Abstract
Background: Trans-nasal evaporative cooling is an effective method to induce intra-arrest therapeutic hypothermia in out-of-hospital cardiac arrest (OHCA). The use of supraglottic airway devices (SGA) instead of endotracheal intubation may enable shorter time intervals to induce cooling. We aimed to study the outcomes in OHCA patients receiving endotracheal intubation (ETI) or a SGA during intra-arrest trans-nasal evaporative cooling. Methods: This is a pre-specified sub-study of the PRINCESS trial (NCT01400373) that included witnessed OHCA patients randomized during resuscitation to trans-nasal intra-arrest cooling vs. standard care followed by temperature control at 33 °C for 24 h. For this study, patients randomized to intra-arrest cooling were stratified according to the use of ETI vs. SGA prior to the induction of cooling. SGA was placed by paramedics in the first-tier ambulance or by physicians or anesthetic nurses in the second tier while ETI was performed only after the arrival of the second tier. Propensity score matching was used to adjust for differences at the baseline between the two groups. The primary outcome was survival with good neurological outcome, defined as cerebral performance category (CPC) 1–2 at 90 days. Secondary outcomes included time to place airway, overall survival at 90 days, survival with complete neurologic recovery (CPC 1) at 90 days and sustained return of spontaneous circulation (ROSC). Results: Of the 343 patients randomized to the intervention arm (median age 64 years, 24% were women), 328 received intra-arrest cooling and had data on the airway method (n = 259 with ETI vs. n = 69 with SGA). Median time from the arrival of the first-tier ambulance to successful airway management was 8 min for ETI performed by second tier and 4 min for SGA performed by the first or second tier (p = 0.001). No significant differences in the probability of good neurological outcome (OR 1.43, 95% CI 0.64–3.01), overall survival (OR 1.26, 95% CI 0.57–2.55), full neurological recovery (OR 1.17, 95% CI 0.52–2.73) or sustained ROSC (OR 0.88, 95% CI 0.50–1.52) were observed between ETI and SGA. Conclusions: Among the OHCA patients treated with trans-nasal evaporative intra-arrest cooling, the use of SGA was associated with a significantly shorter time to airway management and with similar outcomes compared to ETI.
1. Introduction
Out-of-hospital cardiac arrest (OHCA) is a major public health concern, affecting approximately 275,000 individuals each year in Europe [1]. The overall OHCA mortality rate is approximately 90%, with lifelong disabilities being common among the survivors [2]. Airway management and ventilation is an important element of the advanced cardiac life support (ACLS) protocol, which has been formulated in order to improve outcomes for OHCA victims [3]. Currently, most patients receive advanced airway management during resuscitation, either using endotracheal intubation (ETI) or a supraglottic airway (SGA) [4].
ETI has been used by emergency medical services (EMS) since the 1970s [5]. However, several studies have questioned the safety and effectiveness of ETI performed by EMS in the pre-hospital setting [6,7]. The potential harms of pre-hospital ETI include unrecognized tube misplacement or dislodgement, iatrogenic hyperventilation and chest compression interruptions during placement [8,9,10]. SGA insertion is most often simpler and faster to insert than ETI [11], which results in higher success rates and fewer interruptions in the administration of chest compressions [12,13,14]. Despite the supposed benefits of SGA, several observational studies have suggested that ETI may be associated with better outcomes than SGA [15]. However, recent randomized controlled trials have raised some controversies on this issue [13,14]. Thus, the optimal strategy for airway management in OHCA remains unclear.
Targeted temperature management remains an important intervention that may influence survival with good neurological function among cardiac arrest patients [16]. In particular, intra-arrest cooling using trans-nasal evaporative cooling may provide some benefits on neurologic recovery in patients with initial shockable rhythms (i.e., ventricular fibrillation or pulseless ventricular tachycardia) [17,18,19]. Trans-nasal evaporative intra-arrest cooling has emerged as a promising therapeutic strategy in OHCA [18,20,21]. The use of SGA in these cases has the potential to shorten the time to successful airway management and thereby enable a shorter time to start cooling. However, as SGA may be associated with an increased risk for aspiration, it is important to examine the safety of advanced airway management in the setting of trans-nasal evaporative intra-arrest cooling therapy.
In this sub-study of the PRINCESS trial, we aimed to compare the effect on neurologic outcome among OHCA patients that had received airway management with ETI versus SGA prior to trans-nasal evaporative intra-arrest cooling.
2. Methods
2.1. Study Design
We performed a post hoc sub-analysis of data from the PRINCESS trial, which is a multicenter randomized clinical trial that compared trans-nasal evaporative intra-arrest cooling to the standard ACLS in a bystander-witnessed OHCA (Trial registration: NCT01400373) [20]. Ethics and institutional committees in each of the participating countries approved the study protocol [22]. Written informed consent was obtained from the next of kin or a legal representative after hospital admission and from each study participant who regained mental capacity. In this sub-analysis, the patients in the intervention arm in PRINCESS were the primary study population that was subsequently divided into two groups depending on the strategy for airway management.
2.2. Study Participants
We included bystander witnessed OHCA randomized to the intervention arm in the PRINCESS trial. The exclusion criteria were age ≥80 years; an etiology of cardiac arrest due to trauma, head trauma, severe bleeding, drug overdose, cerebrovascular accident, drowning, smoke inhalation, electrocution, or hanging; hypothermia at the time of evaluation; an anatomical barrier preventing the insertion of intra-nasal catheters; an existing do-not-attempt resuscitation order; known terminal illness; known or clinically apparent pregnancy; known coagulopathy (except when therapeutically induced); need for supplemental oxygen; ROSC prior to randomization; and EMS response time (i.e., from collapse to EMS arrival) greater than 15 min. In this sub-study of the PRINCESS trial, we also excluded study participants who were randomized to the control group as well as study participants who were initially randomized to the intervention group but did not receive intra-arrest cooling. Patients were divided into two different treatment groups depending on the airway management technique used prior to trans-nasal evaporative cooling (i.e., those receiving ETI versus those receiving SGA).
2.3. Emergency Medical Services
All study sites had two-tiered EMS systems where the first vehicle used bag mask ventilation only or SGA with bag-valve ventilation connected to the SGA prior to the arrival of the second tier. The second tier was manned by physicians or anesthetic nurses, trained in advance airway management including placing an SGA and endotracheal intubation. In addition, the intra-arrest cooling equipment was carried by the second tier. Thus, patients could have had the SGA placed by paramedics from the first vehicle and subsequently, cooling was started after randomization and application of trans-nasal evaporative cooling by the crew in the second tier. Among patients receiving bag mask ventilation by the paramedics, ETI was performed after the arrival of the second tier. The use of SGA could also be due to ETI being difficult to perform in the field. No data were collected on the number of intubation attempts or change in airway strategy. The confirmation of the tube was undertaken with end tidal CO2, but this was only recorded in a limited number of patients and not presented in this analysis.
2.4. Exposure
The exposure of interest was defined as the type of airway management technique used prior to cooling. All of the study participants included in the PRINCESS trial were treated with advanced airway management prior to randomization using either ETI or an SGA [22]; patients were therefore divided into two different treatment groups depending on the airway management technique used prior to trans-nasal evaporative cooling.
2.5. Treatment
The RhinoChill™ device delivers a mixture of air or oxygen and a chemically inert cooling liquid (perfluorohexane) via nasal catheters directly into the nasal cavity, with the goal of primarily cooling the brain [20,21,23]. Trans-nasal evaporative cooling is maintained until hospital arrival, and whenever possible until systemic cooling is initiated. The study participants received standard post-resuscitation care upon admission to the intensive care unit (ICU). Intravenous sedation, analgesia, and neuromuscular blockade were used according to the institutional cooling protocols. The targets for respiratory management, blood pressure, and glucose control have been previously described [22]. The study participants were treated with targeted temperature management at 32–34 °C for 24 h.
2.6. Outcome
Neurological outcome assessment was performed at 90 days via a structured telephone interview or during a follow-up appointment using the cerebral performance categories (CPC) scale [24]. The primary outcome of this study was survival with good neurologic outcome (CPC 1–2) at 90 days. The secondary outcomes were overall survival at 90 days, survival with complete neurologic recovery (CPC 1) at 90 days, and hospital admission with the sustained return of spontaneous circulation (ROSC) (defined as ROSC >20 min). Additional safety parameters that were investigated included the time until successful airway management, the time until the initiation of intra-arrest cooling, arterial blood gas parameters, and the prevalence of pneumonia. The time until successful airway management was defined as the time interval that elapsed between EMS arrival at the site of the arrest and the time of successful airway device placement. Similarly, we defined the time until the initiation of trans-nasal evaporative cooling as the time duration between EMS arrival and the initiation of intra-arrest cooling.
2.7. Statistical Analysis
Continuous variables were presented as means and standard deviations (SD) if normally distributed, or as medians and interquartile ranges (IQR) if not normally distributed. Categorical variables were reported as counts and percentages. We assessed the group differences in continuous variables using either the Mann–Whitney U-test or Student’s T-test, as appropriate. Group-wise differences in categorical variables were assessed using Pearson’s chi-squared test.
A range of factors may influence the decision of EMS personnel to use one advanced airway device or strategy over any other form of airway management. Therefore, we used propensity score matching to balance known confounding variables across the two treatment groups, in a manner reminiscent of that conducted in the work of Hasegawa et al. and McMullan et al. [4,7]. Propensity scores were calculated using a logistic regression model with the following independent variables: EMS response time, age, sex, bystander CPR, initial rhythm, etiology, and body mass index (BMI). Propensity score matching (1:2) was carried out using the nearest neighbor method with a caliper width of 0.2. We used the standardized mean difference (SMD) to examine group differences in the covariates before and after matching. We fit a series of conditional logistic regression models to evaluate the association between each outcome variable and airway management strategy. We calculated bootstrapped 95% confidence intervals for the odds ratios using 1000 bootstrapped datasets.
We used multiple imputation by chained equations (mice) to impute missing data, generating five imputed datasets [25]. The analysis described above was separately performed in each of the imputed datasets. The resulting regression coefficients and test statistics were subsequently pooled across all imputed datasets [25,26].
3. Results
3.1. Study Population
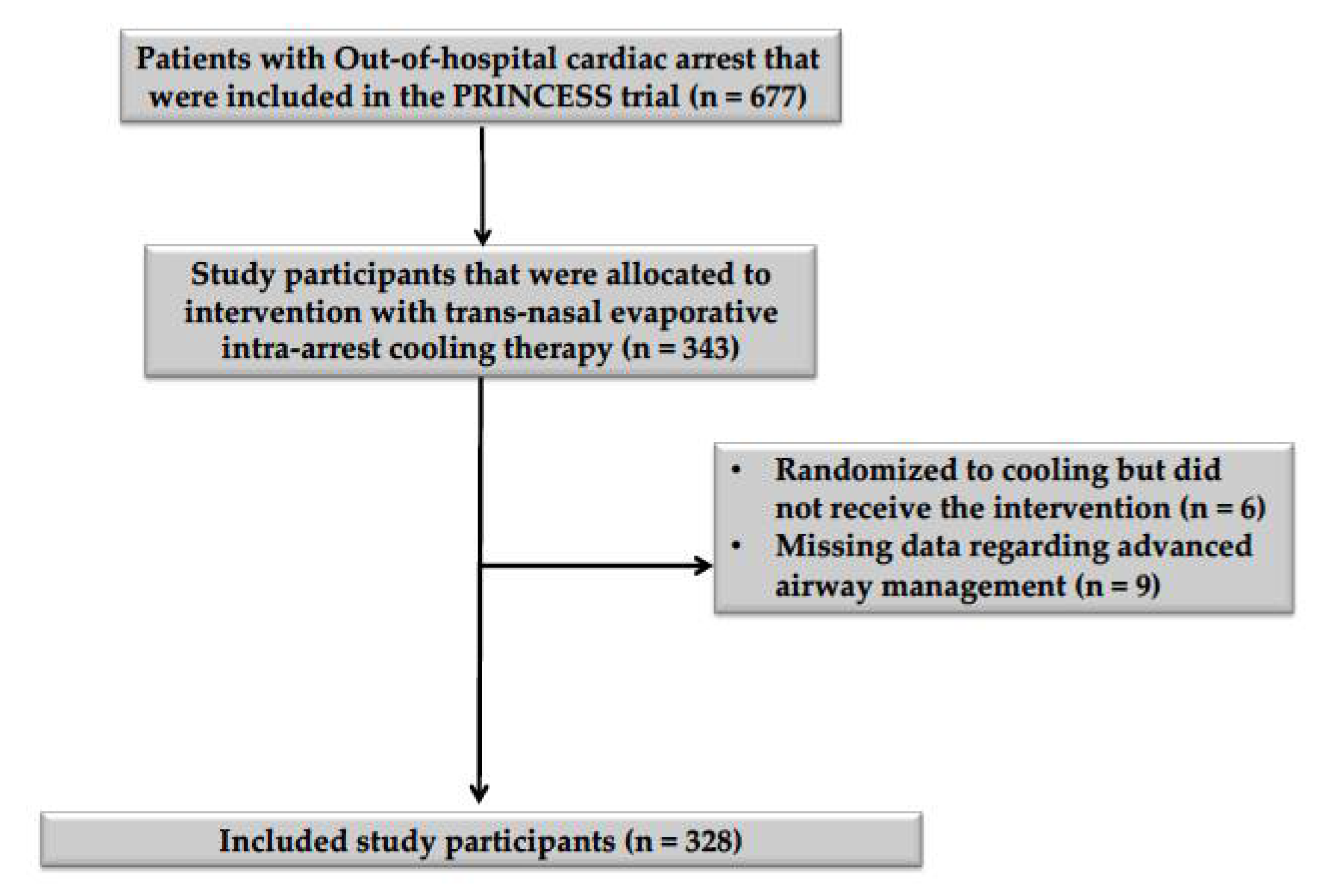
Of the 343 patients who were allocated to intra-arrest cooling in the PRINCESS trial, six did not receive the assigned intervention, and data on airway management were missing for nine study participants (Figure 1). Thus, the study cohort consisted of 328 patients; 259 (79%) received orotracheal intubation (ETI group), while in the SGA group, six were treated using a laryngeal tube and the others (n = 63) with a laryngeal mask airway. Patients who were treated with SGA were older (p = 0.03) and had a higher BMI (p = 0.01) than patients who were treated with ETI (Table 1). After propensity score matching, all baseline characteristics were adequately balanced between groups (Supplementary Materials, Supplementary Figure S1). No significant differences in the arterial blood gas parameters at the time of hospital admission were observed between groups after propensity score matching (Table 2). Missing entries amounted to 3.32% of the included data (Supplementary Materials, Supplementary Table S1).
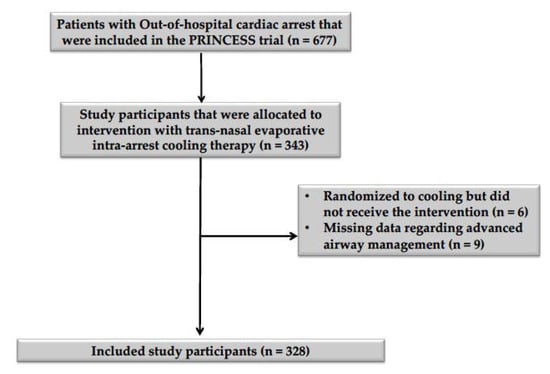
Figure 1.
Flowchart describing patient inclusion and exclusion. A diagram describing the inclusion and exclusion of study participants, according to the inclusion and exclusion criteria mentioned in the methodology section of this article.

Table 1.
Demographics before and after propensity score matching. Data after propensity score matching corresponded to that of one of the five imputed datasets that were generated following multiple imputation by chained equations (mice). Abbreviations: BMI = body mass index, CPR = cardiopulmonary resuscitation, EMS = emergency medical services, ETI = endotracheal intubation, SD = standard deviation, SGA = supraglottic airway, SMD = standardized mean difference, Q1 = first quartile, Q3 = third quartile.

Table 2.
Patient characteristics at hospital admission. The presented results correspond to data obtained after propensity score matching for one of the five imputed datasets that were generated following multiple imputation by chained equations (mice). Abbreviations: PaCO2 = partial pressure of carbon dioxide, PaO2 = partial pressure of oxygen, SD = standard deviation, Q1 = first quartile, Q3 = third quartile.
3.2. Outcome Measures
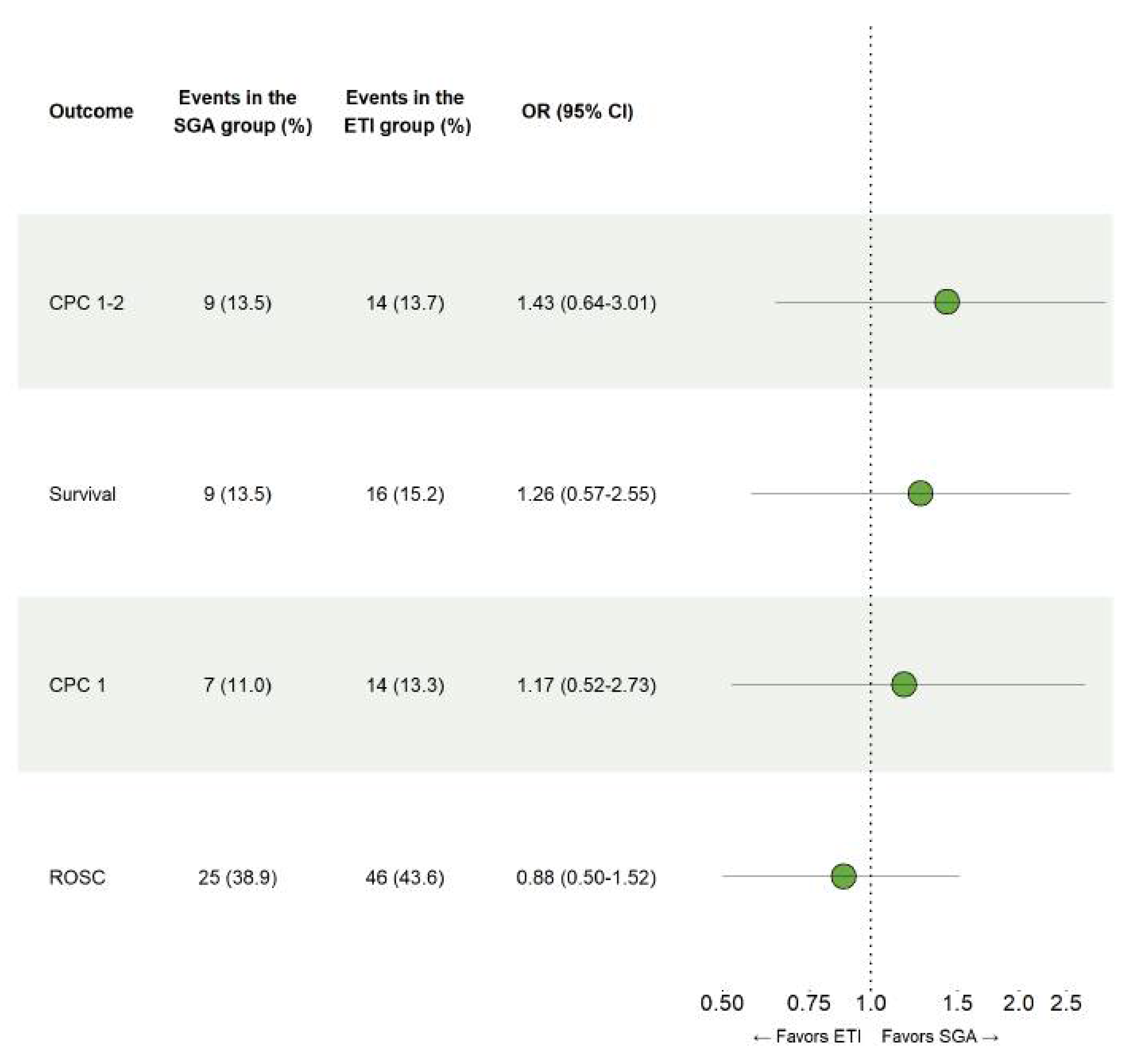
No significant differences between ETI and SGA were observed on the occurrence of survival with good neurologic outcome, CPC 1-2 at 90 days (OR 1.43, 95% CI 0.64–3.01), overall survival at 90 days (OR 1.26, 95% CI 0.57–2.55), survival with complete neurologic recovery at 90 days (OR 1.17, 95% CI 0.52–2.73) or hospital admission following sustained ROSC (OR 0.88, 95% CI 0.50–1.52), as can be seen in Figure 2.
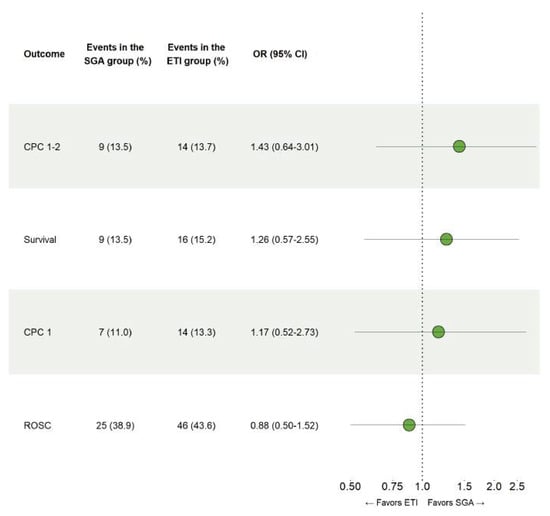
Figure 2.
Comparison of endotracheal intubation and supraglottic airways. Results for the conditional logistic regression models that were fitted using propensity score matched data to examine the safety of ETI and SGA in patients with out-of-hospital cardiac arrest.
3.3. Time until Successful Airway Device Placement and the Initiation of Intra-Arrest Cooling
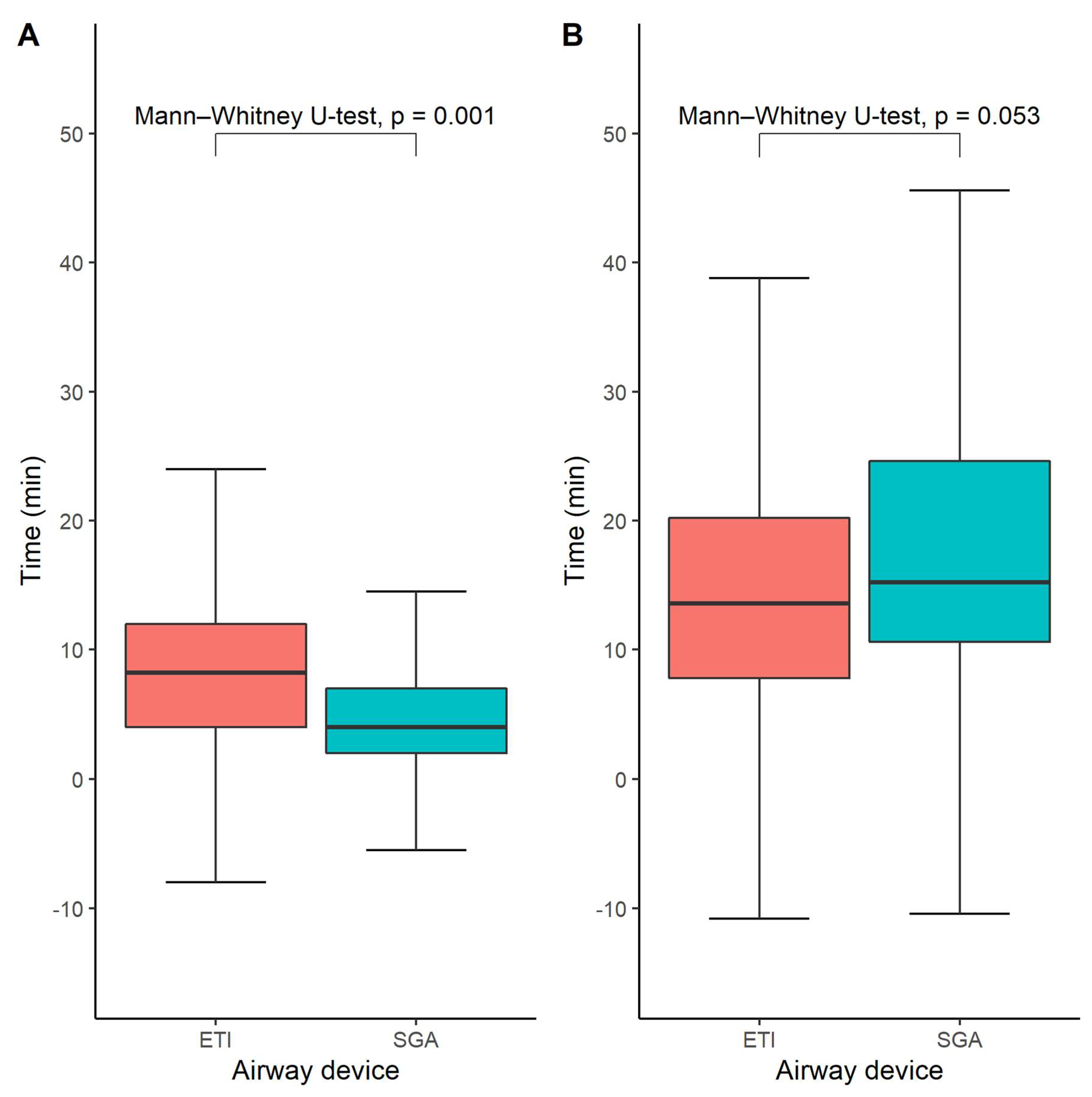
The time until airway management was available for 276 out of 328 study participants (84%). The median time from EMS arrival at the scene until successful airway management was 8 (interquartile range 4–12 min) minutes in the ETI group and 4 min in the SGA group (interquartile range 2–7 min), p = 0.001 (Figure 3A). However, we did not find any statistically significant difference between the SGA- and ETI groups regarding the time until the initiation of hypothermia treatment, which was on average 14 min in the ETI group (interquartile range 8–20) and 15 min in the SGA group (interquartile range 11–25), p = 0.053 (Figure 3B).
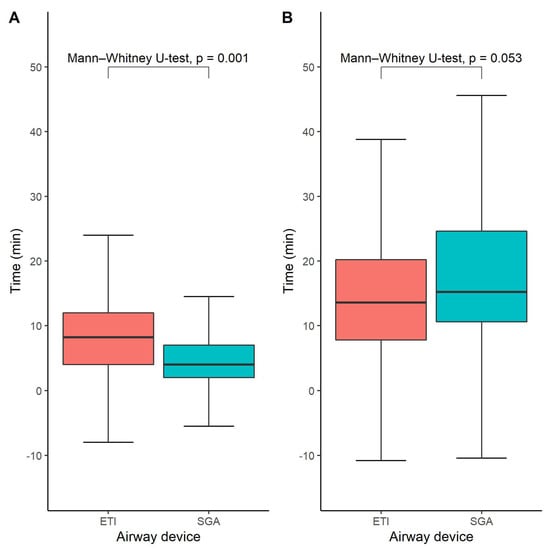
Figure 3.
Time until successful advanced airway management (A) and time to start of intra-arrest cooling (B). Box-and-whisker plots depicting the time until successful airway management in the ETI and SGA groups, respectively. Medians and quartiles were estimated using quantile regression for each imputed dataset and subsequently averaged across all imputed datasets. Likewise, the Mann–Whitney U-test was performed in each imputed dataset and the results were pooled across all available imputed datasets.
The time until the initiation of intra-arrest cooling therapy was associated with survival with good neurological outcome (defined as CPC 1-2, OR 0.96 [0.92–1.00], p = 0.045), overall survival (OR 0.95 [0.91–0.99], p = 0.017), and survival with complete neurologic recovery at 90 days (defined as CPC 1, 0.95 [0.90–0.99], p = 0.018), as shown in the Supplementary Materials (Supplementary Table S2). In contrast, we did not observe any statistically significant association between the likelihood of achieving sustained ROSC and the time of intra-arrest cooling. Moreover, we found no statistically significant association between the endpoints of this study and the time until successful airway device placement. Thus, whereas the time until the initiation of intra-arrest cooling has a statistically significant association with both neurological outcomes and overall survival in OHCA patients, the time until successful airway device placement does not have any significant relationship to patient outcomes.
3.4. Infections
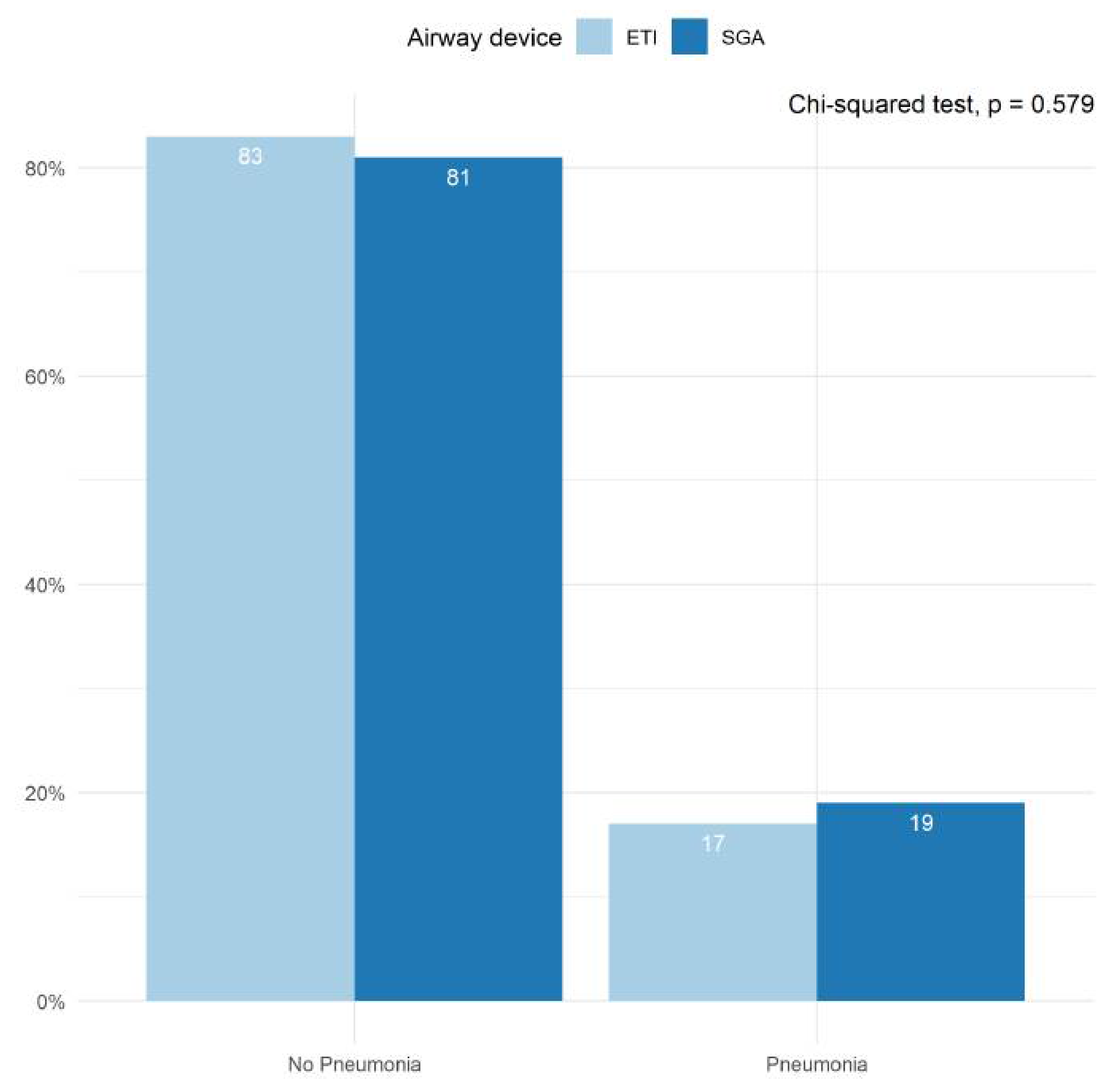
The incidence of infections prior to hospital discharge was reported in conjunction with the PRINCESS trial by all participating centers (Figure 4). Approximately 20% of patients suffered from some form of infection during their hospital stay, with pneumonia accounting for the majority of these cases. We found no statistically significant differences between the ETI- and SGA groups in the proportion of patients who suffered from pneumonia during their hospital stay (p = 0.579).
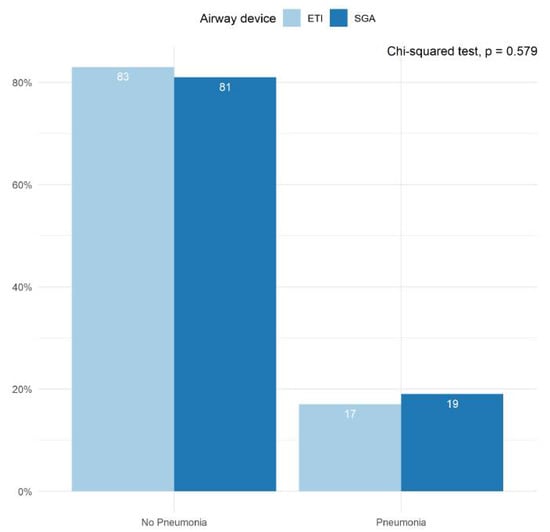
Figure 4.
Pneumonia diagnosed during hospital stay. Bar plots depicting the proportion of patients in whom pneumonia was diagnosed prior to hospital discharge. Results are shown for the ETI group and the SGA group separately. The proportions were averaged across all imputed datasets. The Chi-squared test was performed in each imputed dataset and the results were pooled across all available imputed datasets.
4. Discussion
In this sub-analysis of the PRINCESS trial, we observed that SGA is feasible to use without any safety aspects reported prior to induction of trans-nasal evaporative cooling in OHCA and it significantly shortened the airway management time compared to ETI; SGA was not associated with any worsening in the gas exchange on arterial blood gas or with patient outcomes.
Trans-nasal evaporative intra-arrest cooling is an emerging therapeutic option in the management of OHCA patients which has, as the only cooling method, been shown to be safe and effective in inducing intra-arrest cooling at the scene of the arrest. Although not currently part of routine medical practice, trans-nasal evaporative intra-arrest cooling therapy has already been implemented in some clinical settings for the treatment of OHCA. Furthermore, trans-nasal evaporative intra-arrest cooling will continue to be investigated in clinical trials. Therefore, it is important to establish the safety of advanced airway management in the setting of trans-nasal evaporative intra-arrest cooling. In this study, we observed a shorter time period to successful airway management in the SGA group compared to intubation, but no differences in terms of sustained ROSC, overall survival, neurological recovery, the prevalence of pneumonia, or arterial blood gas parameters.
We observed that patients who were treated with SGA were on average older and had a higher BMI than patients who were treated with ETI. Although these imbalances were adjusted for in the subsequent analyses, we cannot dismiss the possibility of residual confounding due to one or several unmeasured parameters such as the lack of equipoise at the baseline including the expertise of centers. Therefore, we believe that there is a need for an external validation to confirm our results.
Despite concerns regarding an increased aspiration risk following trans-nasal evaporative cooling, we did not find any significant differences between the ETI and SGA groups concerning the proportion of patients suffering from pneumonia or arterial blood gas parameters on admission to hospital. Despite a limited sample size, these findings further support our other results, which suggest that ETI and SGA have similar safety profiles in the setting of trans-nasal evaporative intra-arrest cooling.
We observed a statistically significant difference between the ETI and SGA groups in the time elapsed until successful airway placement in the setting of trans-nasal evaporative intra-arrest cooling therapy. This result is in agreement with that of earlier studies on the topic of advanced airway management in OHCA [5,11]. However, we observed no statistically significant differences concerning the time until the initiation of trans-nasal evaporative intra-arrest cooling therapy. This result is most likely attributed to logistics, as the cooling device was carried by the second-tier vehicles and not by the paramedics in the first-tier ambulances. The pre-hospital physicians generally arrived at the site of the arrest later than the paramedics. Thus, although the patient’s airway may have been secured using a SGA by the paramedic prior to the arrival of the pre-hospital emergency medicine physicians, intra-arrest cooling could only be initiated once the physician had arrived at the site of the arrest, making the time to hypothermia largely independent of the time until successful airway management.
This secondary analysis of the PRINCESS trial had several limitations. The study of two subgroups receiving different airway strategies within the intervention arm of the PRINCESS trial introduced a risk of selection bias. Although the treatment groups had similar baseline characteristics and propensity score matching was performed, the risk of residual confounding could not be eliminated. An additional limitation of this study is the fact that ETI and SGA could not be compared to bag-valve mask ventilation, as successful advanced airway management was specified as a requirement for inclusion in the PRINCESS trial [22]. In addition, the time elapsed from EMS arrival and successful ETI including the time interval between the arrival of the first- and second-tier vehicle and time interval for the ETI procedure, which may have enabled the SGA, could be undertaken by the first-tier team, to be placed faster. Furthermore, data on the quality of CPR such as information regarding chest compression interruption were unavailable and could thus not be included in this analysis. We also lacked information on the intubation attempts and where SGA was placed due to intubation failure.
5. Conclusions
In this sub-study of the PRINCESS trial, a shorter time period to successful airway management was observed in the SGA group when compared to ETI. No differences in clinically relevant outcomes such as survival with good neurologic outcome and overall survival were observed between groups. This study might help to design future trials using trans-nasal evaporative cooling to minimize the time to induce cooling.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11216370/s1. Figure S1: Covariate balance. Standardized mean differences before and after propensity score matching. Standardized Mean Differences (SMDs) at baseline (orange) and after propensity score matching (blue). The adjusted variables are displayed on the y-axis. SMD > 0.1 was considered to be indicative of a statistically significant group difference; Figure S2: Prehospital response times. Time from cardiac arrest to arrival of medical emergency services (EMS) and Acute life support (ALS) vehicle. The figure shows the arrival times (in minutes) of EMS and ALS for the ETI and SGA groups, respectively; Table S1: Missing data for the predictor variables (where present). Abbreviations: BMI = Body mass index in units of kg/m2, CPR = Cardiopulmonary resuscitation, EMS = Emergency medical services; Table S2: The relationship between study endpoints and the time until the initiation of intra-arrest cooling and the time until successful airway device insertion, respectively. These results were obtained using univariate logistic regression in the unmatched dataset. Abbreviations: CPC = Cerebral Performance Category, OR = Odds ratio, CI = Confidence interval, Q1 = First quartile, Q3 = Third quartile.
Author Contributions
Conceptualization, J.T., E.D., F.S.T., A.T., S.F., J.H., M.J., L.S. and P.N.; Formal analysis, J.T. and M.J.; Funding acquisition, L.S. and P.N.; Investigation, T.H., E.D., F.S.T., A.T., S.F., J.H., M.R. and L.S.; Methodology, J.T., T.H., E.D., F.S.T., A.T., S.F., J.H., M.R., M.J., L.S. and P.N.; Resources, P.N.; Supervision, P.N.; Writing—original draft, J.T.; Writing—review & editing, T.H., E.D., F.S.T., A.T., S.F., J.H., M.R., M.J., L.S. and P.N. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was provided by the Swedish Heart and Lung Foundation (Grant No. 20160637) and the Laerdal Foundation for Acute Medicine. The funders of the study had no role in the study design, data collection, analysis, or writing of the article.
Institutional Review Board Statement
Ethics and institutional committees in each participating country approved the study protocol and statistical analysis plan and the rationale and design of the trial have been published. An independent data and safety monitoring committee reviewed predefined end points at interim analyses after recruitment of 200 and 500 randomized patients.
Informed Consent Statement
The study was conducted according to the requirements of the Declaration of Helsinki. Written informed consent was obtained from the closest relative or a legal representative of each patient after hospital admission and, at a later stage, from each patient who regained mental capacity.
Data Availability Statement
The study present data from The PRINCESS randomized controlled Trial. The data are not publicly available in accordance with ethical approval and institutional regulations of patient data management.
Acknowledgments
We thank the study participants of the PRINCESS trial for making this study possible.
Conflicts of Interest
Nordberg reported grants from Swedish Heart-Lung Foundation and the Laerdal Foundation and nonfinancial support from BrainCool AB during the conduct of the study. Taccone reported personal fees from BARD outside the submitted work. Truhlar reported nonfinancial support from the Karolinska Institute during the conduct of the study. Svensson reported grants from the Swedish Heart and Lung foundation during the conduct of the study. No other disclosures were reported.
References
- Atwood, C.; Eisenberg, M.S.; Herlitz, J.; Rea, T.D. Incidence of EMS-treated out-of-hospital cardiac arrest in Europe. Resuscitation 2005, 67, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Gräsner, J.T.; Wnent, J.; Herlitz, J.; Perkins, G.D.; Lefering, R.; Tjelmeland, I.; Koster, R.W.; Masterson, S.; Rossell-Ortiz, F.; Maurer, H.; et al. Survival after out-of-hospital cardiac arrest in Europe—Results of the EuReCa TWO study. Resuscitation 2020, 148, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Link, M.S.; Berkow, L.C.; Kudenchuk, P.J.; Halperin, H.R.; Hess, E.P.; Moitra, V.K.; Neumar, R.W.; O’Neil, B.J.; Paxton, J.H.; Silvers, S.M.; et al. Part 7: Adult advanced cardiovascular life support: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015, 132, S444–S464. [Google Scholar] [CrossRef] [PubMed]
- McMullan, J.; Gerecht, R.; Bonomo, J.; Robb, R.; McNally, B.; Donnelly, J.; Wang, H.E. Airway management and out-of-hospital cardiac arrest outcome in the CARES registry. Resuscitation 2014, 85, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.D.; Paris, P.M.; Winter, P.M.; Pelton, G.H.; Cannon, G.M. Field endotracheal intubation by paramedical personnel. Success rates and complications. Chest 1984, 85, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.E.; Yealy, D.M. Out-of-Hospital Endotracheal Intubation: Where Are We? Ann. Emerg. Med. 2006, 47, 532–541. [Google Scholar] [CrossRef]
- Hasegawa, K.; Hiraide, A.; Chang, Y.; Brown, D.F.M. Association of prehospital advanced airway management with neurologic outcome and survival in patients with out-of-hospital cardiac arrest. JAMA J. Am. Med. Assoc. 2013, 309, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.E.; Simeone, S.J.; Weaver, M.D.; Callaway, C.W. Interruptions in Cardiopulmonary Resuscitation From Paramedic Endotracheal Intubation. Ann. Emerg. Med. 2009, 54, 645–652.e1. [Google Scholar] [CrossRef] [PubMed]
- Aufderheide, T.P.; Lurie, K.G. Death by hyperventilation: A common and life-threatening problem during cardiopulmonary resuscitation. Crit. Care Med. 2004, 32, S345–S351. [Google Scholar] [CrossRef]
- Katz, S.H.; Falk, J.L. Misplaced endotracheal tubes by paramedics in an urban emergency medical services system. Ann. Emerg. Med. 2001, 37, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Kurola, J.; Harve, H.; Kettunen, T.; Laakso, J.P.; Gorski, J.; Paakkonen, H.; Silfvast, T. Airway management in cardiac arrest—Comparison of the laryngeal tube, tracheal intubation and bag-valve mask ventilation in emergency medical training. Resuscitation 2004, 61, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Kurz, M.C.; Prince, D.K.; Christenson, J.; Carlson, J.; Stub, D.; Cheskes, S.; Lin, S.; Aziz, M.; Austin, M.; Vaillancourt, C.; et al. Association of advanced airway device with chest compression fraction during out-of-hospital cardiopulmonary arrest. Resuscitation 2016, 98, 35–40. [Google Scholar] [CrossRef]
- Benger, J.R.; Kirby, K.; Black, S.; Brett, S.J.; Clout, M.; Lazaroo, M.J.; Nolan, J.P.; Reeves, B.C.; Robinson, M.; Scott, L.J.; et al. Effect of a strategy of a supraglottic airway device vs tracheal intubation during out-of-hospital cardiac arrest on functional outcome the AIRWAYS-2 randomized clinical trial. JAMA J. Am. Med. Assoc. 2018, 320, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.E.; Schmicker, R.H.; Daya, M.R.; Stephens, S.W.; Idris, A.H.; Carlson, J.N.; Riccardo Colella, M.; Herren, H.; Hansen, M.; Richmond, N.J.; et al. Effect of a strategy of initial laryngeal tube insertion vs endotracheal intubation on 72-hour survival in adults with out-of-hospital cardiac arrest a randomized clinical trial. JAMA J. Am. Med. Assoc. 2018, 320, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Benoit, J.L.; Gerecht, R.B.; Steuerwald, M.T.; McMullan, J.T. Endotracheal intubation versus supraglottic airway placement in out-of-hospital cardiac arrest: A meta-analysis. Resuscitation 2015, 93, 20–26. [Google Scholar] [CrossRef]
- Mild Therapeutic Hypothermia to Improve the Neurologic Outcome after Cardiac Arrest. N. Engl. J. Med. 2002, 346, 549–556. [CrossRef]
- Abella, B.S.; Zhao, D.; Alvarado, J.; Hamann, K.; Vanden Hoek, T.L.; Becker, L.B. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation 2004, 109, 2786–2791. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Taccone, F.S.; Jonsson, M.; Forsberg, S.; Hollenberg, J.; Truhlar, A.; Ringh, M.; Abella, B.S.; Becker, L.B.; Vincent, J.-L.; et al. Time to intra-arrest therapeutic hypothermia in out-of-hospital cardiac arrest patients and its association with neurologic outcome: A propensity matched sub-analysis of the PRINCESS trial. Intensive Care Med. 2020, 46, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Taccone, F.S.; Hollenberg, J.; Forsberg, S.; Truhlar, A.; Jonsson, M.; Annoni, F.; Gryth, D.; Ringh, M.; Cuny, J.; Busch, H.J.; et al. Effect of intra-arrest trans-nasal evaporative cooling in out-of-hospital cardiac arrest: A pooled individual participant data analysis. Crit. Care 2021, 25, 198. [Google Scholar] [CrossRef]
- Nordberg, P.; Taccone, F.S.; Truhlar, A.; Forsberg, S.; Hollenberg, J.; Jonsson, M.; Cuny, J.; Goldstein, P.; Vermeersch, N.; Higuet, A.; et al. Effect of Trans-Nasal Evaporative Intra-arrest Cooling on Functional Neurologic Outcome in Out-of-Hospital Cardiac Arrest: The PRINCESS Randomized Clinical Trial. JAMA 2019, 321, 1677–1685. [Google Scholar] [CrossRef]
- Castrén, M.; Nordberg, P.; Svensson, L.; Taccone, F.; Vincent, J.L.; Desruelles, D.; Eichwede, F.; Mols, P.; Schwab, T.; Vergnion, M.; et al. Intra-arrest transnasal evaporative cooling: A randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal cooling effectiveness). Circulation 2010, 122, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, P.; Taccone, F.S.; Castren, M.; Truhlár, A.; Desruelles, D.; Forsberg, S.; Hollenberg, J.; Vincent, J.L.; Svensoon, L. Design of the PRINCESS trial: Pre-hospital resuscitation intra-nasal cooling effectiveness survival study (PRINCESS). BMC Emerg. Med. 2013, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Abou-Chebl, A.; Sung, G.; Barbut, D.; Torbey, M. Local brain temperature reduction through intranasal cooling with the rhinochill device: Preliminary safety data in brain-injured patients. Stroke 2011, 42, 2164–2169. [Google Scholar] [CrossRef] [PubMed]
- Jennett, B.; Bond, M. Assessment of outcome after severe brain damage. Lancet 1975, 1, 480–484. [Google Scholar] [CrossRef]
- van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Ling, A.Y.; Montez-Rath, M.E.; Mathur, M.B.; Kapphahn, K.; Desai, M. How to apply multiple imputation in propensity score matching with partially observed confounders: A simulation study and practical recommendations. J. Mod. Appl. Stat. Methods 2019, 19, eP3439. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).