Meditation and Irritable Bowel Syndrome, a Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
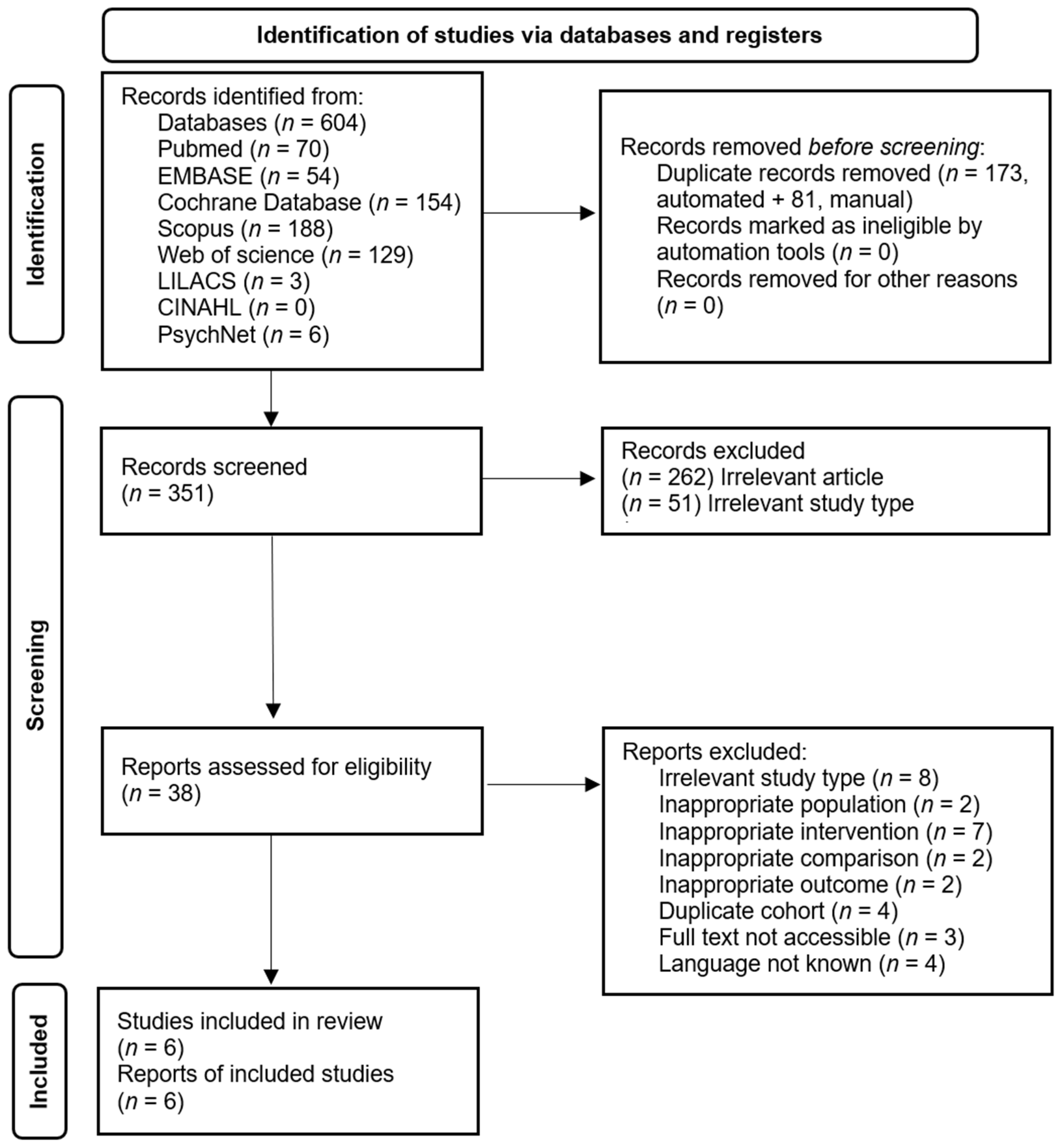
2.4. Selection Process
2.5. Data Collection Process and Data Items
2.6. Study Risk of Bias Assessment
2.7. Effect Measures
2.8. Synthesis Methods
2.9. Reporting Bias Assessment
3. Results
3.1. Study Characteristics
3.2. Treatment Outcomes
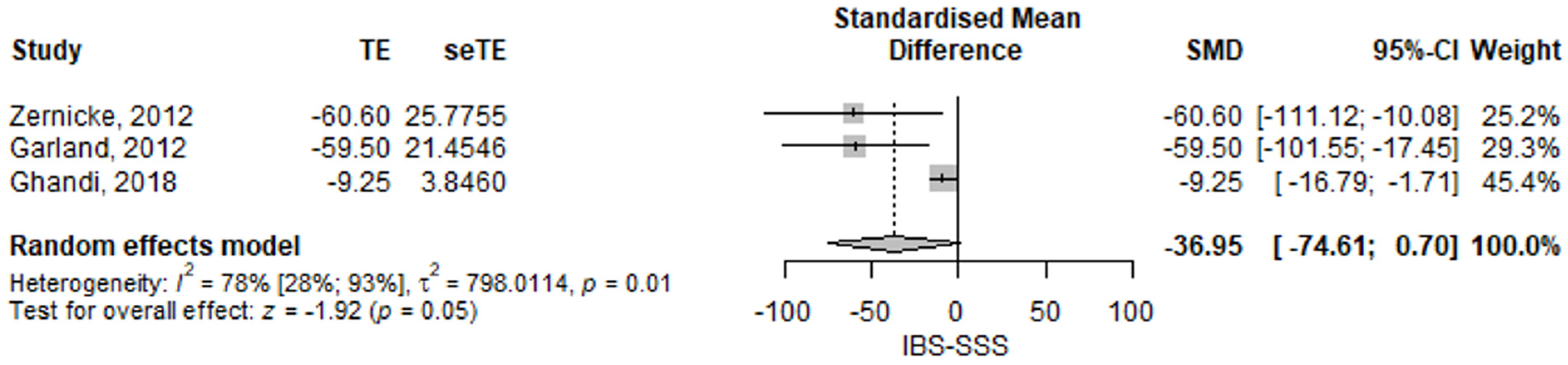
3.2.1. Irritable Bowel Syndrome Severity Symptom Score
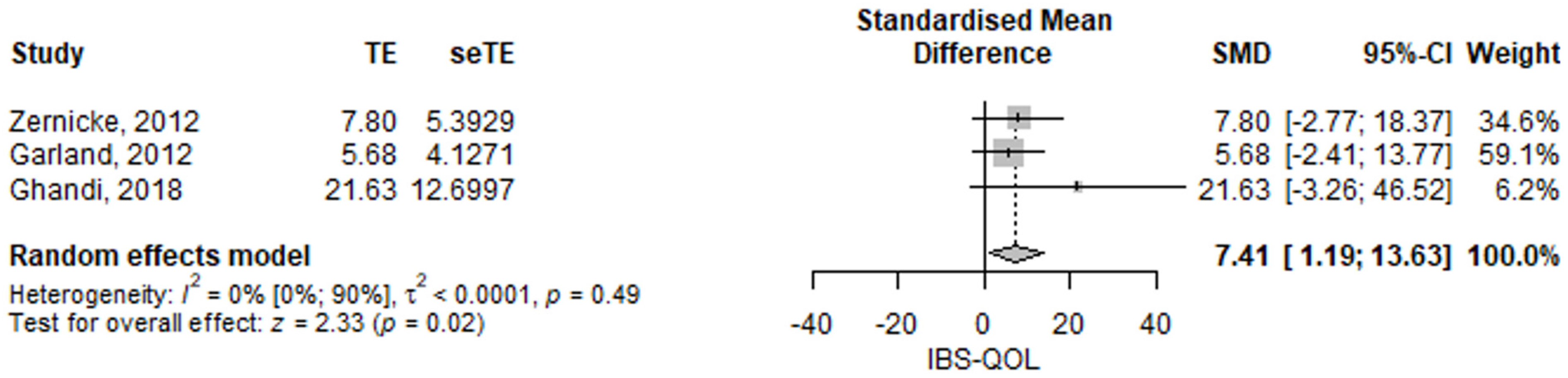
3.2.2. Irritable Bowel Syndrome Quality of Life
3.2.3. Irritable Bowel Syndrome, Perceived Stress
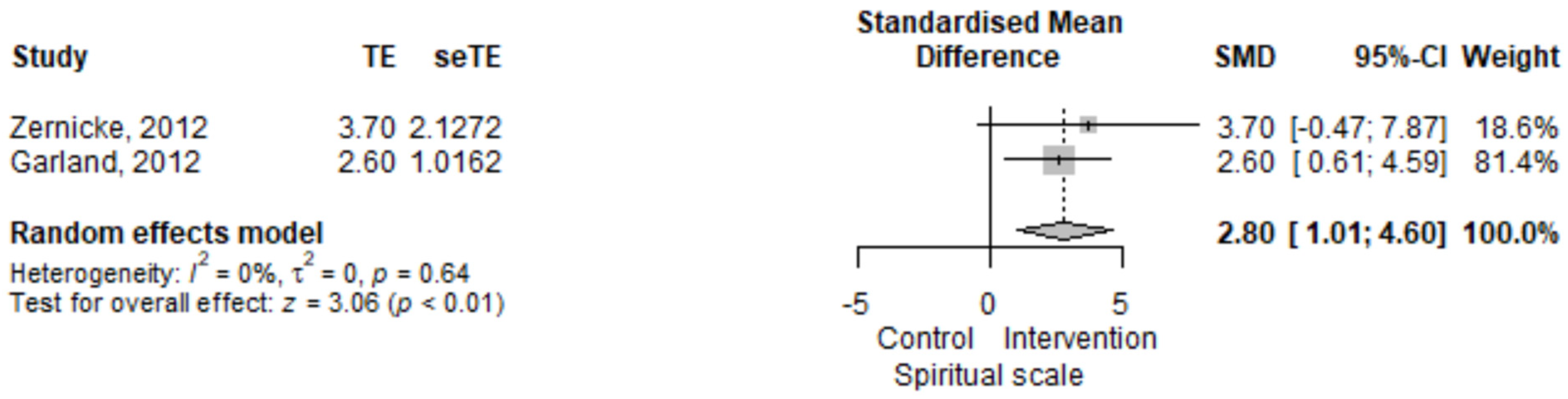
3.2.4. Irritable Bowel Syndrome, Spiritual Scale
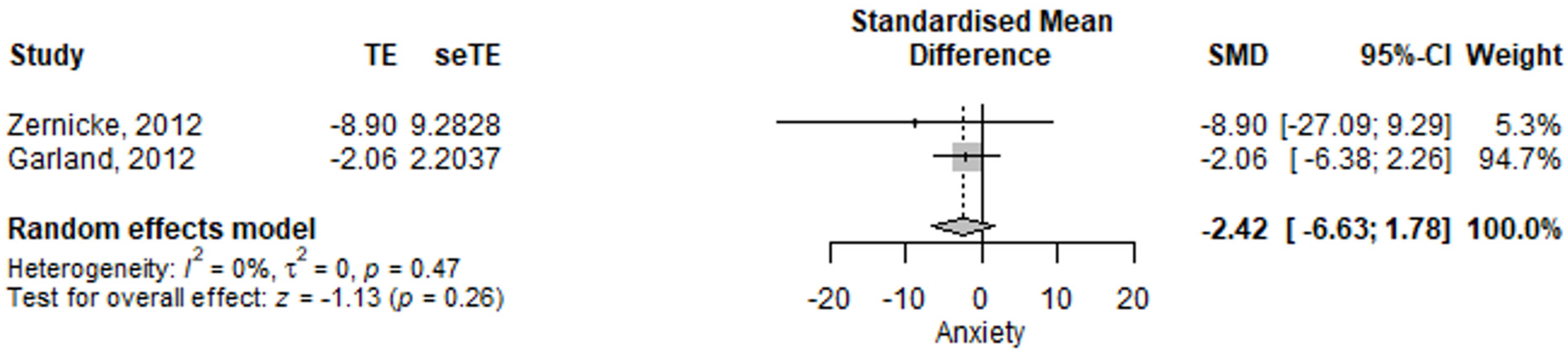
3.2.5. Irritable Bowel Syndrome, Anxiety Assessment
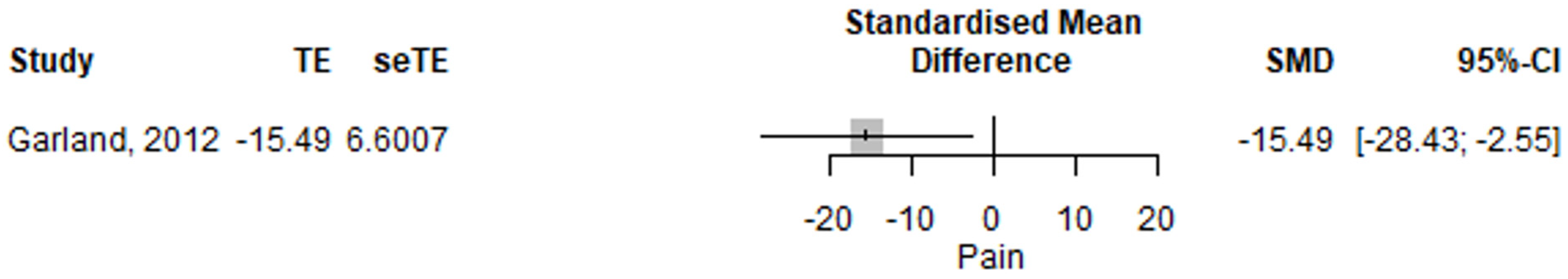
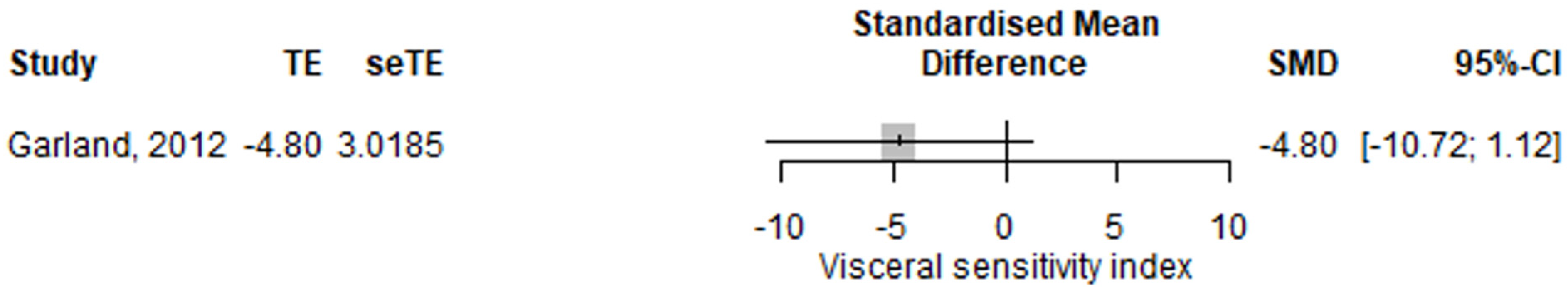
3.2.6. Irritable Bowel Syndrome, Pain and Visceral Sensitivity
3.3. Follow-Up Measurements
3.4. Quality Assessment
3.5. Questionnaires’ Translation and Validity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Palma, G.; Collins, S.M.; Bercik, P. The Microbiota-Gut-Brain Axis in Functional Gastrointestinal Disorders. Gut Microbes 2014, 5, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Aziz, I.; Palsson, O.S.; Törnblom, H.; Sperber, A.D.; Whitehead, W.E.; Simrén, M. The Prevalence and Impact of Overlapping Rome IV-Diagnosed Functional Gastrointestinal Disorders on Somatization, Quality of Life, and Healthcare Utilization: A Cross-Sectional General Population Study in Three Countries. Am. J. Gastroenterol. 2018, 113, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Kearney, D.J.; Brown-Chang, J. Complementary and Alternative Medicine for IBS in Adults: Mind-Body Interventions. Nat. Clin. Pract. Gastroenterol. Hepatol. 2008, 5, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Babos, C.I. Mind-Body Medicine si Recuperarea din Adictii—Lucrare de Disertatie; Universitatea de Medicină și Farmacie: Cluj-Napoca, Romania, 2018. [Google Scholar]
- Chiesa, A.; Serretti, A. Mindfulness-Based Interventions for Chronic Pain: A Systematic Review of the Evidence. J. Altern. Complement. Med. 2011, 17, 83–93. [Google Scholar] [CrossRef] [PubMed]
- D’Silva, S.; Poscablo, C.; Habousha, R.; Kogan, M.; Kligler, B. Mind-Body Medicine Therapies for a Range of Depression Severity: A Systematic Review. Psychosomatics 2012, 53, 407–423. [Google Scholar] [CrossRef]
- Kaliman, P.; Álvarez-López, M.J.; Cosín-Tomás, M.; Rosenkranz, M.A.; Lutz, A.; Davidson, R.J. Rapid Changes in Histone Deacetylases and Inflammatory Gene Expression in Expert Meditators. Psychoneuroendocrinology 2014, 40, 96–107. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series; John Wiley & Sons: Chichester, UK, 2008. [Google Scholar]
- EndNote. Available online: https://access.clarivate.com/login?app=endnote (accessed on 3 March 2022).
- Corporation for Digital Scholarship. Zotero. Available online: https://www.zotero.org/ (accessed on 3 March 2022).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Schwarzer, G. Meta: General Package for Meta-Analysis. R News 2007, 7, 40–45. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Garland, E.L.; Gaylord, S.A.; Palsson, O.; Faurot, K.; Douglas Mann, J.; Whitehead, W.E. Therapeutic Mechanisms of a Mindfulness-Based Treatment for IBS: Effects on Visceral Sensitivity, Catastrophizing, and Affective Processing of Pain Sensations. J. Behav. Med. 2012, 35, 591–602. [Google Scholar] [CrossRef]
- Gaylord, S.A.; Palsson, O.S.; Garland, E.L.; Faurot, K.R.; Coble, R.S.; Mann, D.J.; Frey, W.; Leniek, K.; Whitehead, W.E. Mindfulness Training Reduces the Severity of Irritable Bowel Syndrome in Women: Results of a Randomized Controlled Trial. Am. J. Gastroenterol. 2011, 106, 1678–1688. [Google Scholar] [CrossRef]
- Zernicke, K.A.; Campbell, T.S.; Blustein, P.K.; Fung, T.S.; Johnson, J.A.; Bacon, S.L.; Carlson, L.E. Mindfulness-Based Stress Reduction for the Treatment of Irritable Bowel Syndrome Symptoms: A Randomized Wait-List Controlled Trial. Int. J. Behav. Med. 2013, 20, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Zomorodi, S.; Abdi, S.; Tabatabaee, S.K.R. Comparison of Long-Term Effects of Cognitive-Behavioral Therapy versus Mindfulness-Based Therapy on Reduction of Symptoms among Patients Suffering from Irritable Bowel Syndrome. Gastroenterol. Hepatol. 2014, 7, 118–124. [Google Scholar]
- Zomorrodi, S.; Rasoulzadeh, S.K. Long Term Effects of Mindfulness on Quality of Life in Irritable Bowel Syndrome. Iran. J. Psychiatry 2015, 10, 100–105. [Google Scholar] [PubMed]
- Ghandi, F.; Sadeghi, A.; Bakhtyari, M.; Imani, S.; Abdi, S.; Banihashem, S.S. Comparing the Efficacy of Mindfulness-Based Stress Reduction Therapy with Emotion Regulation Treatment on Quality of Life and Symptoms of Irritable Bowel Syndrome. Iran. J. Psychiatry 2018, 13, 175–183. [Google Scholar]
- Mohamadi, J.; Ghazanfari, F.; Drikvand, F.M. Comparison of the Effect of Dialectical Behavior Therapy, Mindfulness Based Cognitive Therapy and Positive Psychotherapy on Perceived Stress and Quality of Life in Patients with Irritable Bowel Syndrome: A Pilot Randomized Controlled Trial. Psychiatr. Q. 2019, 90, 565–578. [Google Scholar] [CrossRef]
- Kabat-Zinn, J.; Borysenko, J.; Hanh, T.N. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness; Revised edition; Bantam Books: New York, NY, USA, 2013; ISBN 978-0-345-53693-8. [Google Scholar]
- Segal, Z.V.; Williams, J.M.G.; Teasdale, J.D. Mindfulness-Based Cognitive Therapy for Depression: A New Approach to Preventing Relapse, 1st ed.; The Guilford Press: New York, NY, USA, 2001; ISBN 978-1-57230-706-3. [Google Scholar]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The Irritable Bowel Severity Scoring System: A Simple Method of Monitoring Irritable Bowel Syndrome and Its Progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Patrick, D.L.; Drossman, D.A.; Frederick, I.O.; DiCesare, J.; Puder, K.L. Quality of life in persons with irritable bowel syndrome: Development and validation of a new measure. Am. J. Dig. Dis. 1998, 43, 400–411. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A Global Measure of Perceived Stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Peterman, A.H.; Fitchett, G.; Brady, M.J.; Hernandez, L.; Cella, D. Measuring Spiritual Well-Being in People with Cancer: The Functional Assessment of Chronic Illness Therapy--Spiritual Well-Being Scale (FACIT-Sp). Ann. Behav. Med. 2002, 24, 49–58. [Google Scholar] [CrossRef]
- Derogatis, L.R. Brief Symptom Inventory. Eur. J. Psychol. Assess 1978, 13, 595–605. [Google Scholar]
- McNair, D.M.; Lorr, M.; Droppleman, L.F. Profile of Mood States; Educational and Industrial Testing Service: San Diego, CA, USA, 1971. [Google Scholar]
- Rosenstiel, A.K.; Keefe, F.J. The Use of Coping Strategies in Chronic Low Back Pain Patients: Relationship to Patient Characteristics and Current Adjustment. Pain 1983, 17, 33–44. [Google Scholar] [CrossRef]
- Robinson, M.E.; Riley, J.L.; Myers, C.D.; Sadler, I.J.; Kvaal, S.A.; Geisser, M.E.; Keefe, F.J. The Coping Strategies Questionnaire: A Large Sample, Item Level Factor Analysis. Clin. J. Pain 1997, 13, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Labus, J.S.; Bolus, R.; Chang, L.; Wiklund, I.; Naesdal, J.; Mayer, E.A.; Naliboff, B.D. The Visceral Sensitivity Index: Development and Validation of a Gastrointestinal Symptom-Specific Anxiety Scale. Aliment. Pharmacol. Ther. 2004, 20, 89–97. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | SMD (95% CI) | p-Value | Studies |
|---|---|---|---|
| IBS-SSS | −20.2 (−71.57–31.17) | 0.441 | [17] |
| IBS-QOL | 7.41 (95% CI 1.19–13.63) | p = 0.02 | [15,17,20] |
| IBS-PS | −2.51 (−6.7–1.69) | 0.241 | [15,17] |
| Spiritual scale | 2.1 (−2.07–6.27) | 0.324 | [17] |
| Anxiety | −3.49 (−12.43–5.45) | 0.445 | [15,17] |
| Pain | −14.38 (−40.88–12.12) | 0.288 | [15] |
| Visceral sensitivity index | 9.39 (−5.81–24.59) | 0.226 | [15] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baboș, C.-I.; Leucuța, D.-C.; Dumitrașcu, D.L. Meditation and Irritable Bowel Syndrome, a Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 6516. https://doi.org/10.3390/jcm11216516
Baboș C-I, Leucuța D-C, Dumitrașcu DL. Meditation and Irritable Bowel Syndrome, a Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(21):6516. https://doi.org/10.3390/jcm11216516
Chicago/Turabian StyleBaboș, Cristian-Ioan, Daniel-Corneliu Leucuța, and Dan Lucian Dumitrașcu. 2022. "Meditation and Irritable Bowel Syndrome, a Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 21: 6516. https://doi.org/10.3390/jcm11216516
APA StyleBaboș, C.-I., Leucuța, D.-C., & Dumitrașcu, D. L. (2022). Meditation and Irritable Bowel Syndrome, a Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(21), 6516. https://doi.org/10.3390/jcm11216516







