The Impact of Antiseptic-Loaded Bacterial Nanocellulose on Different Biofilms—An Effective Treatment for Chronic Wounds?
Abstract
1. Introduction
2. Materials and Methods
2.1. 24-Hour CDC Bioreactor Model—S. aureus
2.2. Drip-Flow Bioreactor—P. aeruginosa
2.3. 24-Hour CDC Bioreactor Model—C. albicans
2.4. Neutralizer Effectiveness Validation Assay
2.5. In Vivo Tests
2.6. Statistical Analysis
3. Results
3.1. 24-Hour CDC Bioreactor Model—S. aureus
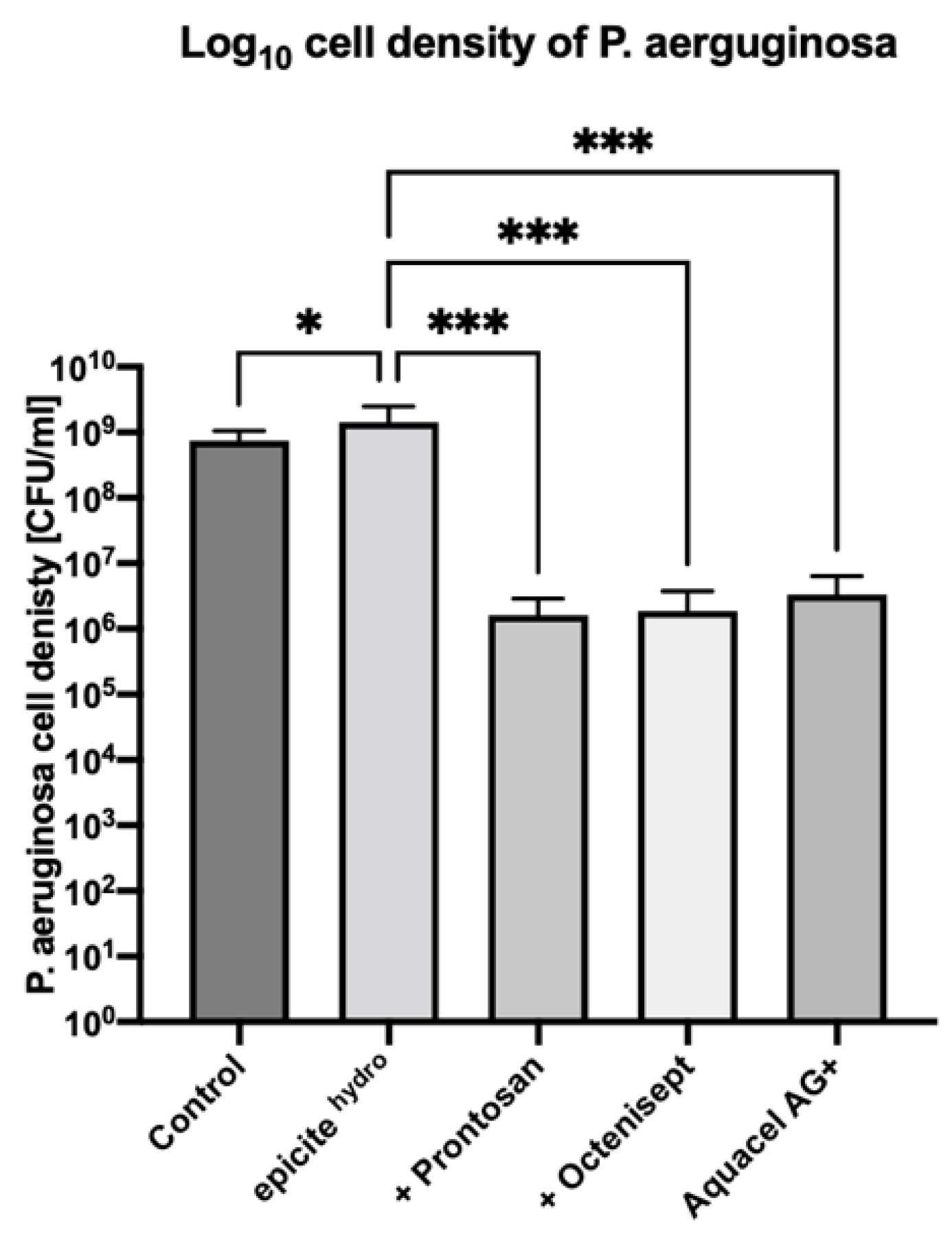
3.2. Drip-Flow Bioreactor—P. aeruginosa
3.3. 24-Hour CDC Bioreactor Model—C. albicans
3.4. Effect of Prolonged Antiseptic Treatment In Vivo
4. Discussion
4.1. Antimicrobial Activity in Gram-Positive Bacteria
4.2. Antimicrobial Activity in Gram-Negative Bacteria
4.3. Antimicrobial Activity in Fungi
4.4. General Assumptions on the Use of Silver-Based Dressings vs. Antiseptic-Loaded BNC
4.5. Wound Healing Process in a Prolonged Antiseptic Treatment Period
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, S.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Alves, P.J.; Barreto, R.T.; Barrois, B.M.; Gryson, L.G.; Meaume, S.; Monstrey, S.J. Update on the role of antiseptics in the management of chronic wounds with critical colonisation and/or biofilm. Int. Wound J. 2021, 18, 342–358. [Google Scholar] [CrossRef] [PubMed]
- White, R.J.; Cutting, K.F. Critical colonization—The concept under scrutiny. Ostomy. Wound. Manag. 2006, 52, 50–56. [Google Scholar]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E.; et al. Quantitative and qualitative assessment methods for biofilm growth: A mini-review. Res. Rev. J. Eng. Technol. 2017, 6, 4. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30214915 (accessed on 18 August 2022).
- Malone, M.; Bjarnsholt, T.; McBain, A.J.; James, G.A.; Stoodley, P.; Leaper, D.; Tachi, M.; Schultz, G.; Swanson, T.; Wolcott, R.D. The prevalence of biofilms in chronic wounds: A systematic review and meta-analysis of published data. J. Wound Care 2017, 26, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.; Shai, S.; Shahane, A.; Kaushik, K.S. Recent advances in non-conventional antimicrobial approaches for chronic wound biofilms: Have we found the ‘chink in the armor’? Biomedicines 2019, 7, 35. [Google Scholar] [CrossRef]
- Percival, S.L.; Vuotto, C.; Donelli, G.; Lipsky, B.A. Biofilms and wounds: An identification algorithm and potential treatment options. Adv. Wound Care 2015, 4, 389–397. [Google Scholar] [CrossRef]
- Pomares, G.; Huguet, S.; Dap, F.; Dautel, G. Contaminated wounds: Effectiveness of debridement for reducing bacterial load. Hand Surg. Rehabil. 2016, 35, 266–270. [Google Scholar] [CrossRef]
- Nischwitz, S.; de Mattos, I.B.; Hofmann, E.; Groeber-Becker, F.; Funk, M.; Mohr, G.; Branski, L.; Mautner, S.; Kamolz, L. Continuous pH monitoring in wounds using a composite indicator dressing—A feasibility study. Burns 2019, 45, 1336–1341. [Google Scholar] [CrossRef]
- Holzer, J.C.; Tiffner, K.; Kainz, S.; Reisenegger, P.; de Mattos, I.B.; Funk, M.; Lemarchand, T.; Laaff, H.; Bal, A.; Birngruber, T.; et al. A novel human ex-vivo burn model and the local cooling effect of a bacterial nanocellulose-based wound dressing. Burns 2020, 46, 1924–1932. [Google Scholar] [CrossRef]
- de Mattos, I.B.; Nischwitz, S.P.; Tuca, A.-C.; Groeber-Becker, F.; Funk, M.; Birngruber, T.; Mautner, S.I.; Kamolz, L.-P.; Holzer, J.C. Delivery of antiseptic solutions by a bacterial cellulose wound dressing: Uptake, release and antibacterial efficacy of octenidine and povidone-iodine. Burns 2020, 46, 918–927. [Google Scholar] [CrossRef] [PubMed]
- de Mattos, I.B.; Holzer, J.C.; Tuca, A.-C.; Groeber-Becker, F.; Funk, M.; Popp, D.; Mautner, S.; Birngruber, T.; Kamolz, L.-P. Uptake of PHMB in a bacterial nanocellulose-based wound dressing: A feasible clinical procedure. Burns 2019, 45, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Ip, M.; Lui, S.L.; Poon, V.K.M.; Lung, I.; Burd, A. Antimicrobial activities of silver dressings: An in vitro comparison. J. Med. Microbiol. 2006, 55, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of acute and chronic wound healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Dreifke, M.B.; Jayasuriya, A.A.; Jayasuriya, A.C. Current wound healing procedures and potential care. Mater. Sci. Eng. C 2015, 48, 651–662. [Google Scholar] [CrossRef]
- Halim, A.S.; Khoo, T.L.; Saad, A.Z.M. Wound bed preparation from a clinical perspective. Indian J. Plast. Surg. 2012, 45, 193–202. [Google Scholar] [CrossRef]
- Schultz, G.; Bjarnsholt, T.; James, G.A.; Leaper, D.J.; McBain, A.J.; Malone, M.; Stoodley, P.; Swanson, T.; Tachi, M.; Wolcott, R.D.; et al. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen. 2017, 25, 744–757. [Google Scholar] [CrossRef]
- Zmejkoski, D.; Spasojević, D.; Orlovska, I.; Kozyrovska, N.; Soković, M.; Glamočlija, J.; Dmitrović, S.; Matović, B.; Tasić, N.; Maksimović, V.; et al. Bacterial cellulose-lignin composite hydrogel as a promising agent in chronic wound healing. Int. J. Biol. Macromol. 2018, 118, 494–503. [Google Scholar] [CrossRef]
- Fjeld, H.; Lingaas, E. Polyheksanid-sikkerhet og effekt som antiseptikum. Tidsskr. Den Nor. Legeforening 2016, 136, 707–711. [Google Scholar] [CrossRef]
- Koburger, T.; Hubner, N.-O.; Braun, M.; Siebert, J.; Kramer, A. Standardized comparison of antiseptic efficacy of triclosan, PVP-iodine, octenidine dihydrochloride, polyhexanide and chlorhexidine digluconate. J. Antimicrob. Chemother. 2010, 65, 1712–1719. [Google Scholar] [CrossRef]
- Kamaruzzaman, N.F.; Chong, S.Q.Y.; Edmondson-Brown, K.M.; Ntow-Boahene, W.; Bardiau, M.; Good, L. Bactericidal and anti-biofilm effects of polyhexamethylene biguanide in models of intracellular and biofilm of staphylococcus aureus isolated from bovine mastitis. Front. Microbiol. 2017, 8, 1518. [Google Scholar] [CrossRef] [PubMed]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2017, 13, 65–71. [Google Scholar] [CrossRef]
- Finley, P.J.; Norton, R.; Austin, C.; Mitchell, A.; Zank, S.; Durham, P. Unprecedented silver resistance in clinically isolated enterobacteriaceae: Major implications for burn and wound management. Antimicrob. Agents Chemother. 2015, 59, 4734–4741. [Google Scholar] [CrossRef]
- Günther, F.; Blessing, B.; Dapunt, U.; Mischnik, A.; Mutters, N.T. Ability of chlorhexidine, octenidine, polyhexanide and chloroxylenol to inhibit metabolism of biofilm-forming clinical multidrug-resistant organisms. J. Infect. Prev. 2021, 22, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Randall, C.P.; Gupta, A.; Jackson, N.; Busse, D.; O’Neill, A.J. Silver resistance in Gram-negative bacteria: A dissection of endogenous and exogenous mechanisms. J. Antimicrob. Chemother. 2015, 70, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Bowler, P.; Woods, E.J. Assessing the effect of an antimicrobial wound dressing on biofilms. Wound Repair Regen. 2008, 16, 52–57. [Google Scholar] [CrossRef]
- Liao, S.; Zhang, Y.; Pan, X.; Zhu, F.; Jiang, C.; Liu, Q.; Cheng, Z.; Dai, G.; Wu, G.; Wang, L.; et al. Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa. Int. J. Nanomed. 2019, 14, 1469–1487. [Google Scholar] [CrossRef]
- Caruso, D.M.; Foster, K.N.; Hermans, M.H.E.; Rick, C. Aquacel Ag® in the management of partial-thickness burns: Results of a clinical trial. J. Burn Care Rehabil. 2004, 25, 89–97. [Google Scholar] [CrossRef]
- Spettel, K.; Bumberger, D.; Camp, I.; Kriz, R.; Willinger, B. Efficacy of octenidine against emerging echinocandin-, azole- and multidrug-resistant Candida albicans and Candida glabrata. J. Glob. Antimicrob. Resist. 2022, 29, 23–28. [Google Scholar] [CrossRef]
- Rippke, F.; Berardesca, E.; Weber, T.M. pH and microbial infections. pH Ski. Issues Chall. 2018, 54, 87–94. [Google Scholar]
- Nešporová, K.; Pavlík, V.; Šafránková, B.; Vágnerová, H.; Odráška, P.; Žídek, O.; Císařová, N.; Skoroplyas, S.; Kubala, L.; Velebný, V. Effects of wound dressings containing silver on skin and immune cells. Sci. Rep. 2020, 10, 15216. [Google Scholar] [CrossRef]
- van Meurs, S.J.; Gawlitta, D.; Heemstra, K.A.; Poolman, R.W.; Vogely, H.C.; Kruyt, M.C. Selection of an optimal antiseptic solution for intraoperative irrigation. J. Bone Jt. Surg. 2014, 96, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Muller, G.; Kramer, A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J. Antimicrob. Chemother. 2008, 61, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Bigliardi, P.L.; Alsagoff, S.A.L.; El-Kafrawi, H.Y.; Pyon, J.-K.; Wa, C.T.C.; Villa, M.A. Povidone iodine in wound healing: A review of current concepts and practices. Int. J. Surg. 2017, 44, 260–268. [Google Scholar] [CrossRef]
- Thongrueang, N.; Liu, S.-S.; Hsu, H.-Y.; Lee, H.-H. An in vitro comparison of antimicrobial efficacy and cytotoxicity between povidone-iodine and chlorhexidine for treating clinical endometritis in dairy cows. PLoS ONE 2022, 17, e0271274. [Google Scholar] [CrossRef]
- Chindera, K.; Mahato, M.; Sharma, A.K.; Horsley, H.; Kloc-Muniak, K.; Kamaruzzaman, N.F.; Kumar, S.; McFarlane, A.; Stach, J.; Bentin, T.; et al. The antimicrobial polymer PHMB enters cells and selectively condenses bacterial chromosomes. Sci. Rep. 2016, 6, 23121. [Google Scholar] [CrossRef] [PubMed]
- Tuca, A.-C.; de Mattos, I.B.; Funk, M.; Winter, R.; Palackic, A.; Groeber-Becker, F.; Kruse, D.; Kukla, F.; Lemarchand, T.; Kamolz, L.-P. Orchestrating the dermal/epidermal tissue ratio during wound healing by controlling the moisture content. Biomedicines 2022, 10, 1286. [Google Scholar] [CrossRef]
- Winter, G.D. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962, 193, 293–294. [Google Scholar] [CrossRef]
- Winter, G.D.; Scales, J.T. Effect of air drying and dressings on the surface of a wound. Nature 1963, 197, 91–99. [Google Scholar] [CrossRef]
- Bishop, S.M.; Walker, M.; Rogers, A.A.; Chen, W.Y.J. Importance of moisture balance at the wound-dressing interface. J. Wound Care 2003, 12, 125–128. [Google Scholar] [CrossRef]
- Okan, D.; Woo, K.; Ayello, E.A.; Sibbald, G. The role of moisture balance in wound healing. Adv. Ski. Wound Care 2007, 20, 39–53. [Google Scholar] [CrossRef]
- Daeschlein, G. Antimicrobial and antiseptic strategies in wound management. Int. Wound J. 2013, 10, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.T.; Gwada, R.F.; Shaheen, A.A.; Saggini, R. Extracorporeal shockwave therapy for the treatment of chronic wound of lower extremity: Current perspective and systematic review. Int. Wound J. 2017, 14, 898–908. [Google Scholar] [CrossRef]
- Takagi, S.; Oyama, T.; Jimi, S.; Saparov, A.; Ohjimi, H. A novel autologous micrografts technology in combination with negative pressure wound therapy (NPWT) for quick granulation tissue formation in chronic/refractory ulcer. Healthcare 2020, 8, 513. [Google Scholar] [CrossRef] [PubMed]
- Wynn, M.; Freeman, S. The efficacy of negative pressure wound therapy for diabetic foot ulcers: A systematised review. J. Tissue Viability 2019, 28, 152–160. [Google Scholar] [CrossRef]
- Nischwitz, S.P.; Popp, D.; Shubitidze, D.; Luze, H.; Zrim, R.; Klemm, K.; Rapp, M.; Haller, H.L.; Feisst, M.; Kamolz, L. The successful use of polylactide wound dressings for chronic lower leg wounds: A retrospective analysis. Int. Wound J. 2021, 19, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Parnell, L.K.S.; Volk, S.W. The evolution of animal models in wound healing research: 1993–2017. Adv. Wound Care 2019, 8, 692–702. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luze, H.; Bernardelli de Mattos, I.; Nischwitz, S.P.; Funk, M.; Tuca, A.C.; Kamolz, L.-P. The Impact of Antiseptic-Loaded Bacterial Nanocellulose on Different Biofilms—An Effective Treatment for Chronic Wounds? J. Clin. Med. 2022, 11, 6634. https://doi.org/10.3390/jcm11226634
Luze H, Bernardelli de Mattos I, Nischwitz SP, Funk M, Tuca AC, Kamolz L-P. The Impact of Antiseptic-Loaded Bacterial Nanocellulose on Different Biofilms—An Effective Treatment for Chronic Wounds? Journal of Clinical Medicine. 2022; 11(22):6634. https://doi.org/10.3390/jcm11226634
Chicago/Turabian StyleLuze, Hanna, Ives Bernardelli de Mattos, Sebastian Philipp Nischwitz, Martin Funk, Alexandru Cristian Tuca, and Lars-Peter Kamolz. 2022. "The Impact of Antiseptic-Loaded Bacterial Nanocellulose on Different Biofilms—An Effective Treatment for Chronic Wounds?" Journal of Clinical Medicine 11, no. 22: 6634. https://doi.org/10.3390/jcm11226634
APA StyleLuze, H., Bernardelli de Mattos, I., Nischwitz, S. P., Funk, M., Tuca, A. C., & Kamolz, L.-P. (2022). The Impact of Antiseptic-Loaded Bacterial Nanocellulose on Different Biofilms—An Effective Treatment for Chronic Wounds? Journal of Clinical Medicine, 11(22), 6634. https://doi.org/10.3390/jcm11226634






