Abstract
The rising prevalence of cardiovascular (CV) risk factors in Portugal has translated into more than 35,000 annual deaths due to CV diseases. We performed a multicenter observational cohort study encompassing clinical activities performed between 2000 and 2019 to characterize the CV risk profile and LDL-C management of patients in every CV risk category using electronic health records of a regional population in Portugal. We analyzed data from 14 health centers and 1 central hospital in the north of Portugal of patients between 40 and 80 years that had at least 1 family medicine appointment at these institutions. Living patients were characterized on 31 December 2019. CV risk assessment was computed according to the 2019 ESC/EAS Guidelines. Lipid-lowering therapy (LLT) and achievement of LDL-C targets were assessed. In total, the analysis included 78,459 patients. Patient proportions were 33%, 29%, 22%, and 17% for low, intermediate, high, and very high CV risk, respectively. Moderate-intensity statins were the most frequently used medication across all CV risk categories. High-intensity statins were used in 5% and 10% of high and very high CV risk patients, respectively. Ezetimibe was used in 6% and 10% of high and very high CV risk patients, respectively. LDL-C targets were achieved in 44%, 27%, 7%, and 3% of low, intermediate, high, and very high CV risk patients, respectively. For uncontrolled patients in the high and very high CV risk categories, a median LDL-C reduction of 44% and 53%, respectively, would be required to meet LDL-C targets. There are clear opportunities to optimize LDL-C management in routine clinical practice. The prescription of LLT according to CV risk represents an important missed treatment opportunity.
1. Introduction
The rising prevalence of cardiovascular (CV) risk factors in Portugal has translated into more than 35,000 annual deaths due to CV diseases, representing 29% of the total mortality in 2017 [1,2]. With stroke and ischemic heart disease as the leading causes of morbidity and death, reducing the incidence of atherosclerotic CV disease (ASCVD) is a major public health priority [3]. Recent studies confirmed the high burden and costs of atherosclerosis in Portugal, being responsible for 14% of all deaths and 12% of overall years of life lost (YLL) in 2016 while related direct and indirect costs were estimated at 1.9 billion euros per year (11% of Portuguese healthcare expenditure) [4,5]. Diseases and Injuries Collaborators. Global Burd The 2019 European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) Guidelines [6] recommend the assessment of cardiovascular risk using the Systematic Coronary Risk Evaluation (SCORE) system for adults >40 years of age, except for people with documented ASCVD, type 1 diabetes mellitus (T1D) or type 2 diabetes mellitus (T2D), chronic kidney disease (CKD), familial hypercholesterolemia (FH), or very high levels of individual risk (carotid or femoral plaques, coronary artery calcium score >100, or extreme Lp(a) elevation), which are automatically at high or very high CVD risk and should be managed accordingly.
Lowering of low-density lipoprotein cholesterol (LDL-C) is paramount to halt atherosclerotic plaque progression [7]. Thus, dyslipidemia has become a primary target for the prevention of atherosclerotic CV events [7,8]. A recent epidemiological study conducted in mainland Portugal estimated the prevalence of dyslipidemia by adapting the LDL-C reference levels from the NCEP-ATP III 2002 criteria and reported that 31.5% and 51.5% of the population aged 18–79 years had LDL-C >160 mg/dL and LDL-C >130 mg/dL, respectively [1,2]. Still, little is known regarding patient characteristics, lipid-lowering therapy (LLT), and LDL-C levels according to the CV risk categories used in current clinical practice.
This study aimed to describe the clinical characteristics, LLT, and control of LDL-C of patients according to the CVD risk categories defined in the 2019 ESC/EAS Guidelines, by leveraging the electronic health records produced in a regional population from northern Portugal over 20 years.
2. Materials and Methods
2.1. Study Design and Participant Selection
This was an observational cohort study using the electronic health records (EHRs) of patients followed at the Unidade Local de Saúde de Matosinhos (ULSM). ULSM is a large healthcare institution that includes 14 primary care centers assisted by 1 hospital that provides secondary and tertiary care services to the region of Matosinhos, reflecting the activity of more than 1000 doctors from different specialties. We considered a 20-year time window from 1 January 2000 to 31 December 2019 to scan EHRs for eligible patients. In order for a patient to be included in this study, the following criteria had to be met at the same point in time: (i) age between 40 and 80 years; (ii) at least one appointment with a ULSM primary care physician in the 3 years preceding the index date; and (iii) at least one record in the last year before the index date. The index date was defined as the calendar day of 31 December 2019. A total of 78,459 patients had enough EHR data to classify them in one category of CV risk and were thus characterized at the index date. The definition of the criteria was designed to be in line with the official government indicator used to determine whether a patient is routinely followed or not [9]. These inclusion criteria maximize the overlap of the study population with the resident population, which we believe to account for approximately 90% of the resident population of Matosinhos, according to the 2021 Portuguese Census [10]. Matosinhos is the eighth most inhabited municipality in the country and the fourth in the northern region [10].
This study was approved by the Ethical Committee and Data Protection Officer of ULSM (translated from Comissão de Ética para a Saúde da Unidade Local de Saúde de Matosinhos) under the approval codes No. 21/CE/JAS of 12 February 2021. All data processing and analysis were performed exclusively by analytic programs developed for this purpose and sent for execution at the ULSM data center. No data was extracted outside ULSM, and no direct access to the data took place. As an additional degree of security, processed data were de-identified by the ULSM Information Technology Department prior to the analytic code execution according to the Health Insurance Portability and Accountability Act (HIPAA) safe harbor standard [11].
2.2. Patient Characterization
All relevant conditions were identified using the ICPC-2, International Classification of Disease, 9th Revision (ICD-9) and International Classification of Disease, 10th Revision (ICD-10) codes. CV and diabetes medications were registered according to the Anatomical Therapeutic Chemical Classification System. Whenever possible, all patient conditions and criteria for risk assessment were reconstructed using the most granular available records of clinical measurements and laboratory results recorded at ULSM. Since DNA-based evidence was not available, FH was classified as definite (defined using ICD-10 code E78.01 and ICD-9 code 272.0) or possible (defined by ICD-10 code Z83.42 OR (TC > 290 mg/dL OR LDL-C >190 mg/dL AND (mother or father family history of TC >290 mg/dL OR family history of early heart disease in mother, ≤55 years-old, or father, ≤65 years-old (ICPC-2, ICD-9 or ICD-10 codes for myocardial infarction or unstable angina))) according to the Simon and Broome criteria [12,13]. In order to compute the family history of relevant diseases, we reconstructed familial relationships from primary care family information. We did not use carotid or coronary imaging data nor ankle brachial index, as this was not available. Patient demographic and clinical characteristics were described for the total cohort and for each CVD risk category. CVD risk assessment was computed according to the 2019 ESC/EAS Guidelines for the management of dyslipidemias [6]. Source data was harmonized according to the Observational Medical Outcomes Partnership (OMOP) Common Data Model (CDM) version 5.3 [14].
2.3. LDL-C Levels, Lipid-Lowering Therapy, and LDL-C Target Achievement
Patient LDL-C level was defined as the LDL-C value reported in the latest available laboratory test relative to the index date. When LDL-C values were not available, we computed them using the Friedewald formula [15]. The proportion of patients under any LLT and specifically treated with statins, ezetimibe, fibrates, combination therapy of ezetimibe with statins, and others was assessed for each CVD risk category. The intensity of statin therapy (low, moderate, or high) was also assessed. Since PCSK-9 inhibitors were not available for use in ULSM within the study period they were not considered for the analysis.
To ascertain LDL-C target achievement, we considered ESC/EAS 2019 risk-based recommended targets: ≤116 mg/dL for low risk; <100 mg/dL for intermediate risk; <70 mg/dL for high risk; and <55 mg/dL for very high risk [6]. The same analysis was performed considering the ESC/EAS 2016 targets [16]. The proportion of patients achieving the ESC/EAS 2019 and 2016 LDL-C targets was calculated for each CVD risk category and according to LLT.
2.4. LLT Exposure
LLT exposure was computed from medication records filed by ULSM physicians in both primary and secondary care. Individual drugs were mapped to LLT categories using the Anatomical Therapeutic Chemical Classification System (ATC). The potency group, considering both the drug and the dosage, was attributed according to the classification of the American College of Cardiology and the American Heart Association [17]. We considered that a patient was under treatment for a given LLT category if the patient had been prescribed one LLT drug within 365 days from the index date.
2.5. Statistical Analysis
All analyses were descriptive with no comparison within patient groups. Continuous variables were reported as the mean and standard deviation (SD) or median and interquartile range (IQR). Categorical variables were presented as frequency counts and percentages. Missing data was considered a separate level and described through frequency counts and percentages. Apache Spark Framework version 2.4.5 and R version 4.0.3 were used to perform the statistical analysis.
3. Results
3.1. Patient Characteristics and CVD Risk Profile
We identified 81,727 eligible patients, from which 4% were excluded due to either no record of LDL-C measurement or an absence of enough information to stratify into any CVD risk category. A total of 78,459 patients were included in the analysis. The mean (SD) age of the overall cohort was 59 (11) years and 42% of the patients were male. The most common comorbidities were hypercholesterolemia (50%) and hypertension (50%), followed by obesity (25%) and T2D (17%). Approximately 1% of the patients had FH (definite or possible). A detailed characterization of the cohort is shown in Table 1.

Table 1.
Sociodemographic and clinical characteristics by cardiovascular risk category on 31 December 2019.
According to the 2019 ESC/EAS Guidelines definitions for CVD risk stratification, 33%, 29%, 22%, and 17% of the patients had low, intermediate, high, and very high CVD risk, respectively.
Within the 39% of the patients with high or very high risk, 57% were male and the mean (SD) age was 59 (19) years. Hypertension was the most prevalent comorbidity in both high (66%) and very-high-risk (79%) patients, followed by hypercholesterolemia (51% and 46%, respectively). Considering very-high-risk patients, 63% had T2D and 38% had ASCVD. Within ASCVD patients, the most common type was ischemic stroke (63%), followed by myocardial infarction (33%), peripheral artery disease (PAD) (11%), and unstable angina (6%).
Considering ASCVD patients who were treated with any LLT (n = 4428), 14% were treated with a high-intensity statin and 11% with ezetimibe. A significant proportion of patients in the high and very high CV risk categories were not receiving any LLT (27% and 15%, respectively). At the time of this analysis, no patients were under proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i). Moderate-intensity statins were the most frequently used LLT for all risk categories, followed by low-intensity statins. The proportion of patients using high-intensity statins was smaller, ranging from 4% to 12% for the low and very high CV risk categories respectively. More detailed information on LLT use by CVD risk category is shown in Table 2.

Table 2.
Lipid-lowering therapy use, overall and by cardiovascular risk category on 31 December 2019.
3.2. LDL-C Levels and LDL-C Targets Achieved by CVD Risk
The study population had a median (IQR) LDL-C of 117 (52) mg/dL. Median LDL-C levels differed according to the CVD risk category, being lower in the low-risk group (115 (46) mg/dL) and higher in the high-risk group (122 (70) mg/dL). Considering ASCVD patients, the median (IQR) LDL-C was 105 (55) mg/dL. Table 3 summarizes the lipid values in the overall population and by CVD risk category.

Table 3.
Lipid panel for patients in every cardiovascular risk category on 31 December 2019.
Despite the described LLT use, only 24% of the patients achieved the LDL-C targets set by the 2019 ESC/EAS Guidelines for the management of dyslipidemias. In patients with very high CVD risk, only 3% achieved LDL-C levels <55 mg/dL (5% in patients with ASCVD). With respect to patients with high, intermediate, and low CVD risk, 7%, 27%, and 44% achieved their CVD level LDL-C targets, respectively (Figure 1).
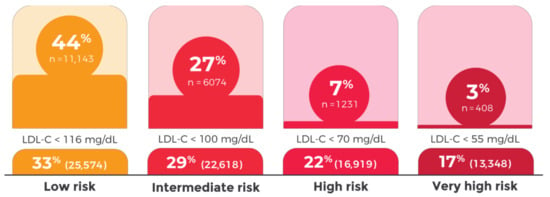
Figure 1.
Low-density lipoprotein cholesterol (LDL-C) control by cardiovascular risk category, 2019 ESC/EAS Guidelines.
Considering the uncontrolled patients in each CVD risk category, the median relative LDL-C reduction required to meet the LDL-C target according to the 2019 ESC Guidelines for the management of dyslipidemias was 15%, 25%, 44%, and 53% for the low, intermediate, high, and very high CV risk categories, respectively. Considering the 2016 ESC/EAS Guidelines, 34% of the patients achieved their LDL-C targets (10%, 28%, 42%, and 44% of the patients in the very high, high, intermediate, and low CVD risk categories, respectively).
4. Discussion
This cross-sectional study showed that more than one in every three individuals followed at ULSM were determined to be in either the high or very high CV risk categories, yet they had very poor control of LDL-C. Only 7% of the high-risk patients and 3% of the very-high-risk patients reached the ESC/EAS 2019 LDL-C targets. Moderate-intensity statins were the most frequently used LLT, irrespective of the CV risk category, and a substantial proportion of patients had no LLT despite meeting the criteria to start medication.
LDL-C is a key determinant of CVD risk and multiple studies have documented a linear relationship between LDL-C levels and CV events [8], underscoring LDL-C as the primary treatment target in clinical recommendations [6,8]. Moreover, a reduction in LDL-C is clearly linked to a substantial decrease in the risk of major adverse cardiovascular events and CV death [18,19].
We characterized more than 95% of the eligible patients at ULSM. Considering the high usage rate of ULSM by the resident population, the low population migration rates, and the large data collection period spanning more than a decade, we believe that these findings can be generalized to the population served in this region, and to populations of a similar composition. This is further substantiated by the correspondence between the sex distribution for the age groups under analysis for the Matosinhos population and the overall Portuguese population [10].
Patients with high and very high risk accounted for 39% of the studied population. Particularly regarding those at higher risk, a clear disparity between real-world routine clinical practice and the ESC/EAS 2019 guidelines was observed regarding LDL-C control and LLT prescription. A substantial proportion of high- and very-high-risk patients have never received any LLT. This proportion is similar to what was found in a recent study [20] that assessed temporal trends in the use of LLT in very-high-risk patients followed in the Cardiology Department of the Central University Hospital and concluded that about one-third of the study population was not receiving any LLT [21,22,23]. These findings underscore the need to support clinicians to systematically identify factors other than the SCORE that allow for direct stratification in high or very high CVD risk. The preferential use of moderate-intensity statins LLT irrespective of the CV risk category represents an important missed treatment opportunity and a likely avenue to significantly improve LDL-C control in high- and very-high-risk patients.
LDL-C target achievement was suboptimal as only 24% of the patients attained the corresponding 2019 ESC/EAS guideline target. The LDL-C target was more frequently achieved in patients at lower risk, and only 3% of the very-high-risk patients had an LDL-C value that was within the recommended target. These results are in line with previously published studies: (i) the DA VINCI study showed that only 33% of the patients followed in selected primary and secondary care European centers reached the LDL-C targets recommended by the 2019 ESC/EAS guidelines [24]; (ii) the EUROASPIRE survey reported that 29% of very-high-risk patients had reached the 2016 ESC/EAS LDL-C targets [25]; and (iii) Araújo et al. [20] reported a LDL-C control of 24% in a specialized tertiary center. Ours is a real-world study that is representative of the overall population followed in both primary and secondary care; thus, the degree of LDL-C control and adequate LLT use were expected to be lower. Based on these findings, we believe that studies conducted in this fashion will yield results that are closer to ours.
Several reasons may contribute to explain our findings: (i) Firstly, there was a high proportion of patients who were not receiving any LLT. Since those included in this study had at least one appointment with a ULSM family medicine physician in the three years preceding the index date, it was expected that at least in the high and very high CV risk categories, LLT would always be prescribed. This observation suggests that CV risk stratification with criteria other than SCORE and history of MACE may be difficult to perform; (ii) secondly, failure to optimize the use of high-intensity statins represented a major missed treatment opportunity, which likely explains a substantial proportion of the marginal degree of LDL-C control in high- and very-high-risk patients; and (iii) finally, residual use of ezetimibe is also expected to contribute substantially to the marginal degree of LDL-C control in high- and very-high-risk patients.
Despite this evidence, it is important to acknowledge that the 2019 ESC/EAS LDL-C targets for patients with high and very high risk are difficult to achieve with high-intensity statin monotherapy, which underscores the need to resort to combination therapies, especially with ezetimibe [6,8]. Our results showed a substantially higher proportion of fibrate use when compared to ezetimibe, which may be explained by the lack of awareness of the most recent recommendations in this area, and the growing evidence that fibrates may play a potential beneficial role in “atherogenic dyslipidemia”, which is particularly present in diabetic patients [26,27]. Previous CV outcome trials demonstrated that the risk reduction with fibrates tends to be proportional to the degree of non-HDL-C lowering [18]; moreover, the CV benefits of this therapy are ambiguous [28] and thus, they currently hold a class IIb recommendation in the 2019 ESC/EAS Guidelines [6]. There are ongoing studies to clarify the CV benefits of fibrates [29].
One of the strengths of our study resides in the fact that the EHR data used integrates primary, secondary, and tertiary health care units; thus, our cohort more closely represents the general CV risk population than cohorts from only one of those settings separately. Another strength is that we scanned all electronic data from the past 20 years, giving this analysis a very robust and detailed patient and family history. Regarding limitations, it must be mentioned that generalization of these results to dissimilar populations is limited as these data are relative to a specific population in the north of Portugal. However, the high degree of patient inclusion and the high rate of patient reconstruction of a stable regional population provides a detailed and unconstrained real-world perspective of patient populations under primary and secondary care followed in similar settings. Another limitation is that LLT medication may have been marginally overestimated as it was not possible to confirm whether prescriptions were filled.
In light of these results, one must consider whether: (i) the lack of awareness of recommendations is a factor contributing to suboptimal lipid management; (ii) treatment inertia may play a substantial role in reviewing LLT; (iii) patient beliefs and misconceptions regarding LLT may hinder therapy intensification; and (iv) patient CVD risk category stratification may be hard to perform in routine clinical practice that is expected to be performed in an incomplete fashion. The recently released 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice position CVD risk estimation as the cornerstone for a tailored intervention at the individual level, with a stepwise approach to risk factor treatment and treatment intensification. This includes apparently healthy people, patients with established ASCVD, patients with T2D, and patients with specific risk factors such as CKD and FH [30]. Thus, it becomes paramount to study the impact of the enumerated considerations to increase the real-world applicability of the recommendations.
5. Conclusions
Our study identified major missed treatment opportunities to optimize LDL-C management for every CVD risk category, with particular emphasis on high- and very-high-risk patients. It also highlighted the need to use high-intensity statins and combination therapy, putting the most recent recommendations into clinical practice. In this regard, investment in adequate control of LDL-C seems to be the most promising solution to decrease the high burden of ASCVD in Portugal.
Author Contributions
C.G., D.S.C., M.P., M.A.-S., D.G., R.J.D.-O., F.A., and T.T.-G. contributed to the study conception and design, selection of bibliography, and revision and final approval of the final version for submission. M.A.-S. wrote the first version of the manuscript and. T.T.-G. developed the analytic code used in the study and takes responsibility for the assessment of data integrity and for the accuracy of the data analysis. All authors contributed to the interpretation of data, to the critical revision of the manuscript for important intellectual content. All authors attest that listed authors meet authorship criteria and that no others meeting the criteria have been omitted. All authors have read and agreed to the published version of the manuscript.
Funding
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This study was carried out within the scope of a collaboration protocol in clinical research between Novartis Farma, Produtos Farmacêuticos SA, and Sociedade Portuguesa de Aterosclerose.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee and Data Protection Officer of ULSM (translated from Comissão de Ética para a Saúde da Unidade Local de Saúde de Matosinhos) (under the approval codes No. 21/CE/JAS of 12 February 2021).
Informed Consent Statement
Patient consent was waived as this is a study using de-identified electronic healthcare records.
Data Availability Statement
All aggregate statistical results are incorporated into this article. Patient level data used in this study is not publicly available.
Acknowledgments
The authors would like to acknowledge the editorial support, namely the constructive review of the manuscript and raised comments. The authors also would like to acknowledge Pedro Hispano Hospital for granted permissions for this study, to Daniel Santos, Tiago Morais and José Castanheira from the Department of Information Technologies for conducting data extraction, and to Mariana Pais for critical review of the manuscript.
Conflicts of Interest
C.G. declares speaker and consulting fees from AstraZeneca, Bayer, BIAL, Boehringer-Ingelheim, Daiichi-Sankyo, Lilly, MSD, Novartis and Novo Nordisk. D.S. declares speaker fees from Daiichi-Sankyo. F.A. declares speaker and consulting fees from AstraZeneca, Bayer, BIAL, Daiichi-Sankyo, Ferrer, MSD, Novartis, Novo Nordisk and Servier. M.A.-S. and D.G. are employees of Novartis Farma, Produtos Farmacêuticos SA. M.P. was an employee of Novartis Farma, Produtos Farmacêuticos SA at the time the study was conducted. T.T.-G. declares speaker and consulting fees from AstraZeneca, BIAL, Daiichi-Sankyo, MSD and Medinfar. T.T.-G. holds shares in MTG. R.J.D.-O. declares no interest conflict. The results presented in this paper have not been published previously in whole or part.
References
- Bourbon, M.; Alves, A.C.; Rato, Q. Prevalência de Fatores de Risco Cardiovascular Na População Portuguesa; Instituto Nacional de Saúde Doutor Ricardo Jorge (INSA, IP): Lisbon, Portugal, 2019. [Google Scholar]
- GBD 2019 Diseases and Injuries Collaborators Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990-2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [CrossRef]
- Gavina, C.; Carvalho, D.S.; Valente, F.; Bernardo, F.; Dinis-Oliveira, R.J.; Santos-Araújo, C.; Taveira-Gomes, T. 20 Years of Real-World Data to Estimate the Prevalence of Heart Failure and Its Subtypes in an Unselected Population of Integrated Care Units. J. Cardiovasc. Dev. Dis. 2022, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Alarcão, J.; Amaral-Silva, A.; Araújo, F.; Ascenção, R.; Caldeira, D.; Cardoso, M.F.; Correia, M.; Fiorentino, F.; Gavina, C.; et al. Os Custos Da Aterosclerose Em Portugal. Rev. Port. Cardiol. 2021, 40, 409–419. [Google Scholar] [CrossRef]
- Costa, J.; Alarcão, J.; Araujo, F.; Ascenção, R.; Caldeira, D.; Fiorentino, F.; Gil, V.; Gouveia, M.; Lourenço, F.; Mello E Silva, A.; et al. The Burden of Atherosclerosis in Portugal. Eur. Heart J. Qual. Care Clin. Outcomes 2021, 7, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; John, M.; Backer, D.; Guy, G.; Delgado, V.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2019, 41, 111–188. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-Density Lipoproteins Cause Atherosclerotic Cardiovascular Disease. 1. Evidence from Genetic, Epidemiologic, and Clinical Studies. A Consensus Statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef]
- Ference, B.A.; Yoo, W.; Alesh, I.; Mahajan, N.; Mirowska, K.K.; Mewada, A.; Kahn, J.; Afonso, L.; Williams, K.A., Sr.; Flack, J.M. Effect of Long-Term Exposure to Lower Low-Density Lipoprotein Cholesterol Beginning Early in Life on the Risk of Coronary Heart Disease: A Mendelian Randomization Analysis. J. Am. Coll. Cardiol. 2012, 60, 2631–2639. [Google Scholar] [CrossRef]
- Administração Central do Sistema de Saúde Operacionalização Da Contratualização Nos Cuidados de Saúde Primários Para 2019. Available online: https://www.acss.min-saude.pt/wp-content/uploads/2019/02/20190214_Operacionalizacao_CSP_2019_vf.pdf (accessed on 11 November 2022).
- INE—Plataforma de Divulgação Dos Censos 2021—Resultados Provisórios. Available online: https://www.ine.pt/scripts/db_censos_2021.html (accessed on 1 November 2022).
- Portability, I.; Act, A. Guidance Regarding Methods for de-Identification of Protected Health Information in Accordance with the Health Insurance Portability and Accountability Act. Available online: https://privacysecurityacademy.com/wp-content/uploads/2021/03/HHS-OCR-Guidance-on-De-Identification-of-PHI-2012.pdf (accessed on 11 November 2022).
- Scientific Steering Committee on Behalf of the Simon Broome Register Group. Risk of Fatal Coronary Heart Disease in Familial Hypercholesterolaemia. BMJ 1991, 303, 893–896. [Google Scholar] [CrossRef]
- Al-Rasadi, K.; Al-Waili, K.; Al-Sabti, H.A.; Al-Hinai, A.; Al-Hashmi, K.; Al-Zakwani, I.; Banerjee, Y. Criteria for Diagnosis of Familial Hypercholesterolemia: A Comprehensive Analysis of the Different Guidelines, Appraising Their Suitability in the Omani Arab Population. Oman Med. J. 2014, 29, 85–91. [Google Scholar] [CrossRef]
- OMOP CDM v5.3. Available online: https://ohdsi.github.io/CommonDataModel/cdm53.html (accessed on 11 November 2022).
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur. Heart J. 2016, 37, 2999–3058. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, S1–S45. [Google Scholar] [CrossRef]
- Silverman, M.G.; Ference, B.A.; Im, K.; Wiviott, S.D.; Giugliano, R.P.; Grundy, S.M.; Braunwald, E.; Sabatine, M.S. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-Analysis. JAMA 2016, 316, 1289–1297. [Google Scholar] [CrossRef]
- Morieri, M.L.; Avogaro, A.; Fadini, G.P. The DARWIN-T2D Network of the Italian Diabetes Society Cholesterol Lowering Therapies and Achievement of Targets for Primary and Secondary Cardiovascular Prevention in Type 2 Diabetes: Unmet Needs in a Large Population of Outpatients at Specialist Clinics. Cardiovasc. Diabetol. 2020, 19, 190. [Google Scholar]
- Araújo, P.M.; Nunes, A.; Torres, S.; Resende, C.X.; Leite, S.M.; Rodrigues, J.D.; Amorim, S.; Martins, E.; Campelo, M.; Maciel, M.J. Temporal Trends of Lipid Control in Very High Cardiovascular Risk Patients. Rev. Port. Cardiol. 2021, 40, 641–648. [Google Scholar] [CrossRef]
- Lu, Z.; Kou, W.; Du, B.; Wu, Y.; Zhao, S.; Brusco, O.A.; Morgan, J.M.; Capuzzi, D.M.; Chinese Coronary Secondary Prevention Study Group; Li, S. Effect of Xuezhikang, an Extract from Red Yeast Chinese Rice, on Coronary Events in a Chinese Population with Previous Myocardial Infarction. Am. J. Cardiol. 2008, 101, 1689–1693. [Google Scholar] [CrossRef]
- Presta, V.; Figliuzzi, I.; Miceli, F.; Coluccia, R.; Fogacci, F.; Cicero, A.F.G.; Ferrucci, A.; Borghi, C.; Volpe, M.; Tocci, G.; et al. Achievement of Low Density Lipoprotein (LDL) Cholesterol Targets in Primary and Secondary Prevention: Analysis of a Large Real Practice Database in Italy. Atherosclerosis 2019, 285, 40–48. [Google Scholar] [CrossRef]
- März, W.; Dippel, F.-W.; Theobald, K.; Gorcyca, K.; Iorga, Ş.R.; Ansell, D. Utilization of Lipid-Modifying Therapy and Low-Density Lipoprotein Cholesterol Goal Attainment in Patients at High and Very-High Cardiovascular Risk: Real-World Evidence from Germany. Atherosclerosis 2018, 268, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Molemans, B.; Schoonen, W.M.; Giovas, P.; Bray, S.; Kiru, G.; Murphy, J.; Banach, M.; De Servi, S.; Gaita, D.; et al. EU-Wide Cross-Sectional Observational Study of Lipid-Modifying Therapy Use in Secondary and Primary Care: The DA VINCI Study. Eur. J. Prev. Cardiol. 2021, 28, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Kotseva, K.; Wood, D.; De Bacquer, D.; De Backer, G.; Rydén, L.; Jennings, C.; Gyberg, V.; Amouyel, P.; Bruthans, J.; Castro Conde, A.; et al. EUROASPIRE IV: A European Society of Cardiology Survey on the Lifestyle, Risk Factor and Therapeutic Management of Coronary Patients from 24 European Countries. Eur. J. Prev. Cardiol. 2016, 23, 636–648. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, S.G. Fibrates Revisited: Potential Role in Cardiovascular Risk Reduction. Diabetes Metab. J. 2020, 44, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Morieri, M.L.; Pipino, C.; Doria, A. Pharmacogenetics of Cardiovascular Prevention in Diabetes: From Precision Medicine to Identification of Novel Targets. J. Pers. Med. 2022, 12, 1402. [Google Scholar] [CrossRef] [PubMed]
- Keene, D.; Price, C.; Shun-Shin, M.J.; Francis, D.P. Effect on Cardiovascular Risk of High Density Lipoprotein Targeted Drug Treatments Niacin, Fibrates, and CETP Inhibitors: Meta-Analysis of Randomised Controlled Trials Including 117,411 Patients. BMJ 2014, 349, g4379. [Google Scholar] [CrossRef]
- Pradhan, A.D.; Paynter, N.P.; Everett, B.M.; Glynn, R.J.; Amarenco, P.; Elam, M.; Ginsberg, H.; Hiatt, W.R.; Ishibashi, S.; Koenig, W.; et al. Rationale and Design of the Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT) Study. Am. Heart J. 2018, 206, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).