, B.; Barbato, E.; Tonino, P.A.L.; Piroth, Z.; Jagic, N.; Möbius-Winkler, S.; Rioufol, G.; Witt, N.; et al. Fractional Flow Reserve–Guided PCI versus Medical Therapy in Stable Coronary Disease. N. Engl. J. Med. 2012, 367, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization. J. Am. Coll. Cardiol. 2022, 79, e21–e129. [Google Scholar] [CrossRef] [PubMed]
- Hueb, W.; Lopes, N.; Gersh, B.J.; Soares, P.R.; Ribeiro, E.E.; Pereira, A.C.; Favarato, D.; Rocha, A.S.C.; Hueb, A.C.; Ramires, J.A.F. Ten-Year Follow-Up Survival of the Medicine, Angioplasty, or Surgery Study (MASS II). Circulation 2010, 122, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Spertus, J.A.; Jones, P.G.; Maron, D.J.; O’Brien, S.M.; Reynolds, H.R.; Rosenberg, Y.; Stone, G.W.; Harrell, F.E.; Boden, W.E.; Weintraub, W.S.; et al. Health-Status Outcomes with Invasive or Conservative Care in Coronary Disease. N. Engl. J. Med. 2020, 382, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.R.; Shaw, L.J.; Min, J.K.; Page, C.B.; Berman, D.S.; Chaitman, B.R.; Picard, M.H.; Kwong, R.Y.; O’Brien, S.M.; Huang, Z.; et al. Outcomes in the ISCHEMIA Trial Based on Coronary Artery Disease and Ischemia Severity. Circulation 2021, 144, 1024–1038. [Google Scholar] [CrossRef]
- Perera, D.; Clayton, T.; O’Kane, P.D.; Greenwood, J.P.; Weerackody, R.; Ryan, M.; Morgan, H.P.; Dodd, M.; Evans, R.; Canter, R.; et al. Percutaneous Revascularization for Ischemic Left Ventricular Dysfunction. N. Engl. J. Med. 2022, 387, 1351–1360. [Google Scholar] [CrossRef]
- Mäkikallio, T.; Holm, N.R.; Lindsay, M.; Spence, M.S.; Erglis, A.; Menown, I.B.A.; Trovik, T.; Eskola, M.; Romppanen, H.; Kellerth, T.; et al. Percutaneous Coronary Angioplasty versus Coronary Artery Bypass Grafting in Treatment of Unprotected Left Main Stenosis (NOBLE): A Prospective, Randomised, Open-Label, Non-Inferiority Trial. Lancet 2016, 388, 2743–2752. [Google Scholar] [CrossRef]
- Holm, N.R.; Mäkikallio, T.; Lindsay, M.M.; Spence, M.S.; Erglis, A.; Menown, I.B.A.; Trovik, T.; Kellerth, T.; Kalinauskas, G.; Mogensen, L.J.H.; et al. Percutaneous Coronary Angioplasty versus Coronary Artery Bypass Grafting in the Treatment of Unprotected Left Main Stenosis: Updated 5-Year Outcomes from the Randomised, Non-Inferiority NOBLE Trial. Lancet 2020, 395, 191–199. [Google Scholar] [CrossRef]
- Park, D.-W.; Ahn, J.-M.; Park, H.; Yun, S.-C.; Kang, D.-Y.; Lee, P.H.; Kim, Y.-H.; Lim, D.-S.; Rha, S.-W.; Park, G.-M.; et al. Ten-Year Outcomes After Drug-Eluting Stents Versus Coronary Artery Bypass Grafting for Left Main Coronary Disease. Circulation 2020, 141, 1437–1446. [Google Scholar] [CrossRef]
- Stone, G.W.; Kappetein, A.P.; Sabik, J.F.; Pocock, S.J.; Morice, M.-C.; Puskas, J.; Kandzari, D.E.; Karmpaliotis, D.; Brown, W.M.; Lembo, N.J.; et al. Five-Year Outcomes after PCI or CABG for Left Main Coronary Disease. N. Engl. J. Med. 2019, 381, 1820–1830. [Google Scholar] [CrossRef] [PubMed]
- Scandinavian Simvastatin Survival Study Group. Randomised Trial of Cholesterol Lowering in 4444 Patients with Coronary Heart Disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 1994, 344, 1383–1389. [Google Scholar] [CrossRef]
- Schwartz, G.G. Effects of Atorvastatin on Early Recurrent Ischemic Events in Acute Coronary Syndromes; The MIRACL Study: A Randomized Controlled Trial. JAMA 2001, 285, 1711. [Google Scholar] [CrossRef] [PubMed]
- Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of Cardiovascular Events and Death with Pravastatin in Patients with Coronary Heart Disease and a Broad Range of Initial Cholesterol Levels. N. Engl. J. Med. 1998, 339, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Pfeffer, M.A.; Moye, L.A.; Rouleau, J.L.; Rutherford, J.D.; Cole, T.G.; Brown, L.; Warnica, J.W.; Arnold, J.M.O.; Wun, C.-C.; et al. The Effect of Pravastatin on Coronary Events after Myocardial Infarction in Patients with Average Cholesterol Levels. N. Engl. J. Med. 1996, 335, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Morris, P.B.; Ballantyne, C.M.; Birtcher, K.K.; Covington, A.M.; DePalma, S.M.; Minissian, M.B.; Orringer, C.E.; Smith, S.C.; Waring, A.A.; et al. 2022 ACC Expert Consensus Decision Pathway on the Role of Nonstatin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk. J. Am. Coll. Cardiol. 2022, 80, 1366–1418. [Google Scholar] [CrossRef]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.J.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Bays, H.E.; Catapano, A.L.; Lalwani, N.D.; Bloedon, L.T.; Sterling, L.R.; Robinson, P.L.; Ballantyne, C.M. Safety and Efficacy of Bempedoic Acid to Reduce LDL Cholesterol. N. Engl. J. Med. 2019, 380, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Fanaroff, A.C.; Hasselblad, V.; Roe, M.T.; Bhatt, D.L.; James, S.K.; Steg, P.G.; Gibson, C.M.; Ohman, E.M. Antithrombotic Agents for Secondary Prevention after Acute Coronary Syndromes: A Systematic Review and Network Meta-Analysis. Int. J. Cardiol. 2017, 241, 87–96. [Google Scholar] [CrossRef] [PubMed]
- CAPRIE Steering Committee. A Randomised, Blinded, Trial of Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events (CAPRIE). Lancet 1996, 348, 1329–1339. [Google Scholar] [CrossRef]
- Kang, J.; Park, K.W.; Lee, H.; Hwang, D.; Yang, H.-M.; Rha, S.-W.; Bae, J.-W.; Lee, N.H.; Hur, S.H.; Han, J.-K.; et al. Aspirin vs. Clopidogrel for Chronic Maintenance Monotherapy after Percutaneous Coronary Intervention: The HOST-EXAM Extended Study. Circulation 2022, 397, 2487–2496. [Google Scholar] [CrossRef]
- Udell, J.A.; Bonaca, M.P.; Collet, J.-P.; Lincoff, A.M.; Kereiakes, D.J.; Costa, F.; Lee, C.W.; Mauri, L.; Valgimigli, M.; Park, S.-J.; et al. Long-Term Dual Antiplatelet Therapy for Secondary Prevention of Cardiovascular Events in the Subgroup of Patients with Previous Myocardial Infarction: A Collaborative Meta-Analysis of Randomized Trials. Eur. Heart J. 2016, 37, 390–399. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Bhatt, D.L.; Cohen, M.; Steg, P.G.; Storey, R.F.; Jensen, E.C.; Magnani, G.; Bansilal, S.; Fish, M.P.; Im, K.; et al. Long-Term Use of Ticagrelor in Patients with Prior Myocardial Infarction. N. Engl. J. Med. 2015, 372, 1791–1800. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330. [Google Scholar] [CrossRef]
- Heart Outcomes Prevention Evaluation Study Investigators; Yusuf, S.; Sleight, P.; Pogue, J.; Bosch, J.; Davies, R.; Dagenais, G. Effects of an Angiotensin-Converting–Enzyme Inhibitor, Ramipril, on Cardiovascular Events in High-Risk Patients. N. Engl. J. Med. 2000, 342, 145–153. [Google Scholar] [CrossRef]
- The PEACE Trial Investigators. Angiotensin-Converting–Enzyme Inhibition in Stable Coronary Artery Disease. N. Engl. J. Med. 2004, 351, 2058–2068. [Google Scholar] [CrossRef]
- Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators; Yusuf, S.; Teo, K.; Anderson, C.; Pogue, J.; Dyal, L.; Copland, I.; Schumacher, H.; Dagenais, G.; Sleight, P. Effects of the Angiotensin-Receptor Blocker Telmisartan on Cardiovascular Events in High-Risk Patients Intolerant to Angiotensin-Converting Enzyme Inhibitors: A Randomised Controlled Trial. Lancet 2008, 372, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The Effect of Spironolactone on Morbidity and Mortality in Patients with Severe Heart Failure. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.C.; Tendal, B.; Mustafa, R.A.; Vandvik, P.O.; Li, S.; Hao, Q.; Tunnicliffe, D.; Ruospo, M.; Natale, P.; Saglimbene, V.; et al. Sodium-Glucose Cotransporter Protein-2 (SGLT-2) Inhibitors and Glucagon-like Peptide-1 (GLP-1) Receptor Agonists for Type 2 Diabetes: Systematic Review and Network Meta-Analysis of Randomised Controlled Trials. BMJ 2021, 372, m4573. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Nidorf, M. Colchicine and the Heart. Eur. Heart J. 2021, 42, 2745–2760. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Eikelboom, J.W.; Budgeon, C.A.; Thompson, P.L. Low-Dose Colchicine for Secondary Prevention of Cardiovascular Disease. J. Am. Coll. Cardiol. 2013, 61, 404–410. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.-F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Parker, J.D.; Parker, J.O. Nitrate Therapy for Stable Angina Pectoris. N. Engl. J. Med. 1998, 338, 520–531. [Google Scholar] [CrossRef]
- Divakaran, S.; Loscalzo, J. The Role of Nitroglycerin and Other Nitrogen Oxides in Cardiovascular Therapeutics. J. Am. Coll. Cardiol. 2017, 70, 2393–2410. [Google Scholar] [CrossRef]
- Münzel, T.; Steven, S.; Daiber, A. Organic Nitrates: Update on Mechanisms Underlying Vasodilation, Tolerance and Endothelial Dysfunction. Vasc. Pharmacol. 2014, 63, 105–113. [Google Scholar] [CrossRef]
- Murrell, W. Nitro-glycerine as a remedy for angina pectoris. Lancet 1879, 113, 80–81. [Google Scholar] [CrossRef]
- Feldman, R.L.; Pepine, C.J.; Conti, C.R. Magnitude of Dilatation of Large and Small Coronary Arteries of Nitroglycerin. Circulation 1981, 64, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, M.G. Pharmacokinetics of Organic Nitrates in Man: An Overview. Eur. Heart J. 1988, 9, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Thadani, U. Transdermal Nitroglycerin Patches in Angina Pectoris. Ann. Intern. Med. 1986, 105, 485. [Google Scholar] [CrossRef] [PubMed]
- Zimrin, D.; Reichek, N.; Bogin, K.T.; Aurigemma, G.; Douglas, P.; Berko, B.; Fung, H.L. Antianginal Effects of Intravenous Nitroglycerin over 24 Hours. Circulation 1988, 77, 1376–1384. [Google Scholar] [CrossRef]
- Parker, J.D. Nitrate Tolerance, Oxidative Stress, and Mitochondrial Function: Another Worrisome Chapter on the Effects of Organic Nitrates. J. Clin. Investig. 2004, 113, 352–354. [Google Scholar] [CrossRef]
- Thadani, U.; Rodgers, T. Side Effects of Using Nitrates to Treat Angina. Expert Opin. Drug Saf. 2006, 5, 667–674. [Google Scholar] [CrossRef]
- Christiansen, I.; Iversen, H.; Olesen, J. Headache Characteristics During the Development of Tolerance to Nitrates: Pathophysiological Implications. Cephalalgia 2000, 20, 437–444. [Google Scholar] [CrossRef]
- Buenger, J.W.; Mauro, V.F. Organic Nitrate-Induced Methemoglobinemia. DICP 1989, 23, 283–288. [Google Scholar] [CrossRef]
- Thadani, U.; Whisett, T.; Hamilton, S. Nitrate Therapy for Myocardial Ischemic Syndromes: Current Perspectives Including Tolerance. Curr. Probl. Cardiol. 1988, 13, 729–784. [Google Scholar] [CrossRef]
- Tarkin, J.M.; Kaski, J.C. Nicorandil and Long-Acting Nitrates: Vasodilator Therapies for the Management of Chronic Stable Angina Pectoris. Eur. Cardiol. Rev. 2018, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.J.S.; Ciampricotti, R.J.S.; Schotborgh, C.E.; de Kam, P.-J.E. A Comparison of Nicorandil with Isosorbide Mononitrate in Elderly Patients with Stable Coronary Heart Disease: The SNAPE Study. Am. Heart J. 2000, 139, a103846. [Google Scholar] [CrossRef]
- IONA Study Group. Effect of Nicorandil on Coronary Events in Patients with Stable Angina: The Impact Of Nicorandil in Angina (IONA) Randomised Trial. Lancet 2002, 359, 1269–1275. [Google Scholar] [CrossRef]
- Pascual, I.; Moris, C.; Avanzas, P. Beta-Blockers and Calcium Channel Blockers: First Line Agents. Cardiovasc. Drugs Ther. 2016, 30, 357–365. [Google Scholar] [CrossRef]
- Insel, P.A. Adrenergic Receptors—Evolving Concepts and Clinical Implications. N. Engl. J. Med. 1996, 334, 580–585. [Google Scholar] [CrossRef]
- Billinger, M.; Seiler, C.; Fleisch, M.; Eberli, F.R.; Meier, B.; Hess, O.M. Do Beta-Adrenergic Blocking Agents Increase Coronary Flow Reserve? J. Am. Coll. Cardiol. 2001, 38, 1866–1871. [Google Scholar] [CrossRef]
- Warren, S.G.; Brewer, D.L.; Orgain, E.S. Long-Term Propranolol Therapy for Angina Pectoris. Am. J. Cardiol. 1976, 37, 420–426. [Google Scholar] [CrossRef]
- Kaski, J.C.; Rodriguez-Plaza, L.; Brown, J.; Maseri, A. Efficacy of Carvedilol (BM14, 190), a New Beta-Blocking Drug with Vasodilating Properties, in Exercise-Induced Ischemia. Am. J. Cardiol. 1985, 56, 35–40. [Google Scholar] [CrossRef]
- Pepine, C.J.; Cohn, P.F.; Deedwania, P.C.; Gibson, R.S.; Handberg, E.; Hill, J.A.; Miller, E.; Marks, R.G.; Thadani, U. Effects of Treatment on Outcome in Mildly Symptomatic Patients with Ischemia during Daily Life. The Atenolol Silent Ischemia Study (ASIST). Circulation 1994, 90, 762–768. [Google Scholar] [CrossRef]
- Dargie, H.J.; Ford, I.; Fox, K.M. Total Ischaemic Burden European Trial (TIBET): Effects of Ischaemia and Treatment with Atenolol, Nifedipine SR and Their Combination on Outcome in Patients with Chronic Stable Angina. Eur. Heart J. 1996, 17, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Freemantle, N.; Cleland, J.; Young, P.; Mason, J.; Harrison, J. Beta Blockade after Myocardial Infarction: Systematic Review and Meta Regression Analysis. BMJ 1999, 318, 1730–1737. [Google Scholar] [CrossRef]
- Gottlieb, S.S.; McCarter, R.J.; Vogel, R.A. Effect of Beta-Blockade on Mortality among High-Risk and Low-Risk Patients after Myocardial Infarction. N. Engl. J. Med. 1998, 339, 489–497. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; Fares, H.; Niazi, A.K.; Chatterjee, S.; D’Ascenzo, F.; Cerrato, E.; Biondi-Zoccai, G.; Lavie, C.J.; Bell, D.S.; O’Keefe, J.H. β-Blockers in Hypertension, Diabetes, Heart Failure and Acute Myocardial Infarction: A Review of the Literature. Open Heart 2015, 2, e000230. [Google Scholar] [CrossRef] [PubMed]
- Belsey, J.; Savelieva, I.; Mugelli, A.; Camm, A.J. Relative Efficacy of Antianginal Drugs Used as Add-on Therapy in Patients with Stable Angina: A Systematic Review and Meta-Analysis. Eur. J. Prev. Cardiol. 2015, 22, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Camici, P.G.; Crea, F.; Danchin, N.; Fox, K.; Maggioni, A.P.; Manolis, A.J.; Marzilli, M.; Rosano, G.M.C.; Lopez-Sendon, J.L. A “diamond” Approach to Personalized Treatment of Angina. Nat. Rev. Cardiol. 2018, 15, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Shu, D.F.; Dong, B.R.; Lin, X.F.; Wu, T.X.; Liu, G.J. Long-Term Beta Blockers for Stable Angina: Systematic Review and Meta-Analysis. Eur. J. Prev. Cardiol. 2012, 19, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A. Meta-Analysis of Trials Comparing β-Blockers, Calcium Antagonists, and Nitrates for Stable Angina. JAMA 1999, 281, 1927. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Seeley, S.; Schulz, C.; Fisher, J.; Gururaja Rao, S. Calcium Channels in the Heart: Disease States and Drugs. Cells 2022, 11, 943. [Google Scholar] [CrossRef]
- Abernethy, D.R.; Schwartz, J.B. Calcium-Antagonist Drugs. N. Engl. J. Med. 1999, 341, 1447–1457. [Google Scholar] [CrossRef]
- Frishman, W.; Charlap, S.; Kimmel, B.; Teicher, M.; Cinnamon, J.; Allen, L.; Strom, J. Diltiazem, Nifedipine, and Their Combination in Patients with Stable Angina Pectoris: Effects on Angina, Exercise Tolerance, and the Ambulatory Electrocardiographic ST Segment. Circulation 1988, 77, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Jansen, T.P.J.; Konst, R.E.; de Vos, A.; Paradies, V.; Teerenstra, S.; van den Oord, S.C.H.; Dimitriu-Leen, A.; Maas, A.H.E.M.; Smits, P.C.; Damman, P.; et al. Efficacy of Diltiazem to Improve Coronary Vasomotor Dysfunction in ANOCA. JACC Cardiovasc. Imaging 2022, 15, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Boden, W.E. Nifedipine-Lnduced Hypotension and Myocardial Ischemia in Refractory Angina Pectoris. JAMA J. Am. Med. Assoc. 1985, 253, 1131. [Google Scholar] [CrossRef]
- Rehnqvist, N.; Hjemdahl, P.; Billing, E.; Bjorkander, I.; Eriksson, S.V.; Forslund, L.; Held, C.; Nasman, P.; Wallen, N.H. Effects of Metoprolol vs Verapamil in Patients with Stable Angina Pectoris: The Angina Prognosis Study in Stockholm (APSIS). Eur. Heart J. 1996, 17, 76–81. [Google Scholar] [CrossRef]
- Pepine, C.J.; Handberg, E.M.; Cooper-DeHoff, R.M.; Marks, R.G.; Kowey, P.; Messerli, F.H.; Mancia, G.; Cangiano, J.L.; Garcia-Barreto, D.; Keltai, M.; et al. A Calcium Antagonist vs a Non–Calcium Antagonist Hypertension Treatment Strategy for Patients With Coronary Artery Disease. JAMA 2003, 290, 2805. [Google Scholar] [CrossRef]
- Hossack, K.F.; Pool, P.E.; Steele, P.; Crawford, M.H.; DeMaria, A.N.; Cohen, L.S.; Ports, T.A.; Skalland, L. Efficacy of Diltiazem in Angina on Effort: A Multicenter Trial. Am. J. Cardiol. 1982, 49, 567–572. [Google Scholar] [CrossRef]
- Strauss, W.E.; McIntyre, K.M.; Parisi, A.F.; Shapiro, W. Safety and Efficacy of Diltiazem Hydrochloride for the Treatment of Stable Angina Pectoris: Report of a Cooperative Clinical Trial. Am. J. Cardiol. 1982, 49, 560–566. [Google Scholar] [CrossRef]
- Poole-Wilson, P.A.; Lubsen, J.; Kirwan, B.-A.; van Dalen, F.J.; Wagener, G.; Danchin, N.; Just, H.; Fox, K.A.; Pocock, S.J.; Clayton, T.C.; et al. Effect of Long-Acting Nifedipine on Mortality and Cardiovascular Morbidity in Patients with Stable Angina Requiring Treatment (ACTION Trial): Randomised Controlled Trial. Lancet 2004, 364, 849–857. [Google Scholar] [CrossRef]
- Nissen, S.E.; Tuzcu, E.M.; Libby, P.; Thompson, P.D.; Ghali, M.; Garza, D.; Berman, L.; Shi, H.; Buebendorf, E.; Topol, E.J.; et al. Effect of Antihypertensive Agents on Cardiovascular Events in Patients with Coronary Disease and Normal Blood Pressure. JAMA 2004, 292, 2217. [Google Scholar] [CrossRef]
- Taylor, S.H. Usefulness of Amlodipine for Angina Pectoris. Am. J. Cardiol. 1994, 73, A28–A33. [Google Scholar] [CrossRef]
- Sueta, D.; Tabata, N.; Hokimoto, S. Clinical Roles of Calcium Channel Blockers in Ischemic Heart Diseases. Hypertens. Res. 2017, 40, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Rayner-Hartley, E.; Sedlak, T. Ranolazine: A Contemporary Review. J. Am. Heart Assoc. 2016, 5, e003196. [Google Scholar] [CrossRef] [PubMed]
- Banon, D.; Filion, K.B.; Budlovsky, T.; Franck, C.; Eisenberg, M.J. The Usefulness of Ranolazine for the Treatment of Refractory Chronic Stable Angina Pectoris as Determined from a Systematic Review of Randomized Controlled Trials. Am. J. Cardiol. 2014, 113, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Thadani, U.; Ezekowitz, M.; Fenney, L.; Chiang, Y.K. Double-Blind Efficacy and Safety Study of a Novel Anti-Ischemic Agent, Ranolazine, versus Placebo in Patients with Chronic Stable Angina Pectoris. Ranolazine Study Group. Circulation 1994, 90, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Weisz, G.; Généreux, P.; Iñiguez, A.; Zurakowski, A.; Shechter, M.; Alexander, K.P.; Dressler, O.; Osmukhina, A.; James, S.; Ohman, E.M.; et al. Ranolazine in Patients with Incomplete Revascularisation after Percutaneous Coronary Intervention (RIVER-PCI): A Multicentre, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2016, 387, 136–145. [Google Scholar] [CrossRef]
- Morrow, D.A. Effects of Ranolazine on Recurrent Cardiovascular Events in Patients With Non–ST-Elevation Acute Coronary Syndromes: The MERLIN-TIMI 36 Randomized Trial. JAMA 2007, 297, 1775. [Google Scholar] [CrossRef]
- Kosiborod, M.; Arnold, S.V.; Spertus, J.A.; McGuire, D.K.; Li, Y.; Yue, P.; Ben-Yehuda, O.; Katz, A.; Jones, P.G.; Olmsted, A.; et al. Evaluation of Ranolazine in Patients With Type 2 Diabetes Mellitus and Chronic Stable Angina. J. Am. Coll. Cardiol. 2013, 61, 2038–2045. [Google Scholar] [CrossRef]
- Guerra, F.; Romandini, A.; Barbarossa, A.; Belardinelli, L.; Capucci, A. Ranolazine for Rhythm Control in Atrial Fibrillation: A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2017, 227, 284–291. [Google Scholar] [CrossRef]
- Oliphant, C.S.; Owens, R.E.; Bolorunduro, O.B.; Jha, S.K. Ivabradine: A Review of Labeled and Off-Label Uses. Am. J. Cardiovasc. Drugs 2016, 16, 337–347. [Google Scholar] [CrossRef]
- Tardif, J.-C.; Ford, I.; Tendera, M.; Bourassa, M.G.; Fox, K. Efficacy of Ivabradine, a New Selective If Inhibitor, Compared with Atenolol in Patients with Chronic Stable Angina. Eur. Heart J. 2005, 26, 2529–2536. [Google Scholar] [CrossRef]
- Fox, K.; Ford, I.; Steg, P.G.; Tardif, J.-C.; Tendera, M.; Ferrari, R. Ivabradine in Stable Coronary Artery Disease without Clinical Heart Failure. N. Engl. J. Med. 2014, 371, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.; Ford, I.; Steg, P.G.; Tendera, M.; Ferrari, R. Ivabradine for Patients with Stable Coronary Artery Disease and Left-Ventricular Systolic Dysfunction (BEAUTIFUL): A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2008, 372, 807–816. [Google Scholar] [CrossRef]
- Mengesha, H.G.; Weldearegawi, B.; Petrucka, P.; Bekele, T.; Otieno, M.G.; Hailu, A. Effect of Ivabradine on Cardiovascular Outcomes in Patients with Stable Angina: Meta-Analysis of Randomized Clinical Trials. BMC Cardiovasc. Disord. 2017, 17, 105. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Kaur, G.; Mehta, P.K.; Morrone, D.; Godoy, L.C.; Bangalore, S.; Sidhu, M.S. Ivabradine in Cardiovascular Disease Management Revisited: A Review. Cardiovasc. Drugs Ther. 2021, 35, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Ussher, J.R.; Keung, W.; Fillmore, N.; Koves, T.R.; Mori, J.; Zhang, L.; Lopaschuk, D.G.; Ilkayeva, O.R.; Wagg, C.S.; Jaswal, J.S.; et al. Treatment with the 3-Ketoacyl-CoA Thiolase Inhibitor Trimetazidine Does Not Exacerbate Whole-Body Insulin Resistance in Obese Mice. J. Pharmacol. Exp. Ther. 2014, 349, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Ciapponi, A.; Pizarro, R.; Harrison, J. Trimetazidine for Stable Angina. In Cochrane Database of Systematic Reviews; Ciapponi, A., Ed.; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2005. [Google Scholar]
- Dézsi, C.A. Trimetazidine in Practice. Am. J. Ther. 2016, 23, e871–e879. [Google Scholar] [CrossRef]
- Ciliberti, G.; Guerra, F.; Coiro, S.; Capucci, A. Is There an ‘Atherosclerotic Continuum’ from Angina with Unobstructed Coronary Arteries to MINOCA? Eur. Heart J. 2019, 40, 1987. [Google Scholar] [CrossRef]
- Ciliberti, G.; Coiro, S.; Tritto, I.; Benedetti, M.; Guerra, F.; del Pinto, M.; Finocchiaro, G.; Cavallini, C.; Capucci, A.; Kaski, J.C.; et al. Predictors of Poor Clinical Outcomes in Patients with Acute Myocardial Infarction and Non-Obstructed Coronary Arteries (MINOCA). Int. J. Cardiol. 2018, 267, 41–45. [Google Scholar] [CrossRef]
- Ong, P.; Camici, P.G.; Beltrame, J.F.; Crea, F.; Shimokawa, H.; Sechtem, U.; Kaski, J.C.; Bairey Merz, C.N. International Standardization of Diagnostic Criteria for Microvascular Angina. Int. J. Cardiol. 2018, 250, 16–20. [Google Scholar] [CrossRef]
- Camici, P.G.; Crea, F. Coronary Microvascular Dysfunction. N. Engl. J. Med. 2007, 356, 830–840. [Google Scholar] [CrossRef]
- Ciliberti, G.; Compagnucci, P.; Urbinati, A.; Bianco, F.; Stronati, G.; Lattanzi, S.; dello Russo, A.; Guerra, F. Myocardial Infarction without Obstructive Coronary Artery Disease (MINOCA): A Practical Guide for Clinicians. Curr. Probl. Cardiol. 2021, 46, 100761. [Google Scholar] [CrossRef] [PubMed]
- Ciliberti, G.; Seshasai, S.R.K.; Ambrosio, G.; Kaski, J.C. Safety of Intracoronary Provocative Testing for the Diagnosis of Coronary Artery Spasm. Int. J. Cardiol. 2017, 244, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Ciliberti, G.; Mancone, M.; Guerra, F.; Capucci, A. Is Invasive Coronary Provocation Testing Cost-Effective among MINOCA Patients? Eur. Heart J. 2018, 39, 3334. [Google Scholar] [CrossRef] [PubMed]
- Ciliberti, G.; Verdoia, M.; Merlo, M.; Zilio, F.; Vatrano, M.; Bianco, F.; Mancone, M.; Zaffalon, D.; Bonci, A.; Boscutti, A.; et al. Pharmacological Therapy for the Prevention of Cardiovascular Events in Patients with Myocardial Infarction with Non-Obstructed Coronary Arteries (MINOCA): Insights from a Multicentre National Registry. Int. J. Cardiol. 2021, 327, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Mannheimer, C. The Problem of Chronic Refractory Angina. Report from the ESC Joint Study Group on the Treatment of Refractory Angina. Eur. Heart J. 2002, 23, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.D.; Bairey Merz, C.N.; Wei, J.; Corban, M.T.; Quesada, O.; Joung, S.; Kotynski, C.L.; Wang, J.; Lewis, M.; Schumacher, A.M.; et al. Autologous CD34+ Stem Cell Therapy Increases Coronary Flow Reserve and Reduces Angina in Patients with Coronary Microvascular Dysfunction. Circ. Cardiovasc. Interv. 2022, 15, e010802. [Google Scholar] [CrossRef] [PubMed]
- Gallone, G.; Baldetti, L.; Tzanis, G.; Gramegna, M.; Latib, A.; Colombo, A.; Henry, T.D.; Giannini, F. Refractory Angina. JACC Cardiovasc. Interv. 2020, 13, 1–19. [Google Scholar] [CrossRef]
| Drug3.6 | Route/Formulation | Dose | Time until Unset of Action | Duration of Action |
|---|---|---|---|---|
| Nitroglycerin | SL spray SL tablet TD patch IV infusion |
|
|
|
| Isosorbide dinitrate | PO IR tablet PO SR tablet |
|
|
|
| Isosorbide mononitrate | PO IR tablet PO SR tablet |
|
|
|
| Sodium nitroprusside | IV |
|
|
|
| Nicorandil | PO tablet |
|
|
|
| Drug. | Cardioselectivity | Membrane Stabilizing Activity | Alpha Blockage | Lipid Solubility | Indications |
|---|---|---|---|---|---|
| Propranolol | Nonselective | Yes | No | High | Angina, hypertension, ventricular arrhythmias, thyrotoxicosis, migraine prophylaxis, HOCM, variceal bleeding, essential tremor, pheochromocytoma |
| Metoprolol | Selective | Yes (high dose) | No | Moderate | Heart failure, ischemic heart disease, hypertension, arrhythmias, migraine prophylaxis, HOCM |
| Nebivolol | Selective | No | No | Low | Hypertension, heart failure |
| Carvedilol | Nonselective | Yes | Yes | Moderate | Heart failure, ischemic heart disease, hypertension |
| Bisoprolol | Selective | No | No | Low | Heart failure, ischemic heart disease, hypertension, arrhythmias |
| Atenolol | Selective | No | No | Low | Hypertension, ischemic heart disease, arrhythmias |
| Labetalol | Nonselective | Yes | Yes | Low | Chronic hypertension, hypertensive emergencies (eclampsia) |
| Nadolol | Nonselective | No | No | Low | Angina, hypertension, ventricular arrhythmias (LQTS, CPVT) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manfredi, R.; Verdoia, M.; Compagnucci, P.; Barbarossa, A.; Stronati, G.; Casella, M.; Dello Russo, A.; Guerra, F.; Ciliberti, G. Angina in 2022: Current Perspectives. J. Clin. Med. 2022, 11, 6891. https://doi.org/10.3390/jcm11236891
Manfredi R, Verdoia M, Compagnucci P, Barbarossa A, Stronati G, Casella M, Dello Russo A, Guerra F, Ciliberti G. Angina in 2022: Current Perspectives. Journal of Clinical Medicine. 2022; 11(23):6891. https://doi.org/10.3390/jcm11236891
Chicago/Turabian StyleManfredi, Roberto, Monica Verdoia, Paolo Compagnucci, Alessandro Barbarossa, Giulia Stronati, Michela Casella, Antonio Dello Russo, Federico Guerra, and Giuseppe Ciliberti. 2022. "Angina in 2022: Current Perspectives" Journal of Clinical Medicine 11, no. 23: 6891. https://doi.org/10.3390/jcm11236891
APA StyleManfredi, R., Verdoia, M., Compagnucci, P., Barbarossa, A., Stronati, G., Casella, M., Dello Russo, A., Guerra, F., & Ciliberti, G. (2022). Angina in 2022: Current Perspectives. Journal of Clinical Medicine, 11(23), 6891. https://doi.org/10.3390/jcm11236891








