The Influence of Physical Training on the Immune System of Rats during N-methyl-N-nitrosourea-Induced Carcinogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Intoxication with MNU
2.3. Physical Training
| Training [Weeks] | Moderate-Intensity Training | High-Intensity Training | ||
|---|---|---|---|---|
| Speed of the Treadmill [km/h] | Duration [min.] | Speed of the Treadmill [km/h] | Duration [min.] | |
| 1 | 0.60 | 10 | 0.72 | 12 |
| 2 | 0.96 | 20 | 1.15 | 24 |
| 3 | 1.20 | 30 | 1.44 | 36 |
| 4 | 1.44 | 40 | 1.73 | 48 |
| 5 | 1.68 | 50 | 2.02 | 60 |
| 6 | 1.68 | 55 | 2.02 | 66 |
| 7 | 1.68 | 60 | 2.02 | 72 |
| 8 | 1.68 | 65 | 2.02 | 78 |
| 9–12 | 1.68 | 30 | 2.02 | 36 |
2.4. Tissue Collection
2.5. Flow Cytometry
- CD3 APC (557030, Becton Dickinson, Belgium) (5 µL/sample), CD4 FITC (554843, Becton Dickinson, Belgium) (5 µL/sample), CD8a PerCP (558824, Becton Dickinson, Belgium) (5 µL/sample) and CD11b/c PE (554862, Becton Dickinson, Belgium) (5 µL/sample)
- CD161 FITC (555008, Becton Dickinson, Belgium) (10 µL/sample), CD3 APC (5 µL/sample) and CD45A PE (551402, Becton Dickinson, Belgium) (5 µL/sample)
- isoFITC (5555743) (5 µL/sample), isoAPC (555745) (5 µL/sample), isoPerCP (559425) (5 µL/sample) and isoPE (555574) (5 µL/sample).
2.6. Tissue Microarrays (TMAs)
2.7. Immunohistochemistry (IHC)
2.8. TUNEL
2.9. Assessment of IHC
2.10. Statistical Analysis
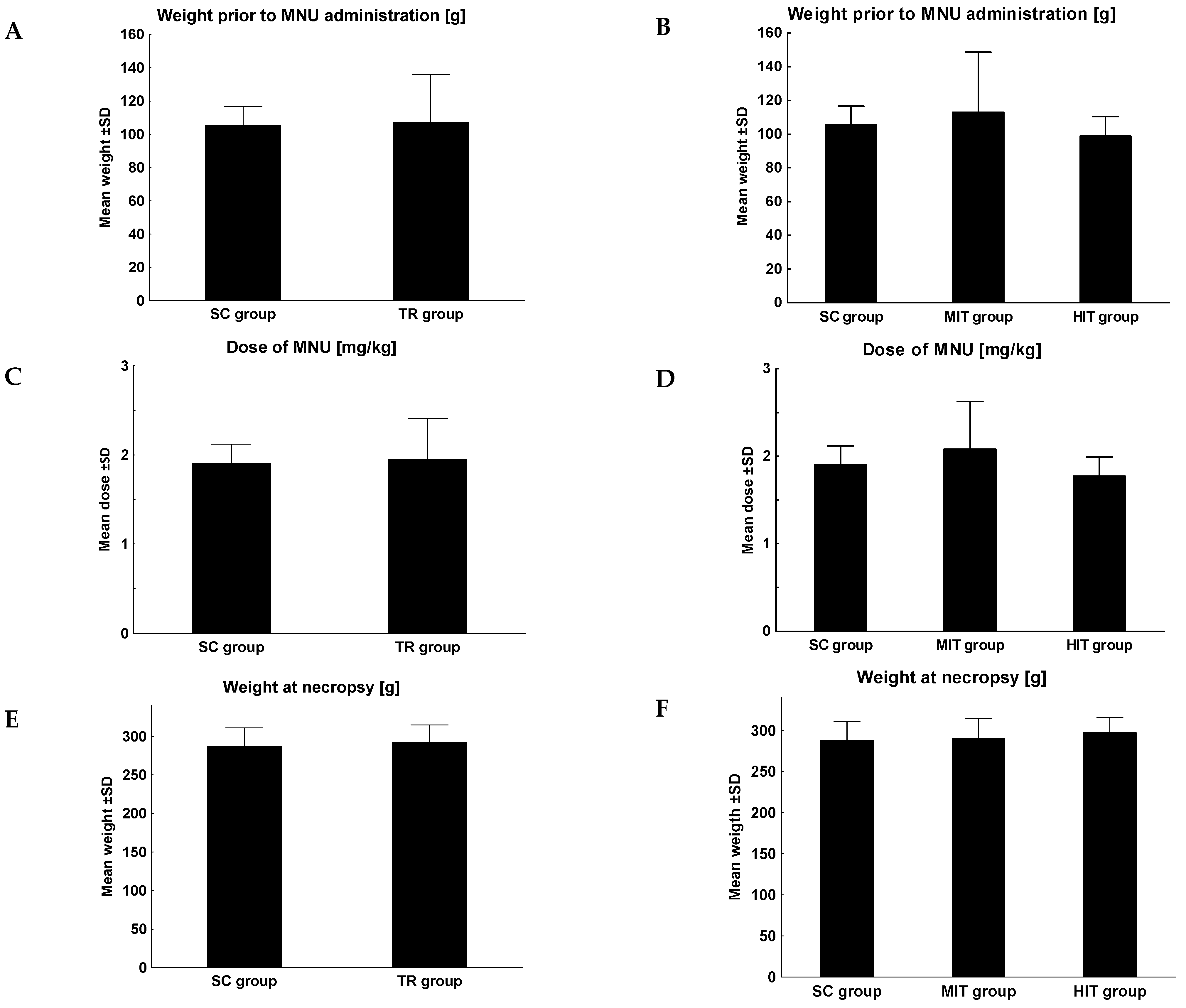
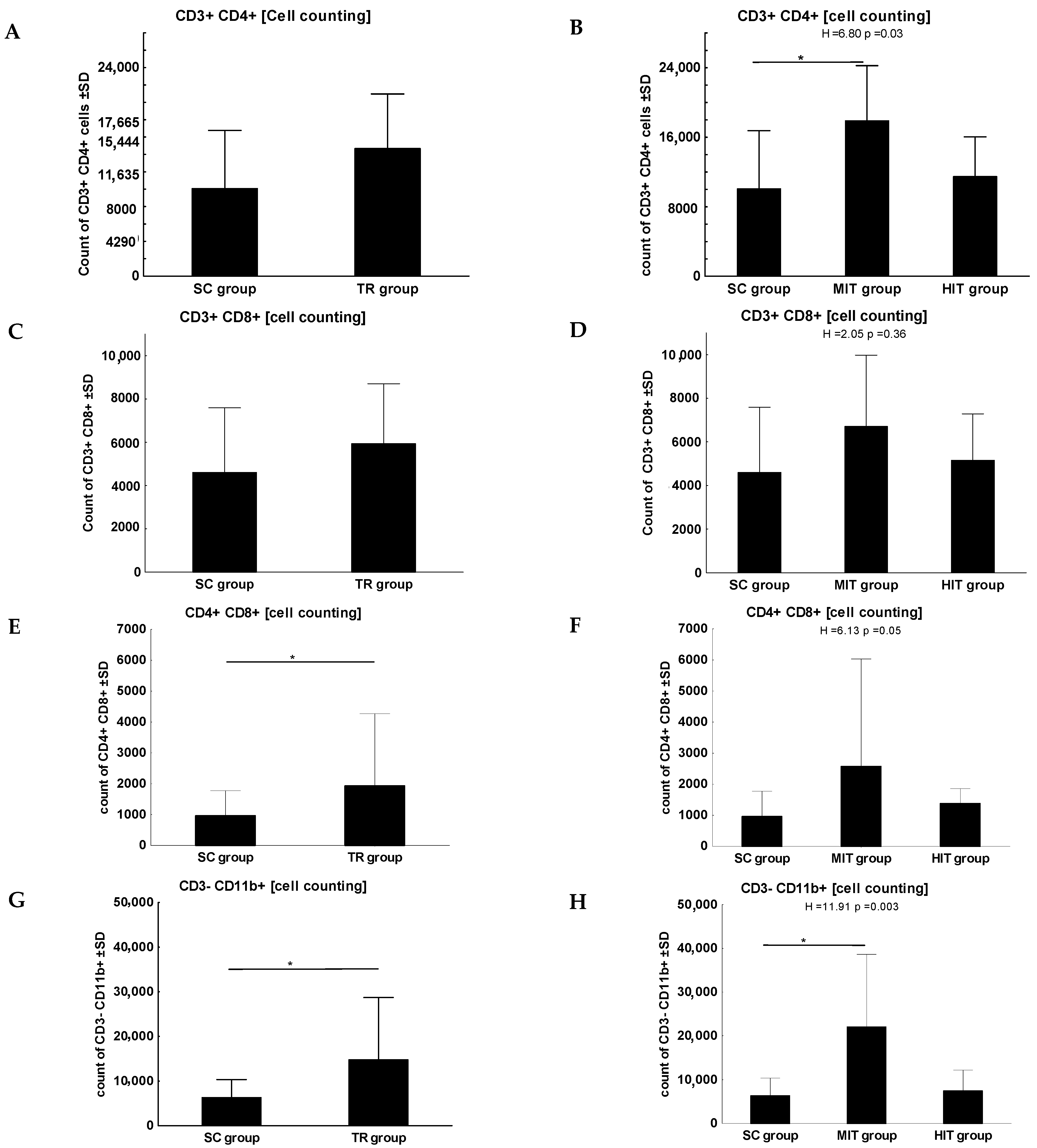
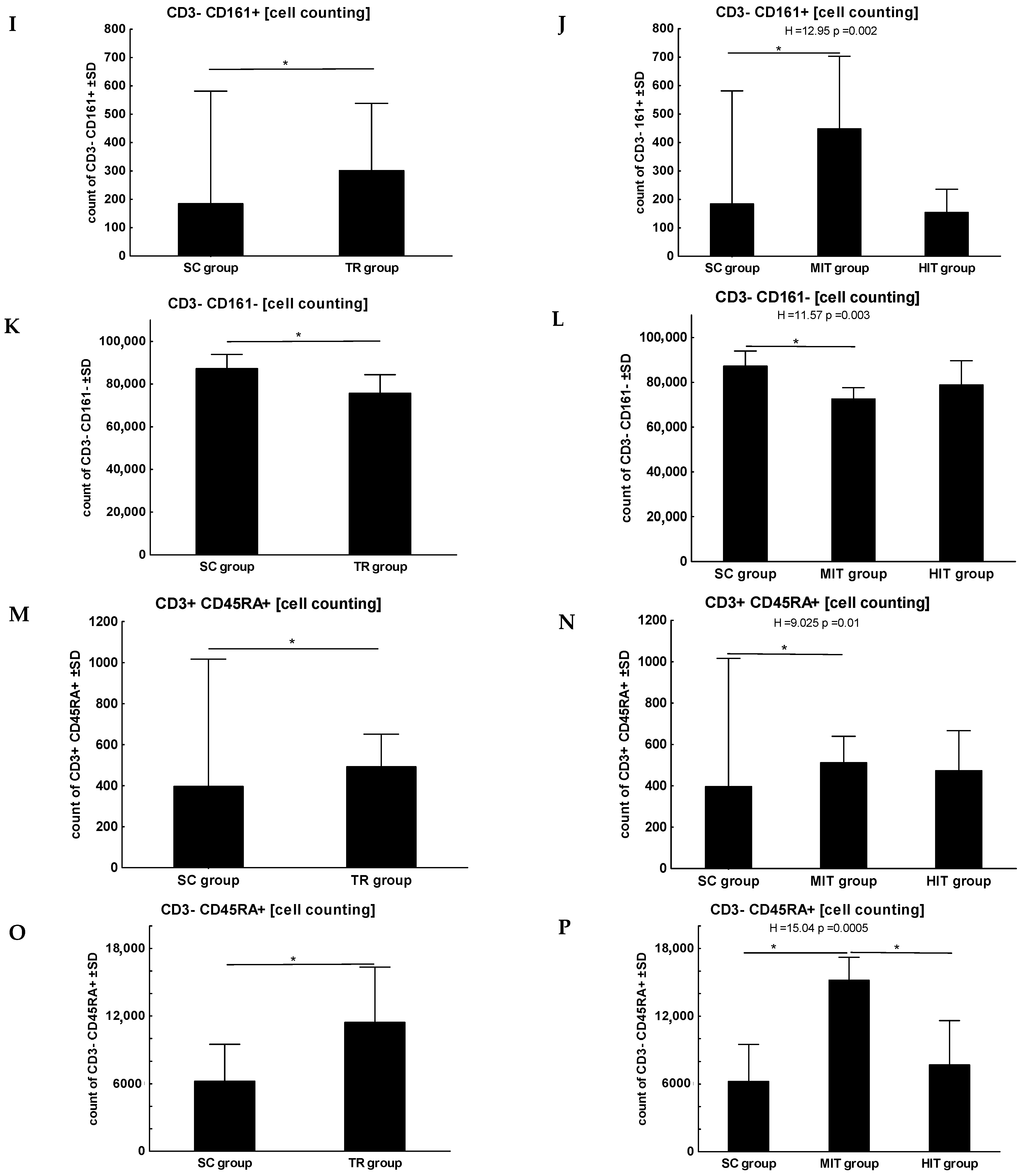
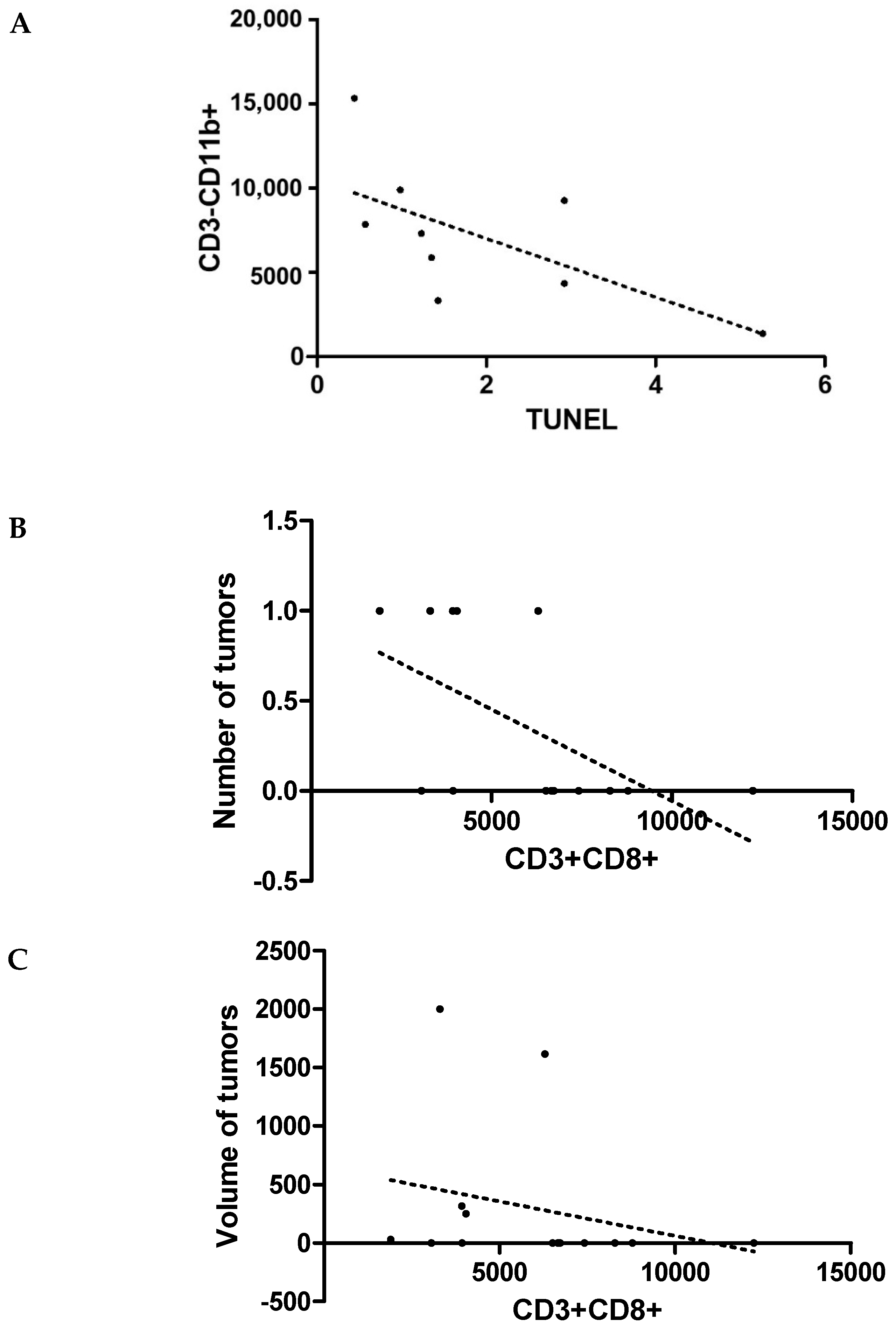
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Starska, K.; Łukomski, M. Rola limfocytów Th i T Rola limfocytów Th i Tc w powstawaniu i progresji cw powstawaniu i progresji nowotworów głowy i szyi. Otorynolaryngologia 2005, 4, 59–63. (In Polish) [Google Scholar]
- Macchetti, A.H.; Marana, H.R.C.; Silva, J.S.; Andrade, J.M.D.; Ribeiro-Silva, A.; Bighetti, S. Tumor-infiltrating CD4+ T lymphocytes in early breast cancer reflect lymph node involvement. Clinics 2006, 61, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Sheu, B.C.; Kuo, W.H.; Chen, R.J.; Huang, S.C.; Chang, K.J.; Chow, S.N. Clinical significance of tumor-infiltrating lymphocytes in neoplastic progression and lymph node metastasis of human breast cancer. Breast 2008, 17, 604–610. [Google Scholar] [CrossRef]
- Matkowski, R.; Gisterek, I.; Halon, A.; Lacko, A.; Szewczyk, K.; Staszek, U.; Pudelko, M.; Szynglarewicz, B.; Szelachowska, J.; Zolnierek, A.; et al. The prognostic role of tumor-infiltrating CD4 and CD8 T lymphocytes in breast cancer. Anticancer. Res. 2009, 29, 2445–2451. [Google Scholar]
- Jung, A.Y.; Behrens, S.; Schmidt, M.; Thoene, K.; Obi, N.; Hüsing, A.; Benner, A.; Steindorf, K.; Chang-Claude, J. Pre-to postdiagnosis leisure-time physical activity and prognosis in postmenopausal breast cancer survivors. Breast Cancer Res. 2019, 21, 117. [Google Scholar] [CrossRef]
- Friedenreich, C.M.; Courneya, K.S.; Bryant, H.E. Influence of physical activity in different age and life periods on the risk of breast cancer. Epidemiology 2001, 12, 604–612. [Google Scholar] [CrossRef]
- Westerlind, K.C.; McCarty, H.L.; Gibson, K.J.; Strange, R. Effect of exercise on the rat mammary gland: Implications for carcinogenesis. Acta Physiol. Scand. 2002, 175, 147–156. [Google Scholar] [CrossRef]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Nieman, D.C. Exercise immunology: Integration and regulation. Immunol. Today 1988, 19, 204–206. [Google Scholar] [CrossRef]
- Campbell, K.L.; McTiernan, A. Exercise and biomarkers for cancer prevention studies. J. Nutr. 2007, 137, 161S–169S. [Google Scholar] [CrossRef] [PubMed]
- Krumrych, W. Wpływ wysiłku fizycznego na mechanizmy odpornościowe u koni. Med. Weter. 2011, 67, 177–180. (In Polish) [Google Scholar]
- Demarzo, M.M.P.; Garcia, S.B. Exhaustive physical exercise increases the number of colonic preneoplastic lesions in untrained rats treated with a chemical carcinogen. Cancer Lett. 2004, 216, 31–34. [Google Scholar] [CrossRef]
- Bacurau, A.V.N.; Belmonte, M.A.; Navarro, F.; Moraes, M.R.; Pontes, J.F.L.; Pesquero, J.; Araujo, R.; Bacurau, R.F.P. Effect of a high-intensity exercise training on the metabolism and function of macrophages and lymphocytes of walker 256 tumor–bearing rats. Exp. Biol. Med. 2007, 232, 1289–1299. [Google Scholar] [CrossRef]
- Cardiff, R.D.; Wellings, S.R. The comparative pathology of human and mouse mammary glands. J. Mammary Gland. Biol. Neoplasia 1999, 4, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.Y.; Lee, S.H.; Kim, H.H.; Kim, S.M.; Shin, M.J.; Kim, N.; Gong, G. Evaluation of tumor angiogenesis with a second-generation US contrast medium in a rat breast tumor model. Korean J. Radiol. 2008, 9, 243–249. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pula, B.; Malicka, I.; Pawlowska, K.; Paslawska, U.; Cegielski, M.; Podhorska-Okolow, M.; Wozniewski, M. Immunohistochemical characterization of N-methyl-N-nitrosourea-induced mammary tumours of Sprague-Dawley rats. In Vivo 2013, 27, 793–801. [Google Scholar]
- Wang, R.; Tian, H.; Guo, D.; Tian, Q.; Yao, T.; Kong, X. Impacts of exercise intervention on various diseases in rats. J. Sport Health Sci. 2020, 9, 211–227. [Google Scholar] [CrossRef]
- Malicka, I.; Siewierska, K.; Kobierzycki, C.; Grzegrzolka, J.; Piotrowska, A.; Paslawska, U.; Cegielski, M.; Dziegiel, P.; Podhorska-Okolow, M.; Wozniewski, M. Impact of physical training on sex hormones and their receptors during n-methyl-n-nitrosourea-induced carcinogenesis in rats. Anticancer. Res. 2017, 37, 3581–3589. [Google Scholar] [CrossRef]
- Podhorska-Okolow, M.; Krajewska, B.; Carraro, U.; Zabel, M. Apoptosis in mouse skeletal muscles after physical exercise. Folia Histochem. Cytobiol. 1999, 37, 127–128. [Google Scholar]
- Malicka, I.; Siewierska, K.; Pula, B.; Kobierzycki, C.; Haus, D.; Paslawska, U.; Cegielski, M.; Dziegiel, P.; Podhorska-Okolow, M.; Wozniewski, M. The effect of physical training on the N-methyl-N-nitrosourea-induced mammary carcinogenesis of Sprague–Dawley rats. Exp. Biol. Med. 2015, 240, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Hoffman-Goetz, L. Exercise and the immune system: Regulation, integration, and adaptation. Physiol. Rev. 2000, 80, 1055–1081. [Google Scholar] [CrossRef] [PubMed]
- Kalicki, B.; Lewicka, A.; Jęderka, K.; Leśniak, M.; Marszałkowska-Jakubik, J.; Lewicki, S. Vitamin B6 improves blood parameters in rats fed a protein-deficient diet and subjected to moderate, long-term exercise. Cent. Eur. J. Immunol. 2019, 44, 23–32. [Google Scholar] [CrossRef]
- Guo, S.; Huang, Y.; Zhang, Y.; Huang, H.; Hong, S.; Liu, T. Impacts of exercise interventions on different diseases and organ functions in mice. J. Sport Health Sci. 2020, 9, 53–73. [Google Scholar] [CrossRef] [PubMed]
- Holmen Olofsson, G.; Jensen, A.W.P.; Idorn, M. Exercise oncology and Immuno-Oncology; a (future) dynamic Duo. Int. J. Mol. Sci. 2020, 21, 3816. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Idorn, M.; Olofsson, G.H.; Lauenborg, B.; Nookaew, I.; Hansen, R.H.; Johannesen, H.H.; Becker, J.C.; Pedersen, K.S.; Dethlefsen, C.; et al. Voluntary running suppresses tumor growth through epinephrine-and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 2016, 23, 554–562. [Google Scholar] [CrossRef]
- Rundqvist, H.; Veliça, P.; Barbieri, L.; A Gameiro, P.; Bargiela, D.; Gojkovic, M.; Mijwel, S.; Reitzner, S.M.; Wulliman, D.; Ahlstedt, E.; et al. Cytotoxic T-cells mediate exercise-induced reductions in tumor growth. Elife 2020, 9, e59996. [Google Scholar] [CrossRef]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The immunobiology of Cancer immunosurveillance and immunoediting. Immunity 2004, 21, 137–148. [Google Scholar] [CrossRef]
- Petersen, S.H.; Odintsova, E.; Haigh, T.A.; Rickinson, A.B.; Taylor, G.S.; Berditchevski, F. The role of tetraspanin CD63 in antigen presentation via MHC class II. Eur. J. Immunol. 2011, 41, 2556–2561. [Google Scholar] [CrossRef]
- Chew, V.; Toh, H.C.; Abastado, J.P. Immune microenvironment in tumor progression: Characteristics and challenges for therapy. J. Oncol. 2012, 2012, 608406. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.P.; Riddell, N.E.; Burns, V.E.; Turner, M.; van Zanten, J.J.; Drayson, M.T.; Bosch, J.A. Acute exercise mobilises CD8+ T lymphocytes exhibiting an effector-memory phenotype. Brain Behav. Immun. 2009, 23, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Santos, I.L.; Amoozgar, Z.; Kumar, A.S.; Ho, W.W.; Roh, K.; Talele, N.P.; Curtis, H.; Kawaguchi, K.; Jain, R.K.; Fukumura, D. Exercise training improves tumor control by increasing CD8+ T-cell infiltration via CXCR3 signaling and sensitizes breast cancer to immune checkpoint blockade. Cancer Immunol. Res. 2021, 9, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Gleeson, M.; Woods, J.A.; Bishop, N.; Simon, P. Position statement part one: Immune function and exercise. Exerc. Immunol. Rev. 2011, 17, 6–63. [Google Scholar]
- Estébanez, B.; Visavadiya, N.P.; de Paz, J.A.; Whitehurst, M.; Cuevas, M.J.; González-Gallego, J.; Huang, C.J. Resistance training diminishes the expression of exosome CD63 protein without modification of plasma miR-146a-5p and cfDNA in the elderly. Nutrients 2021, 13, 665. [Google Scholar] [CrossRef]
- Miki, Y.; Yashiro, M.; Okuno, T.; Kuroda, K.; Togano, S.; Hirakawa, K.; Ohira, M. Clinico-pathological significance of exosome marker CD63 expression on cancer cells and stromal cells in gastric cancer. PLoS ONE 2018, 13, e0202956. [Google Scholar] [CrossRef]
- Goh, J.; Tsai, J.; Bammler, T.K.; Farin, F.M.; Endicott, E.; Ladiges, W.C. Exercise training in transgenic mice is associated with attenuation of early breast cancer growth in a dose-dependent manner. PLoS ONE 2013, 8, e80123. [Google Scholar] [CrossRef]
- Hagar, A.; Wang, Z.; Koyama, S.; Aponte-Serrano, J.O.; Melo, L.; Vargas, S.; Carpenter, R.; Foley, J. Endurance training slows breast tumor growth in mice by suppressing Treg cells recruitment to tumors. BMC Cancer 2019, 19, 536. [Google Scholar] [CrossRef]
- Demarzo, M.M.P.; Martins, L.V.; Fernandes, C.R.; Herrero, F.A.; Perez, S.E.D.A.; Turatti, A.; Garcia, S.B. Exercise Reduces Inflammation and Cell Proliferation in Rat Colon Carchnogenesis. Med. Sci. Sports Exerc. 2008, 40, 618–621. [Google Scholar] [CrossRef]
- Gustafson, M.P.; DiCostanzo, A.C.; Wheatley, C.M.; Kim, C.-H.; Bornschlegl, S.; Gastineau, D.A.; Johnson, B.D.; Dietz, A.B. A systems biology approach to investigating the influence of exercise and fitness on the composition of leukocytes in peripheral blood. J. Immunother. Cancer 2017, 5, 30. [Google Scholar] [CrossRef]

| CD3+/CD4+ | Th lymphocytes |
| CD3+/CD8+ | Tc lymphocytes |
| CD4+/CD8+ | double-positive immature lymphocytes |
| CD3−/CD11b+ | granulocytes, monocytes |
| CD3−/CD161+ | NK |
| CD3−/CD161− | B lymphocytes, monocytes |
| CD3+/CD45RA+ | T lymphocytes |
| CD3−/CD45RA+ | B lymphocytes, monocytes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malicka, I.; Siewierska, K.; Olbromski, M.; Glatzel-Plucinska, N.; Podhorska-Okolow, M.; Dziegiel, P.; Wozniewski, M. The Influence of Physical Training on the Immune System of Rats during N-methyl-N-nitrosourea-Induced Carcinogenesis. J. Clin. Med. 2022, 11, 6371. https://doi.org/10.3390/jcm11216371
Malicka I, Siewierska K, Olbromski M, Glatzel-Plucinska N, Podhorska-Okolow M, Dziegiel P, Wozniewski M. The Influence of Physical Training on the Immune System of Rats during N-methyl-N-nitrosourea-Induced Carcinogenesis. Journal of Clinical Medicine. 2022; 11(21):6371. https://doi.org/10.3390/jcm11216371
Chicago/Turabian StyleMalicka, Iwona, Katarzyna Siewierska, Mateusz Olbromski, Natalia Glatzel-Plucinska, Marzenna Podhorska-Okolow, Piotr Dziegiel, and Marek Wozniewski. 2022. "The Influence of Physical Training on the Immune System of Rats during N-methyl-N-nitrosourea-Induced Carcinogenesis" Journal of Clinical Medicine 11, no. 21: 6371. https://doi.org/10.3390/jcm11216371
APA StyleMalicka, I., Siewierska, K., Olbromski, M., Glatzel-Plucinska, N., Podhorska-Okolow, M., Dziegiel, P., & Wozniewski, M. (2022). The Influence of Physical Training on the Immune System of Rats during N-methyl-N-nitrosourea-Induced Carcinogenesis. Journal of Clinical Medicine, 11(21), 6371. https://doi.org/10.3390/jcm11216371








