Outcomes of Directional Branches of the T-Branch Off-the-Shelf Multi-Branched Stent-Graft
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Procedure
2.3. Endpoints
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
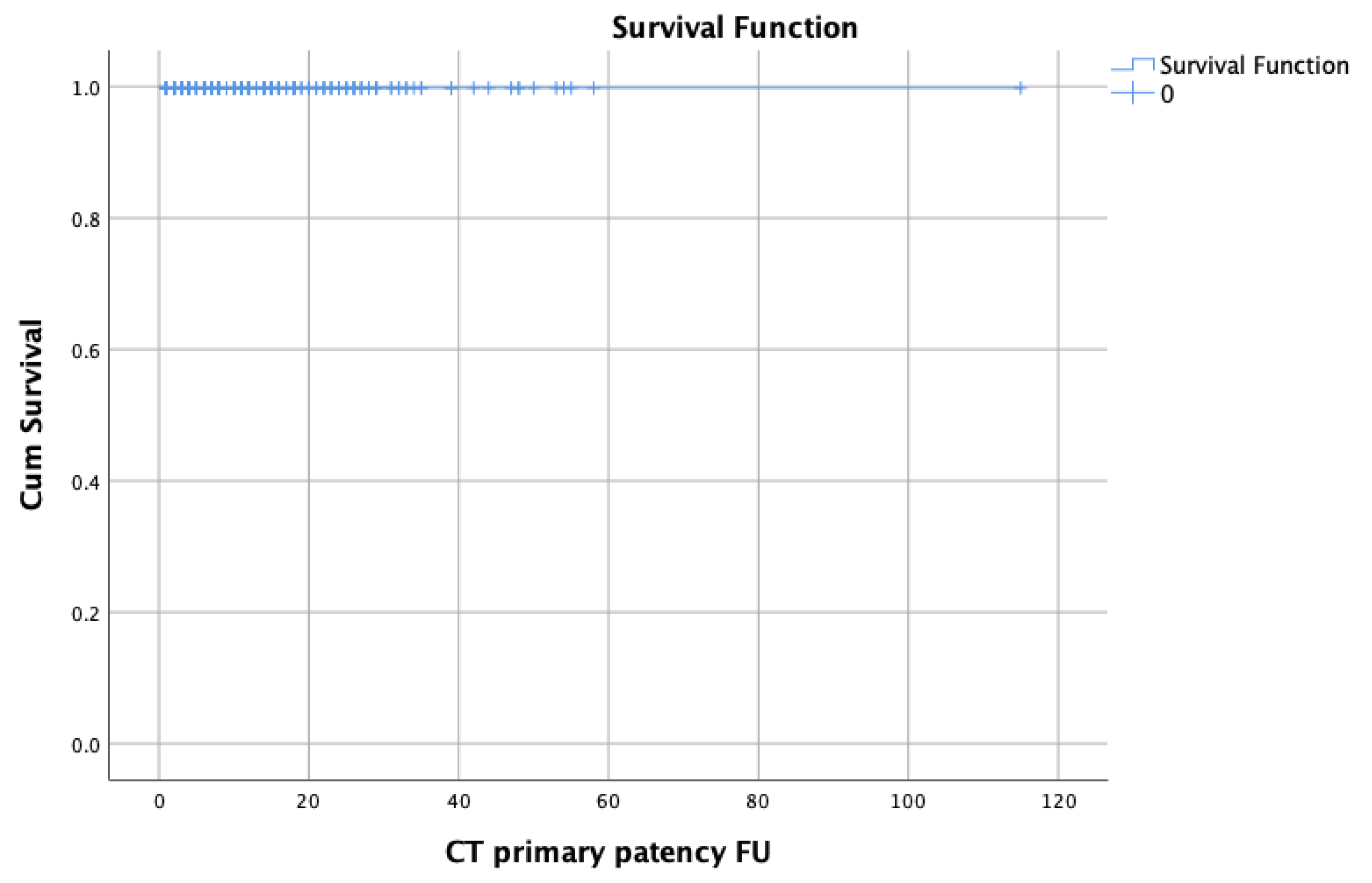
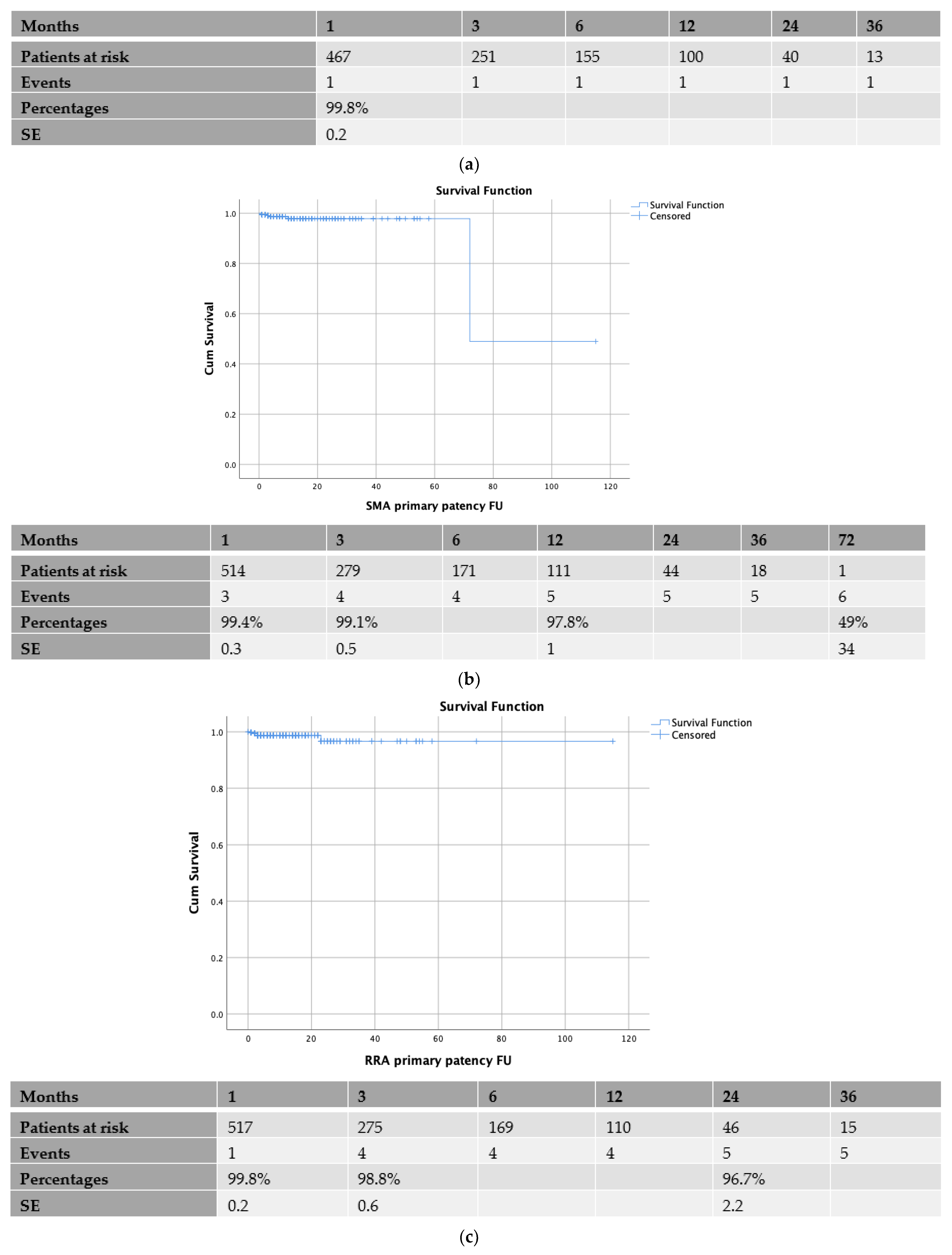
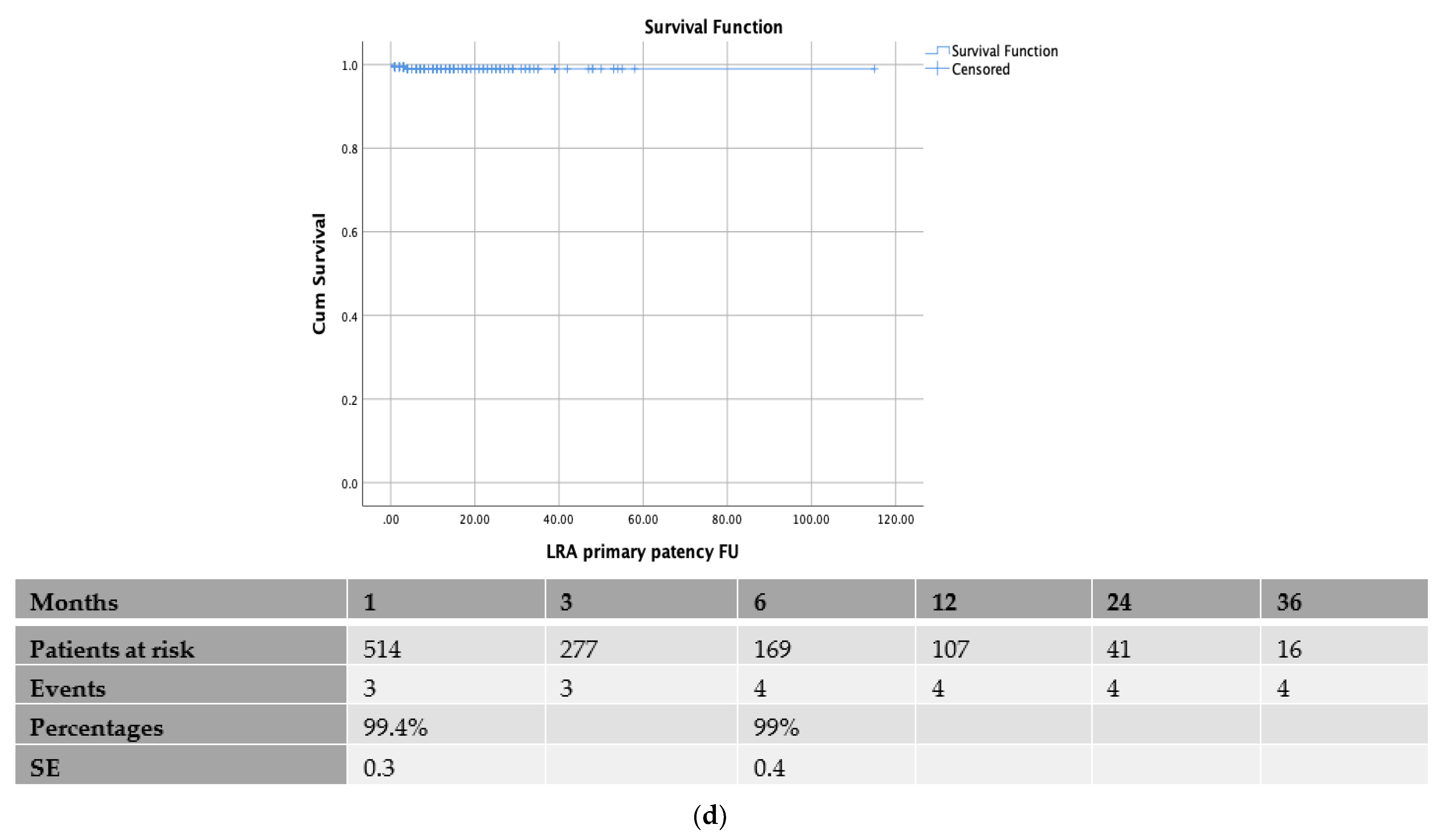
3.2. Patency Rates
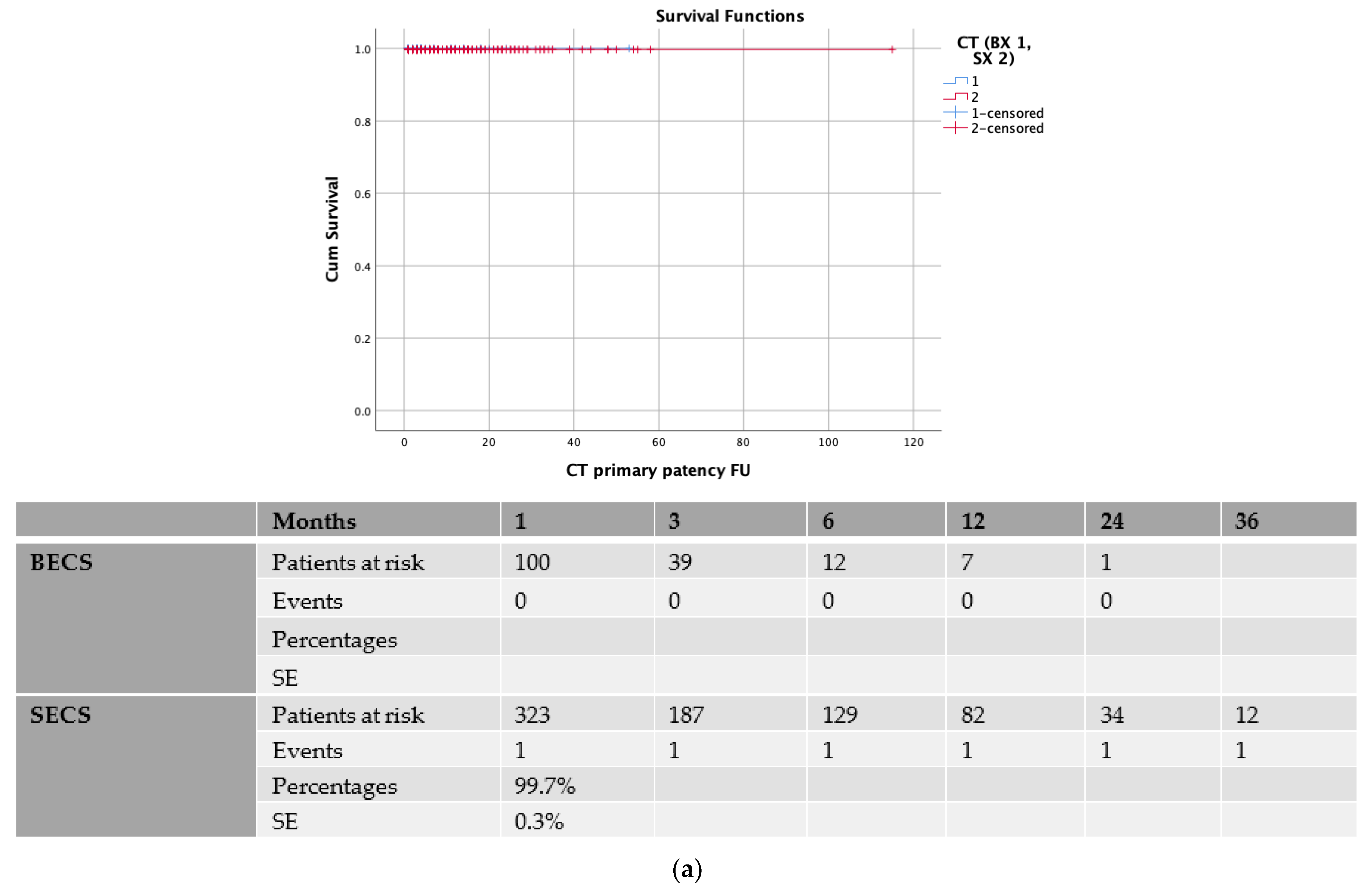
3.3. Balloon-Expandable vs. Self-Expanding Covered Stents
3.4. Early vs. Late Experience
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spanos, K.; Kölbel, T.; Kubitz, J.C.; Wipper, S.; Konstantinou, N.; Heidemann, F.; Rohlffs, F.; Debus, S.E.; Tsilimparis, N. Risk of spinal cord ischemia after fenestrated or branched endovascular repair of complex aortic aneurysms. J. Vasc. Surg. 2019, 69, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Makaloski, V.; Tsilimparis, N.; Panuccio, G.; Spanos, K.; Wyss, T.R.; Rohlffs, F.; Debus, E.S.; Kölbel, T. Perioperative Outcome of Fenestrated and Branched Stent Grafting after Previous Open or Endovascular Abdominal Aortic Repair. Ann. Vasc. Surg. 2021, 74, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Kölbel, T.; Spanos, K.; Jama, K.; Behrendt, C.-A.; Panuccio, G.; Eleshra, A.; Rohlffs, F.; Jakimowicz, T. Early outcomes of the t-Branch off-the-shelf multi-branched stent graft in 542 patients for elective and urgent aortic pathologies: A retrospective observational study. J. Vasc. Surg. 2021, 74, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Eleshra, A.; Hatm, M.; Spanos, K.; Panuccio, G.; Rohlffs, F.; Debus, E.S.; Behrendt, C.-A.; Tsilimparis, N.; Kölbel, T. Early outcomes of t-Branch off-the-shelf multibranched stent-graft in urgent and emergent repair of thoracoabdominal aortic aneurysms. J. Vasc. Surg. 2021, 75, 416–424.e2. [Google Scholar] [CrossRef] [PubMed]
- Spanos, K.; Kölbel, T.; Theodorakopoulou, M.; Heidemann, F.; Rohlffs, F.; Debus, E.S.; Tsilimparis, N. Early Outcomes of the t-Branch Off-the-Shelf Multibranched Stent-Graft in Urgent Thoracoabdominal Aortic Aneurysm Repair. J. Endovasc. Ther. 2018, 25, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Mastracci, T.M.; Eagleton, M.J.; Kuramochi, Y.; Bathurst, S.; Wolski, K. Twelve-year results of fenestrated endografts for juxtarenal and group iv thoracoabdominal aneurysms. J. Vasc. Surg. 2015, 61, 355–364. [Google Scholar] [CrossRef]
- Oderich, G.S.; Ribeiro, M.; Hofer, J.; Wigham, J.; Cha, S.; Chini, J.; Macedo, T.A.; Gloviczki, P. Prospective, nonrandomized study to evaluate endovascular repair of pararenal and thoracoabdominal aortic aneurysms using fenestrated-branched endografts based on supraceliac sealing zones. J. Vasc. Surg. 2017, 65, 1249–1259.e10. [Google Scholar] [CrossRef]
- Walker, J.; Kaushik, S.; Hoffman, M.; Gasper, W.; Hiramoto, J.; Reilly, L.; Chuter, T. Long-term durability of multibranched endovascular repair of thoracoabdominal and pararenal aortic aneurysms. J. Vasc. Surg. 2019, 69, 341–347. [Google Scholar] [CrossRef]
- Spanos, K.; Antoniou, G.A.; Giannoukas, A.D.; Rohlffs, F.; Tsilimparis, N.; Debus, S.E.; Koelbel, T. Durability of fenestrated endovascular aortic repair for juxta-renal abdominal aortic aneurysm repair. J. Cardiovasc. Surg. 2018, 59, 213–224. [Google Scholar] [CrossRef]
- Oderich, G.S.; Farber, M.A.; Schneider, D.; Makaroun, M.; Sanchez, L.A.; Schanzer, A.; Beck, A.W.; Starnes, B.W.; Fillinger, M.; Tenorio, E.R.; et al. Final 5-year results of the United States Zenith Fenestrated prospective multicenter study for juxtarenal abdominal aortic aneurysms. J. Vasc. Surg. 2021, 73, 1128–1138.e2. [Google Scholar] [CrossRef]
- Verhoeven, E.L.G.; Katsargyris, A.; Bekkema, F.; Oikonomou, K.; Zeebregts, C.J.A.M.; Ritter, W.; Tielliu, I.F.J. Editor’s choice—ten-year experience with endovascular repair of thoracoabdominal aortic aneurysms: Results from 166 consecutive patients. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Mastracci, T.M.; Greenberg, R.K.; Eagleton, M.J.; Hernandez, A.V. Durability of branches in branched and fenestrated endografts. J. Vasc. Surg. 2013, 57, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Tsilimparis, N.; Fiorucci, B.; Debus, E.S.; Rohlffs, F.; Kölbel, T. Technical aspects of implanting the t-Branch off-the-shelf multibranched stentgraft for thoracoabdominal aneurysms. J. Endovasc. Ther. 2017, 24, 397–404. [Google Scholar] [CrossRef]
- Tenorio, E.R.; Oderich, G.S.; Kölbel, T.; Gargiulo, M.; Timaran, C.H.; Bertoglio, L.; Modarai, B.; Jama, K.; Eleshra, A.; Lima, G.B.; et al. Trans-Atlantic Aortic Research Consortium. Outcomes of off-the-shelf multi-branched stent grafts with intentional occlusion of directional branches using endovascular plugs during endovascular repair of complex aortic aneurysms. J. Vasc. Surg. 2021, 75, 1142–1150.e4. [Google Scholar] [CrossRef] [PubMed]
- Eleshra, A.; Oderich, G.S.; Spanos, K.; Panuccio, G.; Kärkkäinen, J.M.; Tenorio, E.R.; Kölbel, T. Short-term outcomes of the t-Branch off-the-shelf multibranched stent graft for reintervention after previous infrarenal aortic repair. J. Vasc. Surg. 2020, 72, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Bosiers, M.; Kölbel, T.; Resch, T.; Tsilimparis, N.; Torsello, G.; Austermann, M. Early and mid-term results from a postmarket observational study of Zeenith t-Branch thoracoabdominal endovascular graft. J. Vasc. Surg. 2021, 74, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, N.; Antonopoulos, C.N.; Jerkku, T.; Banafsche, R.; Kölbel, T.; Fiorucci, B.; Tsilimparis, N. Systematic review and meta-analysis of published studies on endovascular repair of thoracoabdominal aortic aneurysms with the t-Branch off-the-shelf multibranched endograft. J. Vasc. Surg. 2020, 72, 716–725.e1. [Google Scholar] [CrossRef]
- Zierler, R.E. Duplex ultrasound follow-up after fenestrated and branched endovascular aneurysm repair (FEVAR and BEVAR). Semin. Vasc. Surg. 2020, 33, 60–64. [Google Scholar] [CrossRef]
- Perini, P.; Sediri, I.; Midulla, M.; Delsart, P.; Gautier, C.; Haulon, S. Contrast-enhanced ultrasound vs. CT angiography in fenestrated EVAR surveillance: A single-center comparison. J. Endovasc. Ther. 2012, 19, 648–655. [Google Scholar] [CrossRef]
- Gargiulo, M.; Gallitto, E.; Serra, C.; Freyrie, A.; Mascoli, C.; Massoni, C.B.; De Matteis, M.; De Molo, C.; Stella, A. Could four-dimensional contrast-enhanced ultrasound replace computed tomography angiography during follow up of fenestrated endografts? Results of a preliminary experience. Eur. J. Vasc. Endovasc. Surg. 2014, 48, 536–542. [Google Scholar] [CrossRef]
- Konstantinou, N.; Kölbel, T.; Dias, N.V.; Verhoeven, E.; Wanhainen, A.; Gargiulo, M.; Oikonomou, K.; Verzini, F.; Heidemann, F.; Sonesson, B.; et al. Revascularization of occluded renal artery stent grafts after complex endovascular aortic repair and its impact on renal function. J. Vasc. Surg. 2021, 73, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Heidemann, F.; Kölbel, T.; Debus, E.S.; Diener, H.; Carpenter, S.W.; Rohlffs, F.; Tsilimparis, N. Renal Function Salvage After Delayed Endovascular Revascularization of Acute Renal Artery Occlusion in Patients with Fenestrated-Branched Endovascular Aneurysm Repair or Visceral Debranching. J. Endovasc. Ther. 2018, 25, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Bertoglio, L.; Loschi, D.; Cambiaghi, T.; Mascia, D.; Kahlberg, A.L.; Melissano, G.; Chiesa, R. Preliminary Outcomes of the Lifestream Balloon-Expandable Covered Stent in Fenestrated and Branched Thoracoabdominal Endovascular Repairs. J. Endovasc. Ther. 2018, 25, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Piazza, M.; Squizzato, F.; Xodo, A.; Gubert, A.; Grego, F.; Antonello, M. Effect of branch length and tortuosity on the outcomes of branched endovascular repair of thoracoabdominal aneurysms using self-expandable bridging stent-graft. J. Vasc. Surg. 2021, 74, 363–371.e3. [Google Scholar] [CrossRef]
- Mastracci, T.; Carrell, T.; Constantinou, J.; Dias, N.; Martin-Gonzalez, T.; Katsargyris, A.; Modarai, B.; Resch, T.; Verhoeven, E.; Burnell, M.; et al. Editor’s Choice-Effect of Branch Stent Choice on Branch-related Outcomes in Complex Aortic Repair. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Tenorio, E.R.; Kärkkäinen, J.M.; Mendes, B.C.; DeMartino, R.R.; Macedo, T.A.; Diderrich, A.; Hofer, J.; Oderich, G.S. Outcomes of directional branches using self-expandable or balloon-expandable stent grafts during endovascular repair of thoracoabdominal aortic aneurysms. J. Vasc. Surg. 2020, 71, 1489–1502.e6. [Google Scholar] [CrossRef]
- Abisi, S.; Gkoutzios, P.; Carmichael, M.; Patel, S.; Sallam, M.; Donati, T.; Zayed, H. The Early Outcomes of BeGraft Peripheral Plus in Branched Endovascular repair of Thoracoabdominal Aneurysms. J. Endovasc. Ther. 2021, 28, 707–715. [Google Scholar] [CrossRef]
- Gallitto, E.; Faggioli, G.; Fenelli, C.; Mascoli, C.; Pini, R.; Ancetti, S.; Logiacco, A.; Sonetto, A.; Gargiulo, M. The combined Use of Distal Self-Expandable and Proximal Balloon Expandable Stent Graft in Bridging Hostile Renal Arteries in Thoracoabdominal Branched Endografting. Ann. Vasc. Surg. 2020, 68, 326–337. [Google Scholar] [CrossRef]
- Motta, F.; Parodi, F.E.; Knowles, M.; Crowner, J.R.; Pascarella, L.; McGinigle, K.L.; Marston, W.A.; Kibbe, M.R.; Ohana, E.; Farber, M.A. Performance of Viabahn balloon-expandable stent compared with self-expandable covered stents for branched endovascular aortic repair. J. Vasc. Surg. 2021, 73, 410–416.e2. [Google Scholar] [CrossRef]
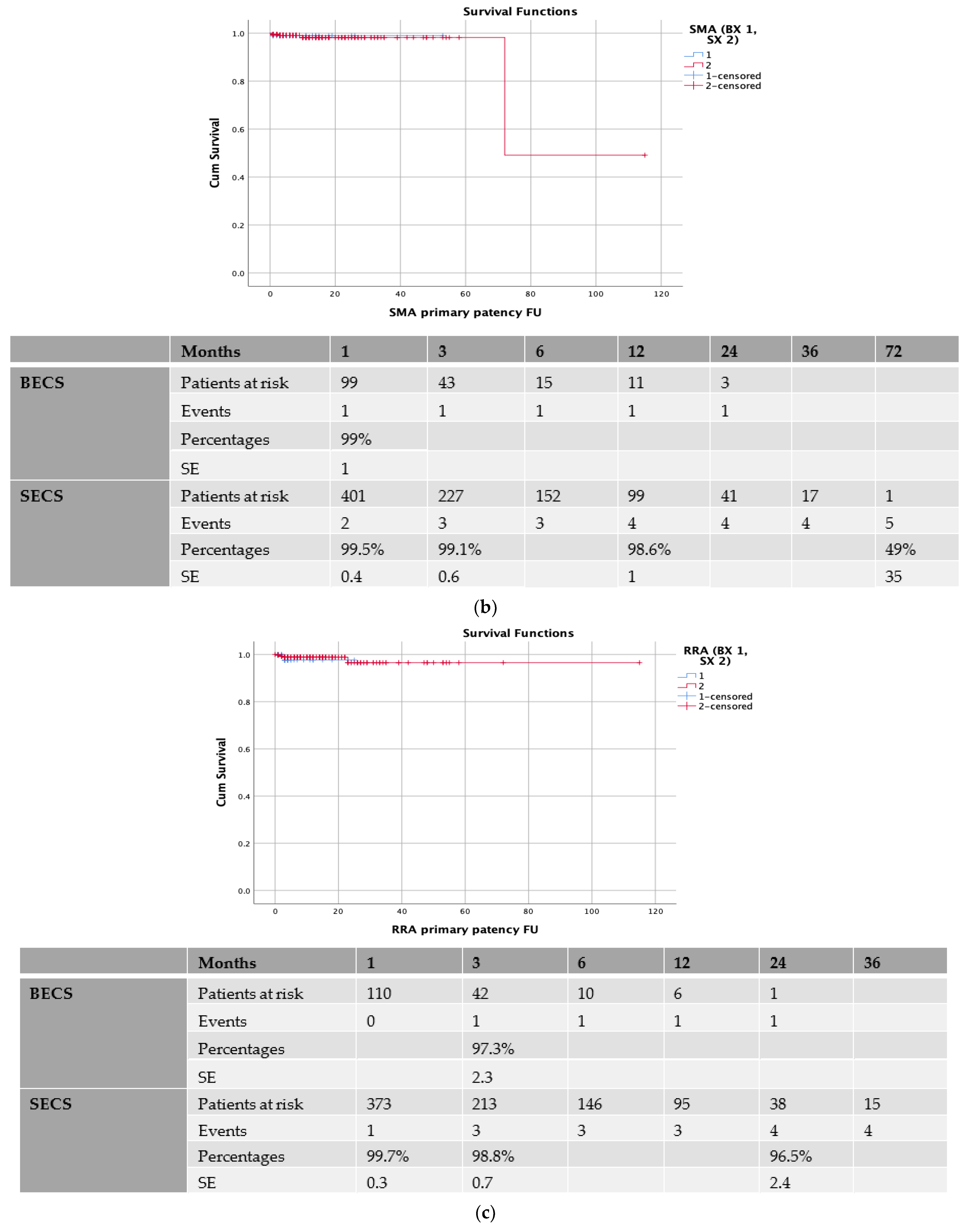
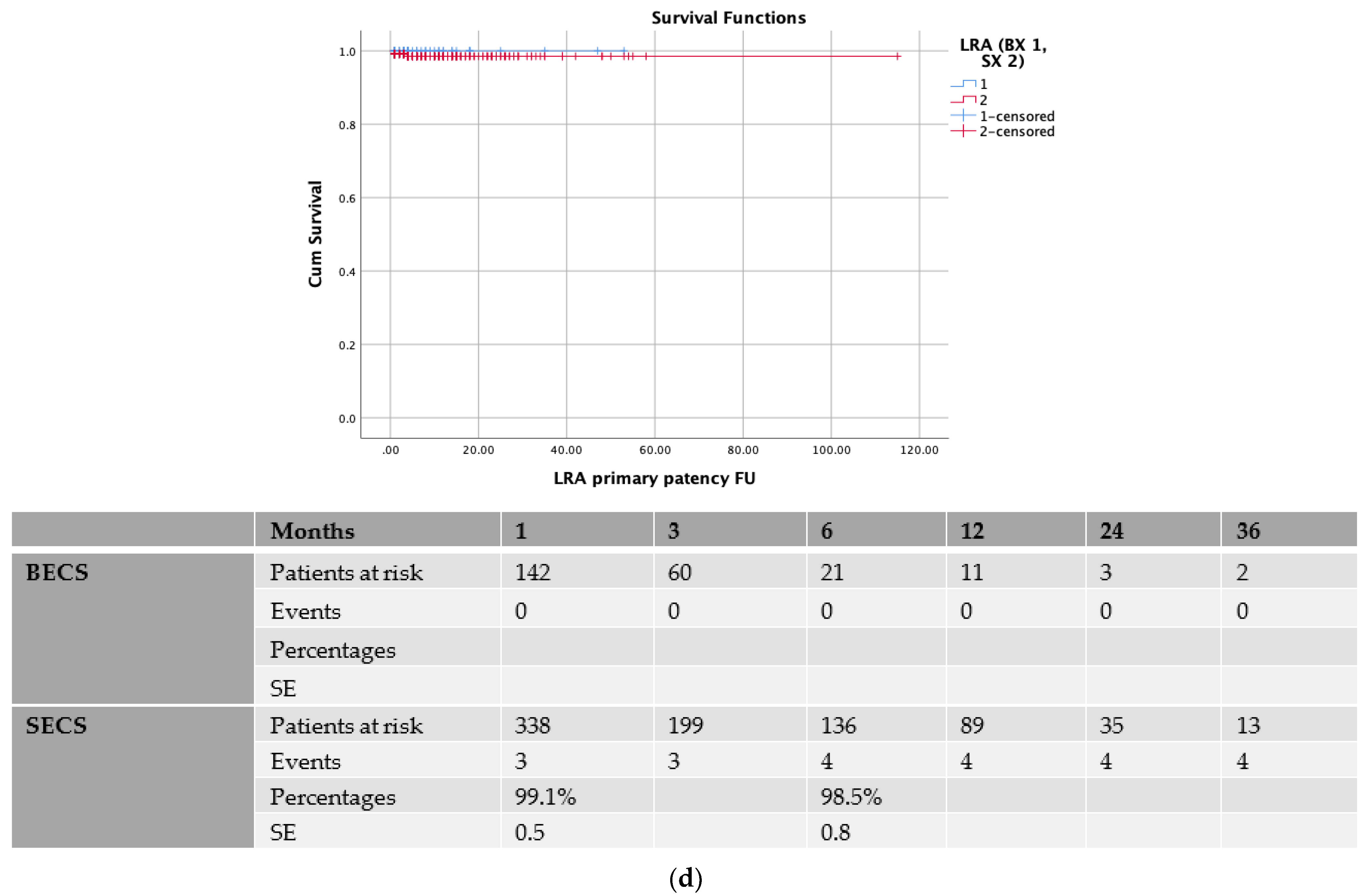
| Demographics | Total (n = 542) | Patency (n = 530) | Loss of Patency (n = 12) | p Value |
|---|---|---|---|---|
| Age | 70.5 ± 8 | 71.3 ± 7 | 69.8 ± 11 | 0.77 |
| BMI | 26 ± 4 | 25 ± 6 | 23.8 ± 3 | 0.08 |
| Aneurysm diameter | 7.5 ± 2.5 | 7.6 ± 2.2 | 7.1 ± 1.6 | 0.76 |
| Male | 388 (72%) | 379 (72%) | 9 (75%) | 0.84 |
| Female | 154 (28%) | 151 (28%) | 3 (25%) | |
| CAD | 287 (53%) | 284 (54%) | 3 (25%) | 0.028 |
| HT | 497 (92%) | 485 (92%) | 11 (92%) | 0.35 |
| HL | 332 (61%) | 329 (62%) | 3 (25%) | 0.004 |
| Smoking | 292 (54%) | 286 (54%) | 6 (50%) | 0.57 |
| COPD | 102 (19%) | 99 (18.6%) | 3 (25%) | 0.57 |
| DM | 88 (16%) | 88 (16.7%) | 0 (0%) | 0.1 |
| Renal insufficiency | ||||
| 0 | 335 | 326 (61%) | 9 (75%) | 0.91 |
| 1 | 197 | 194 (37%) | 3 (25%) | |
| 2 | 9 | 9 (1.8%) | 0 | |
| 3 | 1 | 1 (0.2%) | 0 | |
| Previous ascending or arch repair | 25 (4.6%) | 25 (5%) | 0 | 0.42 |
| Previous EVAR | 78 (14.4%) | 77 (15%) | 1 (8%) | 0.48 |
| Previous AAA | 90 (16.6%) | 85 (16%) | 5 (42%) | 0.033 |
| Previous TAA | 72 (13.3%) | 68 (13%) | 4 (33%) | 0.06 |
| Status of aneurysm | ||||
| Non-ruptured | 339 | 330 (62%) | 9 (75%) | 0.46 |
| Contained | 46 | 45 (8.5%) | 1 (8%) | |
| Symptomatic | 157 | 155 (29%) | 2 (16%) | |
| Type of aneurysm | ||||
| Juxta-renal | 22 | 22 | 0 | |
| Supra-renal | 19 | 19 | 0 | |
| TAAA Type I | 31 | 31 | 0 | |
| TAAA Type II | 73 | 70 | 3 | |
| TAAA Type III | 118 | 117 | 1 | |
| TAAA Type IV | 233 | 228 | 5 | |
| TAAA Type V | 32 | 30 | 2 | |
| Infra-renal | 14 | 13 | 1 |
| Total | BX | 1 | 2 | 3 | Relining | 1 | 2 | SX | 1 | 2 | 3 | Relining | 1 | 2 | 3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CT | 485 | 110 | 97 | 13 | 0 | 30 (27%) | 30 | 0 | 375 | 356 | 17 | 2 | 295 (79%) | 290 | 5 | 0 |
| SMA | 520 | 101 | 86 | 14 | 1 | 27 (27%) | 27 | 0 | 419 | 394 | 21 | 4 | 342 (82%) | 337 | 5 | 0 |
| RRA | 506 | 116 | 107 | 8 | 1 | 42 (36%) | 40 | 2 | 390 | 344 | 45 | 1 | 305 (78%) | 291 | 13 | 1 |
| LRA | 507 | 149 | 126 | 23 | 0 | 54 (36%) | 52 | 2 | 358 | 314 | 41 | 3 | 276 (77%) | 254 | 11 | 1 |
| Total | 2018 | 476 | 416 | 58 | 2 | 153 (32%) | 149 | 4 | 1542 | 1408 | 124 | 10 | 1218 (86%) | 1172 | 34 | 2 |
| Total | CT | SMA | RRA | LRA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mm | N | BX | SX | N | BX | SX | N | BX | SX | N | BX | SX | |
| 5 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 |
| 6 | 500 | 5 | 3 | 2 | 3 | 2 | 1 | 254 | 55 | 199 | 238 | 67 | 171 |
| 7 | 239 | 6 | 3 | 3 | 10 | 2 | 8 | 108 | 17 | 91 | 115 | 24 | 91 |
| 8 | 537 | 230 | 48 | 182 | 210 | 37 | 173 | 51 | 6 | 45 | 46 | 14 | 32 |
| 9 | 201 | 80 | 20 | 60 | 106 | 23 | 83 | 2 | 0 | 2 | 13 | 4 | 9 |
| 10 | 157 | 65 | 10 | 55 | 87 | 14 | 73 | 2 | 1 | 1 | 3 | 1 | 2 |
| 11 | 2 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12 | 26 | 11 | 0 | 11 | 15 | 0 | 15 | 0 | 0 | 0 | 0 | 0 | 0 |
| N | Age | AAA Diameter | Clinical Presentation | Type of Aneurysm | TV Occlusion | Time of Event in Months | Type of BCS | Number of BCS | Relining | Number of BMS |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 74 | 7.4 | Asymptomatic | Type IV TAAA | RRA | 2 | SECS | 1 | 1 | 1 |
| 2 | 85 | 6.2 | Asymptomatic | Type III TAAA | LRA | 1 | SECS | 1 | 1 | 1 |
| SMA | 1 | SECS | 1 | 1 | 1 | |||||
| CT | 1 | SECS | 1 | 0 | 0 | |||||
| 3 | 79 | 7 | Asymptomatic | Type IV TAAA | SMA | 1 | SECS | 1 | 1 | 1 |
| 4 | 66 | 6.8 | Asymptomatic | Type IV TAAA | RRA | 3 | BECS | 1 | 1 | 1 |
| 5 | 82 | 6.7 | Contained ruptured | Type V TAAA | SMA | 1 | BECS | 1 | 0 | 0 |
| 6 | 44 | 12 | Asymptomatic | Type IV TAAA | LRA | 1 | SECS | 1 | 1 | 1 |
| 7 | 68 | 6.5 | Asymptomatic | Type IV TAAA | RRA | 3 | SECS | 1 | 1 | 1 |
| 8 | 66 | 5.7 | Asymptomatic | Type II TAAA | LRA | 4 | SECS | 1 | 0 | 0 |
| SMA | 72 | SECS | 1 | 0 | 0 | |||||
| 9 | 72 | 6.3 | Symptomatic | Type II TAAA | SMA | 10 | SECS | 2 | 0 | 0 |
| 10 | 61 | 5.8 | Asymptomatic | Infrarenal | RRA | 23 | SECS | 1 | 0 | 0 |
| 11 | 57 | 7.1 | Asymptomatic | Type V TAAA | SMA | 3 | SECS | 1 | 0 | 0 |
| 12 | 78 | 6.5 | Symptomatic | Type II TAAA | RRA | 1 | SECS | 1 | 0 | 0 |
| LRA | 1 | SECS | 1 | 0 | 0 |
| Univariate Analysis of SMA Patency | Multivariate | CI | ||||
|---|---|---|---|---|---|---|
| Age | 0.28 | Relining | 0.02 | 8.27 | 1.4 | 49 |
| Clinical presentation | 0.36 | BX vs. SX | 0.26 | |||
| Diameter of aneurysm | 0.72 | |||||
| Gender | 0.23 | |||||
| Previous aortic repair | 0.65 | |||||
| BX vs. SX | 0.11 | |||||
| Relining | 0.01 | |||||
| Numbers of stents | 0.83 | |||||
| Univariate analysis of RRA patency | ||||||
| Age | 0.89 | |||||
| Clinical presentation | 0.44 | |||||
| Diameter of aneurysm | 0.71 | |||||
| Gender | 0.76 | |||||
| Previous aortic repair | 0.17 | |||||
| BX vs. SX | 0.48 | |||||
| Relining | 0.42 | |||||
| Numbers of stents | 0.21 | |||||
| Univariate analysis of LRA patency | ||||||
| Age | 0.62 | |||||
| Clinical presentation | 0.36 | |||||
| Diameter of aneurysm | 0.81 | |||||
| Gender | 0.35 | |||||
| Previous aortic repair | 0.67 | |||||
| BX vs. SX | 0.07 | |||||
| Relining | 0.14 | |||||
| Numbers of stents | 0.3 | |||||
| Univariate analysis of LRA patency | Not feasible with only one event | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spanos, K.; Jakimowicz, T.; Nana, P.; Behrendt, C.-A.; Panuccio, G.; Kouvelos, G.; Jama, K.; Eleshra, A.; Rohlffs, F.; Kölbel, T. Outcomes of Directional Branches of the T-Branch Off-the-Shelf Multi-Branched Stent-Graft. J. Clin. Med. 2022, 11, 6513. https://doi.org/10.3390/jcm11216513
Spanos K, Jakimowicz T, Nana P, Behrendt C-A, Panuccio G, Kouvelos G, Jama K, Eleshra A, Rohlffs F, Kölbel T. Outcomes of Directional Branches of the T-Branch Off-the-Shelf Multi-Branched Stent-Graft. Journal of Clinical Medicine. 2022; 11(21):6513. https://doi.org/10.3390/jcm11216513
Chicago/Turabian StyleSpanos, Konstantinos, Tomasz Jakimowicz, Petroula Nana, Christian-Alexander Behrendt, Giuseppe Panuccio, George Kouvelos, Katarzyna Jama, Ahmed Eleshra, Fiona Rohlffs, and Tilo Kölbel. 2022. "Outcomes of Directional Branches of the T-Branch Off-the-Shelf Multi-Branched Stent-Graft" Journal of Clinical Medicine 11, no. 21: 6513. https://doi.org/10.3390/jcm11216513
APA StyleSpanos, K., Jakimowicz, T., Nana, P., Behrendt, C.-A., Panuccio, G., Kouvelos, G., Jama, K., Eleshra, A., Rohlffs, F., & Kölbel, T. (2022). Outcomes of Directional Branches of the T-Branch Off-the-Shelf Multi-Branched Stent-Graft. Journal of Clinical Medicine, 11(21), 6513. https://doi.org/10.3390/jcm11216513






