On the Role of Stimulus-Response Context in Inhibitory Control in Alcohol Use Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Recruitment Procedure
2.2. Clinical Assessment
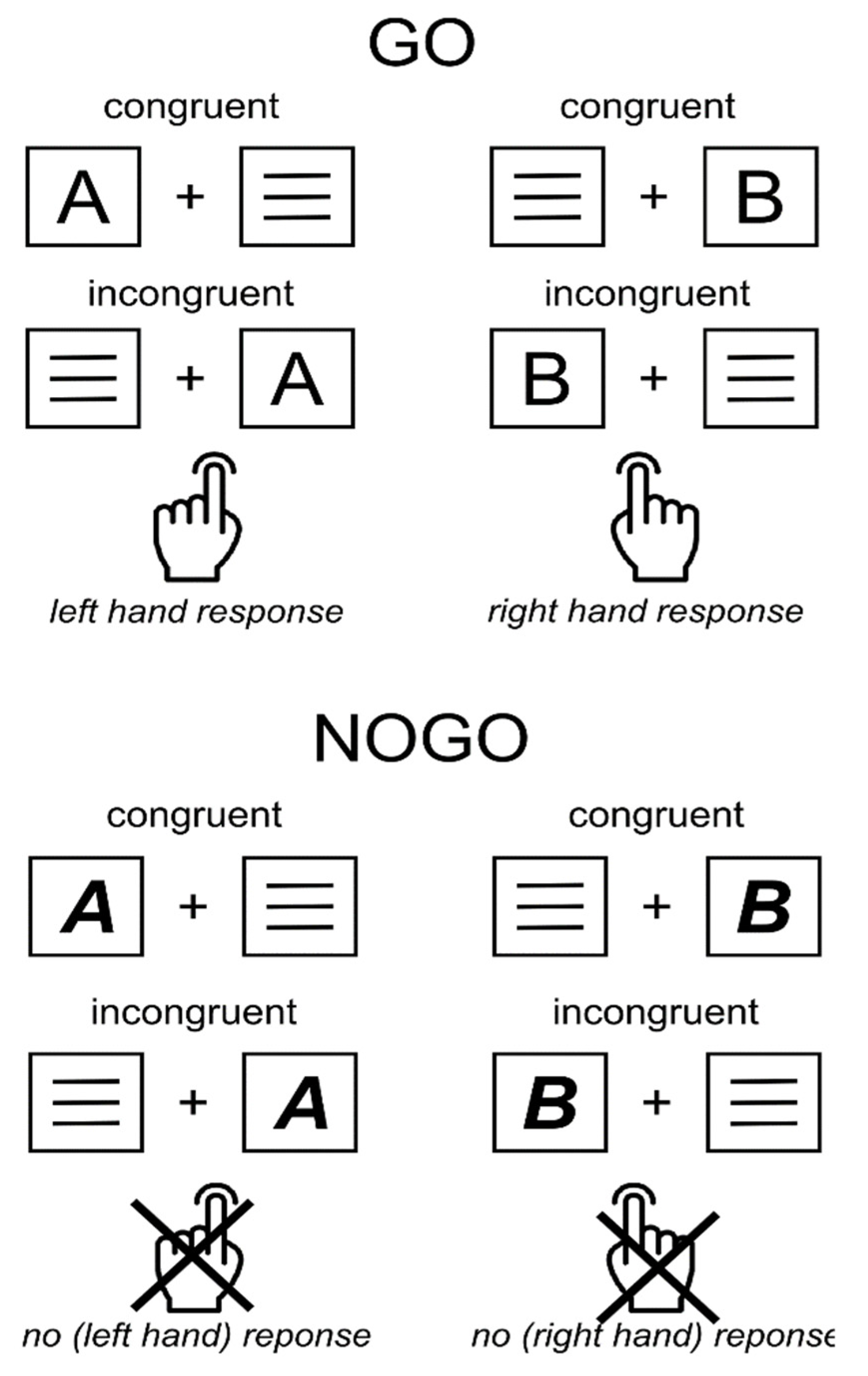
2.3. Behavioral Task
2.4. EEG Recording and Analysis
2.4.1. Residue Iteration Decomposition (RIDE)
2.4.2. Multivariate Pattern Analysis (MVPA)
2.4.3. Source Localization
3. Results
3.1. Sample Characteristics
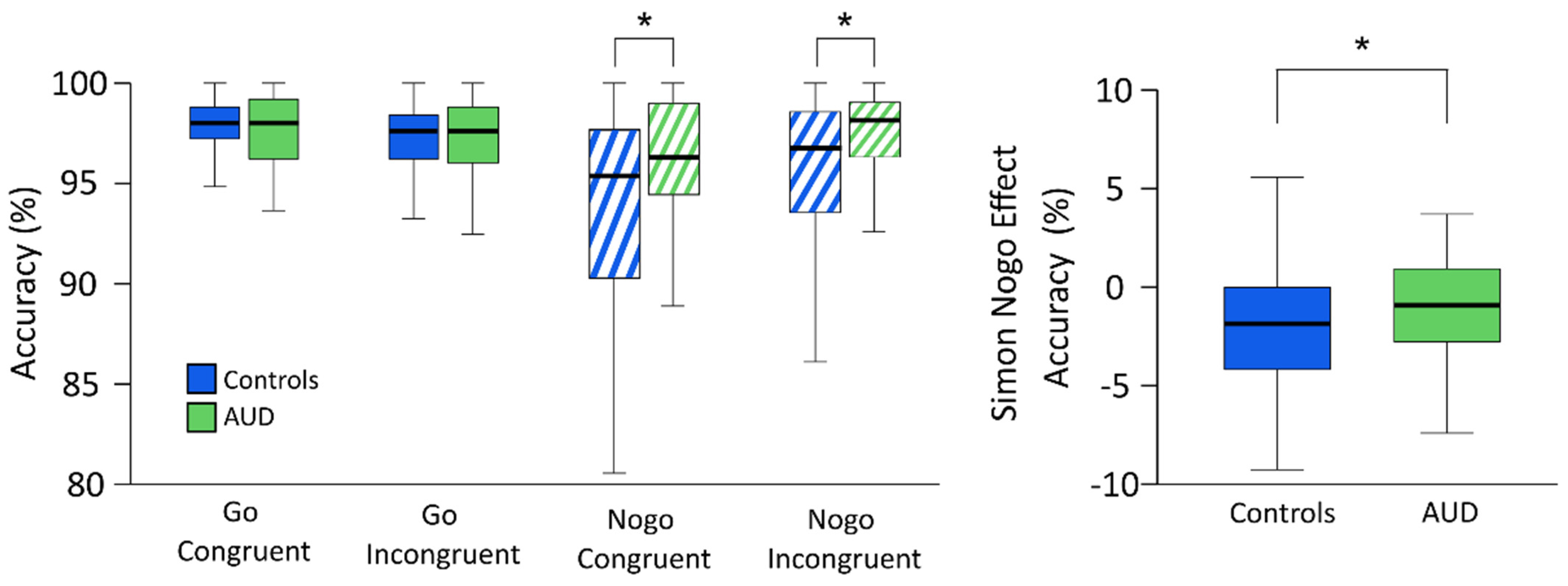
3.2. Behavioral Results
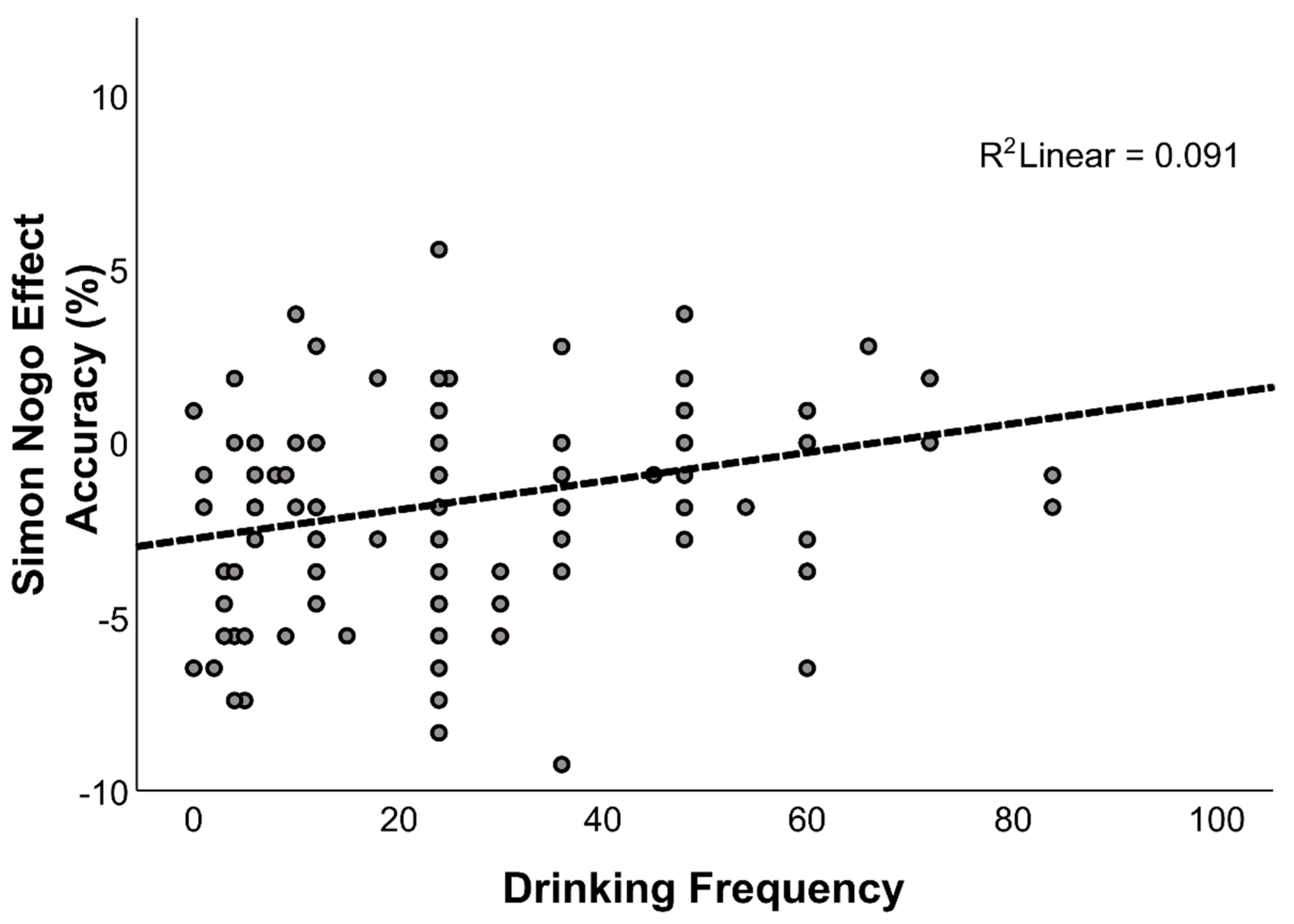
3.2.1. Alcohol-Related Modulators of Response Inhibition
3.2.2. Summary of Behavioral Results
3.3. Neurophysiological Results
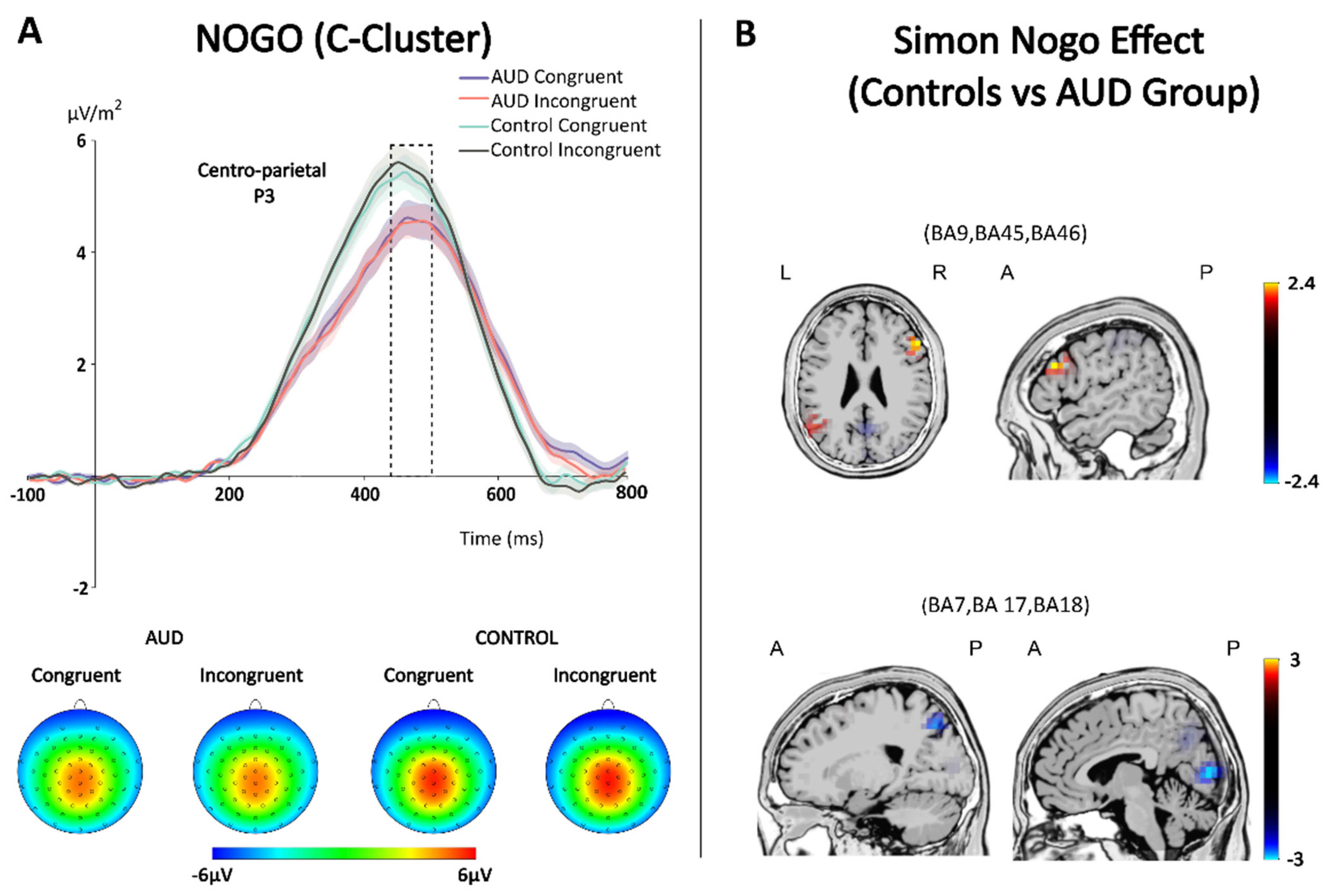
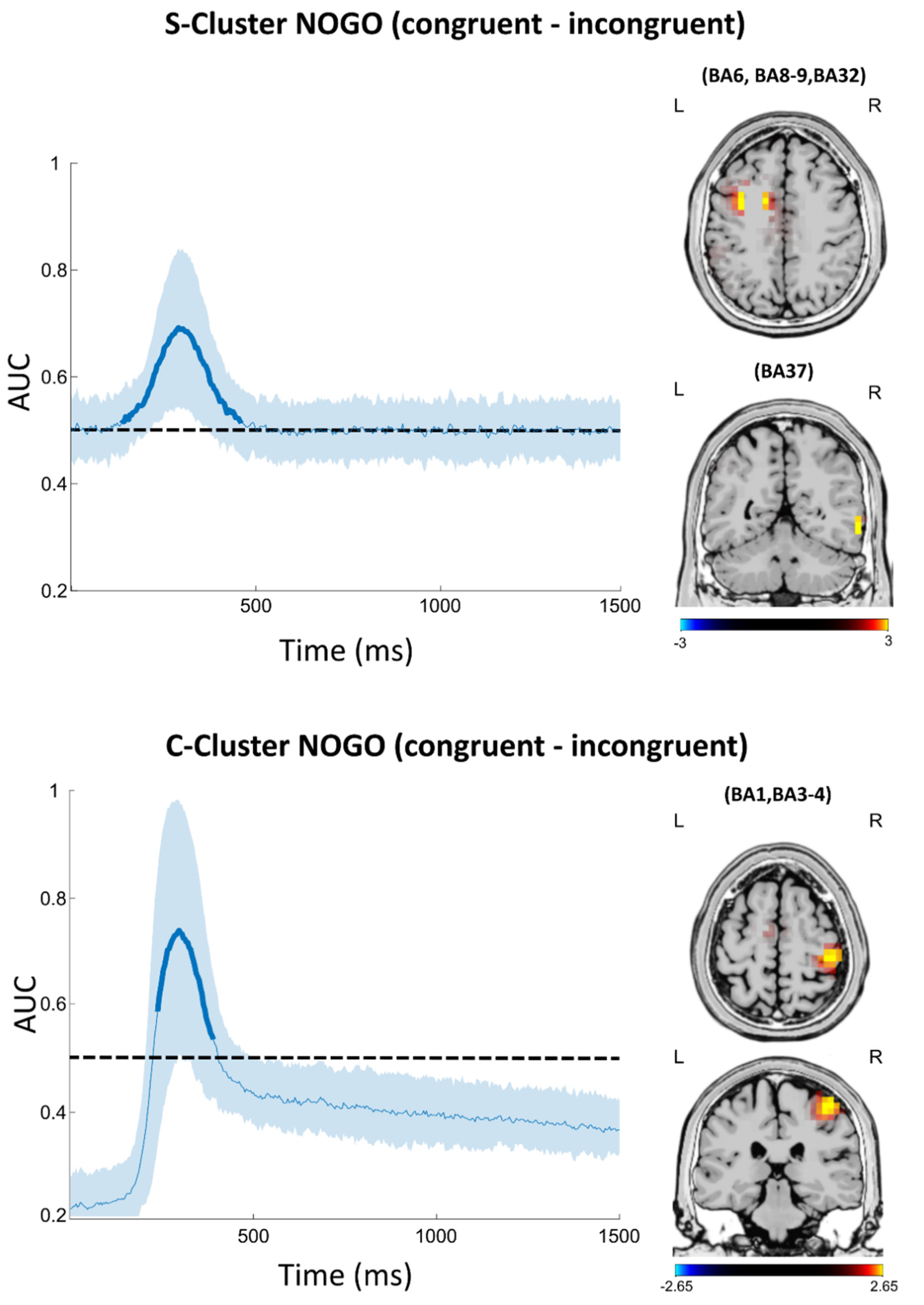
3.3.1. S-Cluster
3.3.2. C-Cluster
3.3.3. Multivariate Pattern Analysis (MVPA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schuckit, M.A. Alcohol-Use Disorders. Lancet 2009, 373, 492–501. [Google Scholar] [CrossRef]
- World Health Organisation. Status Report on Alcohol Consumption Harm Policy Responses 30 European Countries 2019; WHO Regional Office for Europe: Copenhagen, Denmark, 2019. [Google Scholar]
- Kranzler, H.R.; Soyka, M. Diagnosis and Pharmacotherapy of Alcohol Use Disorder: A Review. JAMA 2018, 320, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Olsen, R.W. Alcohol Use Disorders and Current Pharmacological Therapies: The Role of GABAA Receptors. Acta Pharmacol. Sin. 2014, 35, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Lingford-Hughes, A.; Welch, S.; Peters, L.; Nutt, D. BAP Updated Guidelines: Evidence-Based Guidelines for the Pharmacological Management of Substance Abuse, Harmful Use, Addiction and Comorbidity: Recommendations from BAP. J. Psychopharmacol. 2012, 26, 899–952. [Google Scholar] [CrossRef]
- Garbusow, M.; Sebold, M.; Beck, A.; Heinz, A. Too Difficult to Stop: Mechanisms Facilitating Relapse in Alcohol Dependence. Neuropsychobiology 2014, 70, 103–110. [Google Scholar] [CrossRef]
- Grant, B.F.; Goldstein, R.B.; Saha, T.D.; Chou, S.P.; Jung, J.; Zhang, H.; Pickering, R.P.; Ruan, W.J.; Smith, S.M.; Huang, B.; et al. Epidemiology of DSM-5 Alcohol Use Disorder. JAMA Psychiatry 2015, 72, 757–766. [Google Scholar] [CrossRef]
- Heinz, A.; Kiefer, F.; Smolka, M.N.; Endrass, T.; Beste, C.; Beck, A.; Liu, S.; Genauck, A.; Romund, L.; Banaschewski, T.; et al. Addiction Research Consortium: Losing and Regaining Control over Drug Intake (ReCoDe)—From Trajectories to Mechanisms and Interventions. Addict. Biol. 2020, 25, e12866. [Google Scholar] [CrossRef]
- Stock, A.-K. Barking up the Wrong Tree: Why and How We May Need to Revise Alcohol Addiction Therapy. Front. Psychol. 2017, 8, 884. [Google Scholar] [CrossRef]
- Ghin, F.; Beste, C.; Stock, A.-K. Neurobiological Mechanisms of Control in Alcohol Use Disorder—Moving towards Mechanism-Based Non-Invasive Brain Stimulation Treatments. Neurosci. Biobehav. Rev. 2022, 133, 104508. [Google Scholar] [CrossRef]
- Burchi, E.; Makris, N.; Lee, M.R.; Pallanti, S.; Hollander, E. Compulsivity in Alcohol Use Disorder and Obsessive Compulsive Disorder: Implications for Neuromodulation. Front. Behav. Neurosci. 2019, 13, 70. [Google Scholar] [CrossRef]
- Wilcox, C.E.; Dekonenko, C.J.; Mayer, A.R.; Bogenschutz, M.P.; Turner, J.A. Cognitive Control in Alcohol Use Disorder: Deficits and Clinical Relevance. Rev. Neurosci. 2014, 25, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Sherman, J.W.; Gawronski, B.; Gonsalkorale, K.; Hugenberg, K.; Allen, T.J.; Groom, C.J. The Self-Regulation of Automatic Associations and Behavioral Impulses. Psychol. Rev. 2008, 115, 314–335. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, R.; Schröter, H.; Leuthold, H.; Birngruber, T. Automatic and Controlled Stimulus Processing in Conflict Tasks: Superimposed Diffusion Processes and Delta Functions. Cogn. Psychol. 2015, 78, 148–174. [Google Scholar] [CrossRef]
- Botvinick, M.M.; Braver, T.S.; Barch, D.M.; Carter, C.S.; Cohen, J.D. Conflict Monitoring and Cognitive Control. Psychol. Rev. 2001, 108, 624–652. [Google Scholar] [CrossRef] [PubMed]
- Ridderinkhof, R.K. Micro- and Macro-Adjustments of Task Set: Activation and Suppression in Conflict Tasks. Psychol. Res. 2002, 66, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Beste, C.; Moll, C.K.E.; Pötter-Nerger, M.; Münchau, A. Striatal Microstructure and Its Relevance for Cognitive Control. Trends Cogn. Sci. 2018, 22, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Goschke, T. Voluntary Action and Cognitive Control from a Cognitive Neuroscience Perspective. In Voluntary Action: Brains, Minds, and Sociality; Oxford University Press: New York, NY, USA, 2003; pp. 49–85. ISBN 978-0-19-857228-2. [Google Scholar]
- Goschke, T. Dysfunctions of Decision-Making and Cognitive Control as Transdiagnostic Mechanisms of Mental Disorders: Advances, Gaps, and Needs in Current Research. Int. J. Methods Psychiatr. Res. 2014, 23, 41–57. [Google Scholar] [CrossRef]
- Hommel, B. Between Persistence and Flexibility: The Yin and Yang of Action Control. In Advances in Motivation Science; Elliot, A.J., Ed.; Elsevier: New York, NY, USA, 2015; Volume 2, pp. 33–67. [Google Scholar]
- Chmielewski, W.X.; Mückschel, M.; Beste, C. Response Selection Codes in Neurophysiological Data Predict Conjoint Effects of Controlled and Automatic Processes during Response Inhibition. Hum. Brain Mapp. 2018, 39, 1839–1849. [Google Scholar] [CrossRef]
- Opitz, A.; Hubert, J.; Beste, C.; Stock, A.-K. Alcohol Hangover Slightly Impairs Response Selection but Not Response Inhibition. J. Clin. Med. 2019, 8, 1317. [Google Scholar] [CrossRef]
- Wendiggensen, P.; Ghin, F.; Koyun, A.H.; Stock, A.-K.; Beste, C. Pretrial Theta Band Activity Affects Context-Dependent Modulation of Response Inhibition. J. Cogn. Neurosci. 2022, 34, 605–617. [Google Scholar] [CrossRef]
- De Jong, R.; Liang, C.-C.; Lauber, E. Conditional and Unconditional Automaticity: A Dual-Process Model of Effects of Spatial Stimulus-Response Correspondence. J. Exp. Psychol. Hum. Percept. Perform. 1994, 20, 731–750. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, W.X.; Beste, C. Testing Interactive Effects of Automatic and Conflict Control Processes during Response Inhibition—A System Neurophysiological Study. NeuroImage 2017, 146, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Ghin, F.; Stock, A.-K.; Beste, C. The Importance of Resource Allocation for the Interplay between Automatic and Cognitive Control in Response Inhibition—An EEG Source Localization Study. Cortex 2022, 155, 202–217. [Google Scholar] [CrossRef]
- Huster, R.J.; Enriquez-Geppert, S.; Lavallee, C.F.; Falkenstein, M.; Herrmann, C.S. Electroencephalography of Response Inhibition Tasks: Functional Networks and Cognitive Contributions. Int. J. Psychophysiol. 2013, 87, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Eggert, E.; Takacs, A.; Münchau, A.; Beste, C. On the Role of Memory Representations in Action Control: Neurophysiological Decoding Reveals the Reactivation of Integrated Stimulus–Response Feature Representations. J. Cogn. Neurosci. 2022, 34, 1246–1258. [Google Scholar] [CrossRef]
- Petruo, V.; Takacs, A.; Mückschel, M.; Hommel, B.; Beste, C. Multi-Level Decoding of Task Sets in Neurophysiological Data during Cognitive Flexibility. iScience 2021, 24, 103502. [Google Scholar] [CrossRef] [PubMed]
- Prochnow, A.; Bluschke, A.; Weissbach, A.; Münchau, A.; Roessner, V.; Mückschel, M.; Beste, C. Neural Dynamics of Stimulus-Response Representations during Inhibitory Control. J. Neurophysiol. 2021, 126, 680–692. [Google Scholar] [CrossRef]
- Takacs, A.; Mückschel, M.; Roessner, V.; Beste, C. Decoding Stimulus–Response Representations and Their Stability Using EEG-Based Multivariate Pattern Analysis. Cereb. Cortex Commun. 2020, 1, tgaa016. [Google Scholar] [CrossRef]
- Fahrenfort, J.J.; van Driel, J.; van Gaal, S.; Olivers, C.N.L. From ERPs to MVPA Using the Amsterdam Decoding and Modeling Toolbox (ADAM). Front. Neurosci. 2018, 12, 368. [Google Scholar] [CrossRef]
- King, J.-R.; Dehaene, S. Characterizing the Dynamics of Mental Representations: The Temporal Generalization Method. Trends Cogn. Sci. 2014, 18, 203–210. [Google Scholar] [CrossRef]
- Takács, Á.; Yu, S.; Mückschel, M.; Beste, C. Protocol to Decode Representations from EEG Data with Intermixed Signals Using Temporal Signal Decomposition and Multivariate Pattern-Analysis. STAR Protoc. 2022, 3, 101399. [Google Scholar] [CrossRef] [PubMed]
- Treder, M.S. MVPA-Light: A Classification and Regression Toolbox for Multi-Dimensional Data. Front. Neurosci. 2020, 14, 289. [Google Scholar] [CrossRef] [PubMed]
- Bridwell, D.A.; Cavanagh, J.F.; Collins, A.G.E.; Nunez, M.D.; Srinivasan, R.; Stober, S.; Calhoun, V.D. Moving Beyond ERP Components: A Selective Review of Approaches to Integrate EEG and Behavior. Front. Hum. Neurosci. 2018, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Stock, A.-K.; Gohil, K.; Huster, R.J.; Beste, C. On the Effects of Multimodal Information Integration in Multitasking. Sci. Rep. 2017, 7, 4927. [Google Scholar] [CrossRef]
- Yu, S.; Ghin, F.; Mückschel, M.; Ziemssen, T.; Stock, A.-K.; Beste, C. A Role of the Norepinephrine System or Effort in the Interplay of Different Facets of Inhibitory Control. Neuropsychologia 2022, 166, 108143. [Google Scholar] [CrossRef]
- Ouyang, G.; Herzmann, G.; Zhou, C.; Sommer, W. Residue Iteration Decomposition (RIDE): A New Method to Separate ERP Components on the Basis of Latency Variability in Single Trials. Psychophysiology 2011, 48, 1631–1647. [Google Scholar] [CrossRef]
- Ouyang, G.; Sommer, W.; Zhou, C. A Toolbox for Residue Iteration Decomposition (RIDE)—A Method for the Decomposition, Reconstruction, and Single Trial Analysis of Event Related Potentials. J. Neurosci. Methods 2015, 250, 7–21. [Google Scholar] [CrossRef]
- American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders: DSM-5TM, 5th ed.; American Psychiatric Publishing, Inc.: Arlington, VA, USA, 2013; p. xliv. 947. ISBN 978-0-89042-554-1. [Google Scholar]
- World Health Organization; Babor, T.F.; Higgins-Biddle, J.C.; Saunders, J.; Monteiro, M.G. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Health Care, 2nd ed.; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Chmielewski, W.X.; Zink, N.; Chmielewski, K.Y.; Beste, C.; Stock, A.-K. How High-Dose Alcohol Intoxication Affects the Interplay of Automatic and Controlled Processes. Addict. Biol. 2020, 25, e12700. [Google Scholar] [CrossRef]
- Nunez, P.L.; Pilgreen, K.L. The Spline-Laplacian in Clinical Neurophysiology: A Method to Improve EEG Spatial Resolution. J. Clin. Neurophysiol. 1991, 8, 397–413. [Google Scholar] [CrossRef]
- Mückschel, M.; Dippel, G.; Beste, C. Distinguishing Stimulus and Response Codes in Theta Oscillations in Prefrontal Areas during Inhibitory Control of Automated Responses. Hum. Brain Mapp. 2017, 38, 5681–5690. [Google Scholar] [CrossRef]
- Opitz, A.; Beste, C.; Stock, A.-K. Using Temporal EEG Signal Decomposition to Identify Specific Neurophysiological Correlates of Distractor-Response Bindings Proposed by the Theory of Event Coding. NeuroImage 2020, 209, 116524. [Google Scholar] [CrossRef] [PubMed]
- Takács, Á.; Kóbor, A.; Kardos, Z.; Janacsek, K.; Horváth, K.; Beste, C.; Nemeth, D. Neurophysiological and Functional Neuroanatomical Coding of Statistical and Deterministic Rule Information during Sequence Learning. Hum. Brain Mapp. 2021, 42, 3182–3201. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, G.; Hildebrandt, A.; Sommer, W.; Zhou, C. Exploiting the Intra-Subject Latency Variability from Single-Trial Event-Related Potentials in the P3 Time Range: A Review and Comparative Evaluation of Methods. Neurosci. Biobehav. Rev. 2017, 75, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, G.; Schacht, A.; Zhou, C.; Sommer, W. Overcoming Limitations of the ERP Method with Residue Iteration Decomposition (RIDE): A Demonstration in Go/No-Go Experiments. Psychophysiology 2013, 50, 253–265. [Google Scholar] [CrossRef]
- Bradley, A.P. The Use of the Area under the ROC Curve in the Evaluation of Machine Learning Algorithms. Pattern Recognit. 1997, 30, 1145–1159. [Google Scholar] [CrossRef]
- Mazziotta, J.; Toga, A.; Evans, A.; Fox, P.; Lancaster, J.; Zilles, K.; Woods, R.; Paus, T.; Simpson, G.; Pike, B.; et al. A Probabilistic Atlas and Reference System for the Human Brain: International Consortium for Brain Mapping (ICBM). Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 1293–1322. [Google Scholar] [CrossRef]
- Fuchs, M.; Kastner, J.; Wagner, M.; Hawes, S.; Ebersole, J.S. A Standardized Boundary Element Method Volume Conductor Model. Clin. Neurophysiol. 2002, 113, 702–712. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D. Standardized Low-Resolution Brain Electromagnetic Tomography (SLORETA): Technical Details. Methods Find. Exp. Clin. Pharmacol. 2002, 24 (Suppl. D), 5–12. [Google Scholar]
- Sekihara, K.; Sahani, M.; Nagarajan, S.S. Localization Bias and Spatial Resolution of Adaptive and Non-Adaptive Spatial Filters for MEG Source Reconstruction. NeuroImage 2005, 25, 1056–1067. [Google Scholar] [CrossRef]
- Liu, Y.; van den Wildenberg, W.P.M.; de Graaf, Y.; Ames, S.L.; Baldacchino, A.; Bø, R.; Cadaveira, F.; Campanella, S.; Christiansen, P.; Claus, E.D.; et al. Is (Poly-) Substance Use Associated with Impaired Inhibitory Control? A Mega-Analysis Controlling for Confounders. Neurosci. Biobehav. Rev. 2019, 105, 288–304. [Google Scholar] [CrossRef]
- Kamarajan, C.; Porjesz, B.; Jones, K.A.; Choi, K.; Chorlian, D.B.; Padmanabhapillai, A.; Rangaswamy, M.; Stimus, A.T.; Begleiter, H. Alcoholism Is a Disinhibitory Disorder: Neurophysiological Evidence from a Go/No-Go Task. Biol. Psychol. 2005, 69, 353–373. [Google Scholar] [CrossRef] [PubMed]
- Corbit, L.H.; Janak, P.H. Habitual Alcohol Seeking: Neural Bases and Possible Relations to Alcohol Use Disorders. Alcohol. Clin. Exp. Res. 2016, 40, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Garbusow, M.; Schad, D.J.; Sommer, C.; Jünger, E.; Sebold, M.; Friedel, E.; Wendt, J.; Kathmann, N.; Schlagenhauf, F.; Zimmermann, U.S.; et al. Pavlovian-to-Instrumental Transfer in Alcohol Dependence: A Pilot Study. Neuropsychobiology 2014, 70, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Sommer, C.; Garbusow, M.; Jünger, E.; Pooseh, S.; Bernhardt, N.; Birkenstock, J.; Schad, D.J.; Jabs, B.; Glöckler, T.; Huys, Q.M.; et al. Strong Seduction: Impulsivity and the Impact of Contextual Cues on Instrumental Behavior in Alcohol Dependence. Transl. Psychiatry 2017, 7, e1183. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Sharma, A. Compensatory Changes in Cortical Resource Allocation in Adults with Hearing Loss. Front. Syst. Neurosci. 2013, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Geisler, M.W.; Murphy, C. Event-Related Brain Potentials to Attended and Ignored Olfactory and Trigeminal Stimuli. Int. J. Psychophysiol. 2000, 37, 309–315. [Google Scholar] [CrossRef]
- Sugimoto, F.; Katayama, J. Somatosensory P2 Reflects Resource Allocation in a Game Task: Assessment with an Irrelevant Probe Technique Using Electrical Probe Stimuli to Shoulders. Int. J. Psychophysiol. 2013, 87, 200–204. [Google Scholar] [CrossRef]
- Kubo-Kawai, N.; Kawai, N. Elimination of the Enhanced Simon Effect for Older Adults in a Three-Choice Situation: Ageing and the Simon Effect in a Go/No-Go Simon Task. Q. J. Exp. Psychol. 2010, 63, 452–464. [Google Scholar] [CrossRef]
- Correas, A.; Cuesta, P.; Rosen, B.Q.; Maestu, F.; Marinkovic, K. Compensatory Neuroadaptation to Binge Drinking: Human Evidence for Allostasis. Addict. Biol. 2021, 26, e12960. [Google Scholar] [CrossRef]
- Koob, G.; Le Moal, M. Drug Addiction, Dysregulation of Reward, and Allostasis. Neuropsychopharmacology 2001, 24, 97–129. [Google Scholar] [CrossRef]
- Marinkovic, K.; Myers, A.B.A.; Arienzo, D.; Sereno, M.I.; Mason, G.F. Cortical GABA Levels Are Reduced in Young Adult Binge Drinkers: Association with Recent Alcohol Consumption and Sex. NeuroImage Clin. 2022, 35, 103091. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B. Allostasis and Allostatic Load Implications for Neuropsychopharmacology. Neuropsychopharmacology 2000, 22, 108–124. [Google Scholar] [CrossRef]
- Roberto, M.; Varodayan, F.P. Synaptic Targets: Chronic Alcohol Actions. Neuropharmacology 2017, 122, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Haag, L.; Quetscher, C.; Dharmadhikari, S.; Dydak, U.; Schmidt-Wilcke, T.; Beste, C. Interrelation of Resting State Functional Connectivity, Striatal GABA Levels, and Cognitive Control Processes. Hum. Brain Mapp. 2015, 36, 4383–4393. [Google Scholar] [CrossRef] [PubMed]
- Dharmadhikari, S.; Ma, R.; Yeh, C.-L.; Stock, A.-K.; Snyder, S.; Zauber, S.E.; Dydak, U.; Beste, C. Striatal and Thalamic GABA Level Concentrations Play Differential Roles for the Modulation of Response Selection Processes by Proprioceptive Information. Neuroimage 2015, 120, 36–42. [Google Scholar] [CrossRef][Green Version]
- Pérez-Ramírez, Ú.; López-Madrona, V.J.; Pérez-Segura, A.; Pallarés, V.; Moreno, A.; Ciccocioppo, R.; Hyytiä, P.; Sommer, W.H.; Moratal, D.; Canals, S. Brain Network Allostasis after Chronic Alcohol Drinking Is Characterized by Functional Dedifferentiation and Narrowing. J. Neurosci. 2022, 42, 4401–4413. [Google Scholar] [CrossRef]
- Bluschke, A.; Chmielewski, W.X.; Mückschel, M.; Roessner, V.; Beste, C. Neuronal Intra-Individual Variability Masks Response Selection Differences between ADHD Subtypes—A Need to Change Perspectives. Front. Hum. Neurosci. 2017, 11, 329. [Google Scholar] [CrossRef]
- Mückschel, M.; Chmielewski, W.; Ziemssen, T.; Beste, C. The Norepinephrine System Shows Information-Content Specific Properties during Cognitive Control—Evidence from EEG and Pupillary Responses. NeuroImage 2017, 149, 44–52. [Google Scholar] [CrossRef]
- Verleger, R.; Metzner, M.F.; Ouyang, G.; Śmigasiewicz, K.; Zhou, C. Testing the Stimulus-to-Response Bridging Function of the Oddball-P3 by Delayed Response Signals and Residue Iteration Decomposition (RIDE). Neuroimage 2014, 100, 271–280. [Google Scholar] [CrossRef]
- Verleger, R.; Siller, B.; Ouyang, G.; Śmigasiewicz, K. Effects on P3 of Spreading Targets and Response Prompts Apart. Biol. Psychol. 2017, 126, 1–11. [Google Scholar] [CrossRef]
- Wolff, N.; Mückschel, M.; Beste, C. Neural Mechanisms and Functional Neuroanatomical Networks during Memory and Cue-Based Task Switching as Revealed by Residue Iteration Decomposition (RIDE) Based Source Localization. Brain Struct. Funct. 2017, 222, 3819–3831. [Google Scholar] [CrossRef] [PubMed]
- Hommel, B. The Simon Effect as Tool and Heuristic. Acta Psychol. 2011, 136, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Keye, D.; Wilhelm, O.; Oberauer, K.; Stürmer, B. Individual Differences in Response Conflict Adaptations. Front. Psychol 2013, 4, 947. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.R.; Robbins, T.W.; Poldrack, R.A. Inhibition and the Right Inferior Frontal Cortex. Trends Cogn. Sci. 2004, 8, 170–177. [Google Scholar] [CrossRef]
- Aron, A.R.; Cai, W.; Badre, D.; Robbins, T.W. Evidence Supports Specific Braking Function for Inferior PFC. Trends Cogn. Sci. 2015, 19, 711–712. [Google Scholar] [CrossRef]
- Garavan, H.; Hester, R.; Murphy, K.; Fassbender, C.; Kelly, C. Individual Differences in the Functional Neuroanatomy of Inhibitory Control. Brain Res. 2006, 1105, 130–142. [Google Scholar] [CrossRef]
- Kelly, A.M.C.; Hester, R.; Murphy, K.; Javitt, D.C.; Foxe, J.J.; Garavan, H. Prefrontal-Subcortical Dissociations Underlying Inhibitory Control Revealed by Event-Related FMRI. Eur. J. Neurosci. 2004, 19, 3105–3112. [Google Scholar] [CrossRef]
- Konishi, S.; Nakajima, K.; Uchida, I.; Sekihara, K.; Miyashita, Y. No-Go Dominant Brain Activity in Human Inferior Prefrontal Cortex Revealed by Functional Magnetic Resonance Imaging. Eur. J. Neurosci. 1998, 10, 1209–1213. [Google Scholar] [CrossRef]
- Bari, A.; Robbins, T.W. Inhibition and Impulsivity: Behavioral and Neural Basis of Response Control. Prog. Neurobiol. 2013, 108, 44–79. [Google Scholar] [CrossRef]
- Wolff, N.; Chmielewski, W.; Buse, J.; Roessner, V.; Beste, C. Paradoxical Response Inhibition Advantages in Adolescent Obsessive Compulsive Disorder Result from the Interplay of Automatic and Controlled Processes. NeuroImage Clin. 2019, 23, 101893. [Google Scholar] [CrossRef]
- Abrahamse, E.L.; Van der Lubbe, R.H.J. Endogenous Orienting Modulates the Simon Effect: Critical Factors in Experimental Design. Psychol. Res. 2008, 72, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Vahid, A.; Mückschel, M.; Stober, S.; Stock, A.-K.; Beste, C. Applying Deep Learning to Single-Trial EEG Data Provides Evidence for Complementary Theories on Action Control. Commun. Biol. 2020, 3, 112. [Google Scholar] [CrossRef] [PubMed]
- Wascher, E.; Schatz, U.; Kuder, T.; Verleger, R. Validity and Boundary Conditions of Automatic Response Activation in the Simon Task. J. Exp. Psychol. Hum. Percept. Perform. 2001, 27, 731–751. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, K.; Wascher, E. Dynamic Aspects of Stimulus-Response Correspondence: Evidence for Two Mechanisms Involved in the Simon Effect. J. Exp. Psychol. Hum. Percept. Perform. 2005, 31, 453–464. [Google Scholar] [CrossRef]
- Bensmann, W.; Zink, N.; Werner, A.; Beste, C.; Stock, A.-K. Acute Alcohol Effects on Response Inhibition Depend on Response Automatization, but Not on GABA or Glutamate Levels in the ACC and Striatum. J. Clin. Med. 2020, 9, 481. [Google Scholar] [CrossRef]
| AUD | Control | |||||
|---|---|---|---|---|---|---|
| M (SEM) | Range | M (SEM) | Range | z | p | |
| Age (years) | 27.07 (0.8) | 18–40 | 27.06 (0.7) | 18–40 | −0.257 | 0.798 |
| Years of education | 12.70 (0.1) | 9–13 | 12.16 (0.2) | 9–13 | −2.595 | 0.009 * |
| BDI | 6.59 (0.7) | 0–19 | 4.38 (0.5) | 0–17 | −2.216 | 0.027 * |
| MMSE | 29.14 (0.1) | 26–30 | 29.32 (0.1) | 27–30 | −1.258 | 0.208 |
| AUD | Control | |||||
|---|---|---|---|---|---|---|
| M (SEM) | Range | M (SEM) | Range | z | p | |
| 1-year AUD criteria | 4.41 (0.22) | 2–9 | 0.25 (0.05) | 0–1 | −9.894 | <0.001 * |
| Lifetime AUD criteria | 4.17 (0.34) | 0–10 | 0.66 (0.12) | 0–4 | −7.892 | <0.001 * |
| AUDIT score | 14.81 (0.70) | 5–31 | 5.47 (0.39) | 0–16 | −8.608 | <0.001 * |
| Drinking frequency | 41.14 (2.52) | 6–84 | 18.16 (1.85) | 0–60 | −6.270 | <0.001 * |
| Binge drinking frequency | 16.86 (1.89) | 0–66 | 3.10 (0.57) | 0–24 | −6.734 | <0.001 * |
| Main Effects | F | p | η2p |
| Group | 8.120 | 0.005 * | 0.063 |
| Condition | 24.856 | <0.001 * | 0.170 |
| Congruency | 10.533 | 0.002 * | 0.080 |
| Interaction Effects | |||
| Condition × Congruency | 42.296 | <0.001 * | 0.259 |
| Group × Condition | 14.388 | <0.001* | 0.106 |
| Group × Congruency | 1.916 | 0.169 | 0.016 |
| Group × Condition × Congruency | 4.414 | 0.038 * | 0.035 |
| ANCOVA with AUD 1 Year | |||
|---|---|---|---|
| Effect | F | p | η2p |
| Group | 3.116 | 0.080 | 0.025 |
| Condition | 7.819 | 0.006 * | 0.061 |
| Congruency | 0.233 | 0.630 | 0.002 |
| AUD 1 year | 0.171 | 0.680 | 0.001 |
| Interactions | |||
| Condition × Congruency | 11.344 | 0.001 * | 0.086 |
| Group × Condition | 1.809 | 0.181 | 0.015 |
| Group × Congruency | 2.726 | 0.101 | 0.022 |
| Group × Condition × Congruency | 0.383 | 0.537 | 0.003 |
| Condition × AUD 1 year | 0.370 | 0.544 | 0.003 |
| Congruency × AUD 1 year | 1.234 | 0.269 | 0.010 |
| Condition × Congruency × AUD 1 year | 0.232 | 0.631 | 0.002 |
| ANCOVA with AUDIT | |||
| Effect | F | p | η2p |
| Group | 2.461 | 0.119 | 0.020 |
| Condition | 7.867 | 0.006 * | 0.062 |
| Congruency | 1.970 | 0.163 | 0.016 |
| AUDIT | 0.229 | 0.633 | 0.002 |
| Interactions | |||
| Condition × Congruency | 9.500 | 0.003 * | 0.073 |
| Group × Condition | 3.508 | 0.064 | 0.028 |
| Group × Congruency | 0.667 | 0.416 | 0.006 |
| Group × Condition × Congruency | 0.946 | 0.333 | 0.008 |
| Condition × AUDIT | 0.880 | 0.350 | 0.007 |
| Congruency × AUDIT | 0.025 | 0.876 | <0.001 |
| Condition × Congruency × AUDIT | 0.364 | 0.547 | 0.003 |
| ANCOVA with Drinking frequency | |||
| Effect | F | p | η2p |
| Group | 1.046 | 0.308 | 0.009 |
| Condition | 17.127 | <0.001 * | 0.125 |
| Congruency | 21.392 | <0.001 * | 0.151 |
| Drinking frequency | 6.145 | 0.015 * | 0.049 |
| Interactions | |||
| Condition × Congruency | 18.645 | <0.001 * | 0.134 |
| Group × Condition | 4.503 | 0.036 * | 0.036 |
| Group × Congruency | 0.495 | 0.483 | 0.004 |
| Group × Condition × Congruency | 1.103 | 0.296 | 0.009 |
| Condition × Drinking frequency | 3.541 | 0.062 | 0.029 |
| Congruency × Drinking frequency | 11.502 | <0.001 * | 0.087 |
| Condition × Congruency × Drinking frequency | 1.537 | 0.217 | 0.013 |
| ANCOVA with Binge drinking frequency 1 | |||
| Effect | F | p | η2p |
| Group | 5.772 | 0.018 * | 0.046 |
| Condition | 13.267 | <0.001 * | 0.100 |
| Congruency | 5.514 | 0.021 * | 0.044 |
| Binge drinking | <0.001 | 0.984 | <0.001 |
| Interactions | |||
| Condition × Congruency | 31.653 | <0.001 * | 0.210 |
| Group × Condition | 9.686 | 0.002 * | 0.075 |
| Group × Congruency | 1.030 | 0.312 | 0.009 |
| Group × Condition × Congruency | 1.031 | 0.312 | 0.009 |
| Condition × Binge drinking | 0.006 | 0.941 | <0.001 |
| Congruency × Binge drinking | 0.012 | 0.914 | <0.001 |
| Condition × Congruency × Binge drinking | 1.808 | 0.181 | 0.015 |
| B | SE B | β | t | p | |
|---|---|---|---|---|---|
| Constant | −2.764 | 0.539 | −5.129 | <0.001 * | |
| Group | 0.468 | 1.078 | 0.083 | 0.434 | 0.665 |
| AUD 1 year | −0.089 | 0.243 | −0.075 | −0.366 | 0.715 |
| AUDIT | 0.008 | 0.075 | 0.018 | 0.105 | 0.917 |
| Drinking frequency | 0.039 | 0.016 | 0.285 | 2.395 | 0.018 * |
| Binge drinking frequency | −0.001 | 0.031 | −0.006 | −0.045 | 0.964 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghin, F.; Beste, C.; Stock, A.-K. On the Role of Stimulus-Response Context in Inhibitory Control in Alcohol Use Disorder. J. Clin. Med. 2022, 11, 6557. https://doi.org/10.3390/jcm11216557
Ghin F, Beste C, Stock A-K. On the Role of Stimulus-Response Context in Inhibitory Control in Alcohol Use Disorder. Journal of Clinical Medicine. 2022; 11(21):6557. https://doi.org/10.3390/jcm11216557
Chicago/Turabian StyleGhin, Filippo, Christian Beste, and Ann-Kathrin Stock. 2022. "On the Role of Stimulus-Response Context in Inhibitory Control in Alcohol Use Disorder" Journal of Clinical Medicine 11, no. 21: 6557. https://doi.org/10.3390/jcm11216557
APA StyleGhin, F., Beste, C., & Stock, A.-K. (2022). On the Role of Stimulus-Response Context in Inhibitory Control in Alcohol Use Disorder. Journal of Clinical Medicine, 11(21), 6557. https://doi.org/10.3390/jcm11216557








