Clinical Characteristics of Patients with Dental Malocclusion: An Otolaryngologic Perspective
Abstract
1. Introduction
2. Methods
2.1. Patient Enrolment
2.2. Clinical Course of the Referred Patients
2.3. Outcome Measures
2.3.1. Subjective Findings
2.3.2. Objective Findings
2.4. Comparative Analysis with An Age- and Sex-Matched Control Group
2.5. Data Analysis, and Statistics
2.6. Ethical Approval and Consent to Participate
3. Results
3.1. Demographic and Clinical Characteristics of Patients with Dental Malocclusion
3.2. Comparison between Patients with Positive and Negative SPT
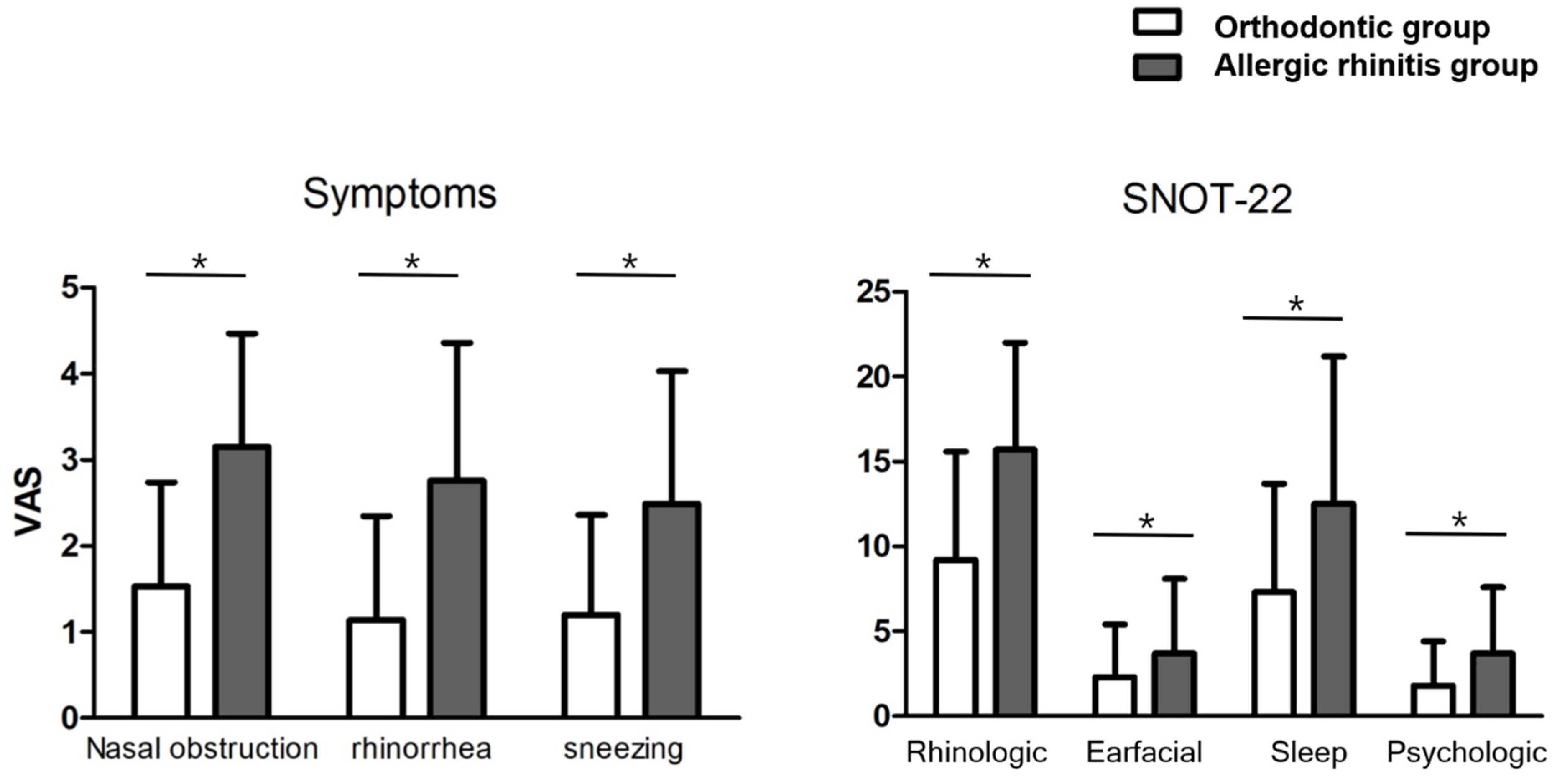
3.3. Comparison between Patients in the Orthodontic and AR Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AR | allergic rhinitis |
| ARIA | Allergic Rhinitis and its Impact on Asthma |
| MPI | minimal persistent inflammation |
| SNOT-22 | sinonasal outcome test-22 questionnaire |
| SPT | skin prick test |
| T&A | tonsil and adenoid |
| VAS | visual analogue scale |
References
- Moss, M.L. A theoretical analysis of the functional matrix. Acta Biotheor. 1968, 18, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Rubin, R.M. Mode of respiration and facial growth. Am. J. Orthod. 1980, 78, 504–510. [Google Scholar] [CrossRef]
- Solow, B.; Kreiborg, S. Soft-tissue stretching: A possible control factor in craniofacial morphogenesis. Scand. J. Dent. Res. 1977, 85, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Valera, F.C.; Travitzki, L.V.; Mattar, S.E.; Matsumoto, M.A.; Elias, A.M.; Anselmo-Lima, W.T. Muscular, functional and orthodontic changes in pre school children with enlarged adenoids and tonsils. Int. J. Pediatr. Otorhinolaryngol. 2003, 67, 761–770. [Google Scholar] [CrossRef]
- Sousa, J.B.; Anselmo-Lima, W.T.; Valera, F.C.; Gallego, A.J.; Matsumoto, M.A. Cephalometric assessment of the mandibular growth pattern in mouth-breathing children. Int. J. Pediatr. Otorhinolaryngol. 2005, 69, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Harvold, E.P.; Tomer, B.S.; Vargervik, K.; Chierici, G. Primate experiments on oral respiration. Am. J. Orthod. 1981, 79, 359–372. [Google Scholar] [CrossRef]
- Vig, K.W. Nasal obstruction and facial growth: The strength of evidence for clinical assumptions. Am. J. Orthod. Dentofac. Orthop. 1998, 113, 603–611. [Google Scholar] [CrossRef]
- Bellanti, J.A.; Wallerstedt, D.B. Allergic rhinitis update: Epidemiology and natural history. Allergy Asthma Proc. 2000, 21, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Shabestari, M.; Jabbari Moghaddam, Y.; Ghaharri, H. Is there any correlation between allergy and adenotonsillar tissue hypertrophy? Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 589–591. [Google Scholar] [CrossRef]
- Luzzi, V.; Ierardo, G.; Viscogliosi, A.; Fabbrizi, M.; Consoli, G.; Vozza, I.; Vestri, A.; Polimeni, A. Allergic rhinitis as a possible risk factor for malocclusion: A case-control study in children. Int. J. Paediatr. Dent. 2013, 23, 274–278. [Google Scholar] [CrossRef]
- Bresolin, D.; Shapiro, P.A.; Shapiro, G.G.; Chapko, M.K.; Dassel, S. Mouth breathing in allergic children: Its relationship to dentofacial development. Am. J. Orthod. 1983, 83, 334–340. [Google Scholar] [CrossRef]
- Brożek, J.L.; Bousquet, J.; Agache, I.; Agarwal, A.; Bachert, C.; Bosnic-Anticevich, S.; Brignardello-Petersen, R.; Canonica, G.W.; Casale, T.; Chavannes, N.H.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J. Allergy Clin. Immunol. 2017, 140, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.L.; Hubbard, M.A.; Huyett, P.; Patrie, J.T.; Borish, L.; Payne, S.C. Sino-nasal outcome test (SNOT-22): A predictor of postsurgical improvement in patients with chronic sinusitis. Ann. Allergy Asthma Immunol. 2013, 111, 246–251.e2. [Google Scholar] [CrossRef] [PubMed]
- Camacho, M.; Zaghi, S.; Certal, V.; Abdullatif, J.; Means, C.; Acevedo, J.; Liu, S.; Brietzke, S.E.; Kushida, C.A.; Capasso, R. Inferior turbinate classification system, grades 1 to 4: Development and validation study. Laryngoscope 2015, 125, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.J.; Cho, I.K.; Lee, K.I.; Bae, S.H.; Lee, J.W.; Chung, P.S.; Mo, J.H. Seasonal specificity of seasonal allergens and validation of the ARIA classification in Korea. Allergy Asthma Immunol. Res. 2013, 5, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Kuprys-Lipinska, I.; Elgalal, A.; Kuna, P. Skin prick test with inhaled allergens in the general population of Lodz province. Pneumonol. Alergol. Pol. 2009, 77, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Kara, C.O.; Ergin, H.; Kocak, G.; Kilic, I.; Yurdakul, M. Prevalence of tonsillar hypertrophy and associated oropharyngeal symptoms in primary school children in Denizli, Turkey. Int. J. Pediatr. Otorhinolaryngol. 2002, 66, 175–179. [Google Scholar] [CrossRef]
- Min, Y.G. Pathophysiology, diagnosis, and treatment of allergic rhinitis. Korean J. Otorhinolaryngol.-Head Neck Surg. 2013, 56, 256–265. [Google Scholar] [CrossRef]
- Bixler, E.O.; Vgontzas, A.N.; Lin, H.M.; Liao, D.; Calhoun, S.; Vela-Bueno, A.; Fedok, F.; Vlasic, V.; Graff, G. Sleep disordered breathing in children in a general population sample: Prevalence and risk factors. Sleep 2009, 32, 731–736. [Google Scholar] [CrossRef]
- Koca, C.F.; Erdem, T.; Bayindir, T. The effect of adenoid hypertrophy on maxillofacial development: An objective photographic analysis. J. Otolaryngol. Head Neck Surg. 2016, 45, 48. [Google Scholar] [CrossRef] [PubMed]
- Kallunki, J.; Marcusson, A.; Ericsson, E. Tonsillotomy versus tonsillectomy—A randomized trial regarding dentofacial morphology and post-operative growth in children with tonsillar hypertrophy. Eur. J. Orthod. 2014, 36, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Gungor, A.Y.; Turkkahraman, H. Effects of airway problems on maxillary growth: A review. Eur. J. Dent. 2009, 3, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Ro, Y.S.; Kang, J.H.; Kim, J.T.; Kwon, D.H. The change of nasal resistance according to the age and sex in Koreans. Korean J. Otorhinolaryngol.-Head Neck Surg. 1995, 38, 711–717. [Google Scholar]
- Cheng, M.C.; Enlow, D.H.; Papsidero, M.; Broadbent, B.H., Jr.; Oyen, O.; Sabat, M. Developmental effects of impaired breathing in the face of the growing child. Angle Orthod. 1988, 58, 309–320. [Google Scholar]
- Freng, A. Restricted nasal respiration, influence on facial growth. Int. J. Pediatr. Otorhinolaryngol. 1979, 1, 249–254. [Google Scholar] [CrossRef]
- Adamidis, I.P.; Spyropoulos, M.N. The effects of lymphadenoid hypertrophy on the position of the tongue, the mandible and the hyoid bone. Eur. J. Orthod. 1983, 5, 287–294. [Google Scholar] [CrossRef]
- Abreu, R.R.; Rocha, R.L.; Lamounier, J.A.; Guerra, A.F. Etiology, clinical manifestations and concurrent findings in mouth-breathing children. J. Pediatr. 2008, 84, 529–535. [Google Scholar] [CrossRef]
- Ciprandi, G.; Buscaglia, S.; Pesce, G.; Pronzato, C.; Ricca, V.; Parmiani, S.; Bagnasco, M.; Canonica, G.W. Minimal persistent inflammation is present at mucosal level in patients with asymptomatic rhinitis and mite allergy. J. Allergy Clin. Immunol. 1995, 96 Pt 1, 971–979. [Google Scholar] [CrossRef]
- Storms, W.W. Minimal persistent inflammation, an emerging concept in the nature and treatment of allergic rhinitis: The possible role of leukotrienes. Ann. Allergy Asthma Immunol. 2003, 91, 131–140. [Google Scholar] [CrossRef]
- Canonica, G.W.; Compalati, E. Minimal persistent inflammation in allergic rhinitis: Implications for current treatment strategies. Clin. Exp. Immunol. 2009, 158, 260–271. [Google Scholar] [CrossRef]
- Occasi, F.; Perri, L.; Saccucci, M.; Di Carlo, G.; Ierardo, G.; Luzzi, V.; De Castro, G.; Brindisi, G.; Loffredo, L.; Duse, M.; et al. Malocclusion and rhinitis in children: An easy-going relationship or a yet to be resolved paradox? A systematic literature revision. Ital. J. Pediatr. 2018, 44, 100. [Google Scholar] [CrossRef] [PubMed]
- Imbaud, T.C.; Mallozi, M.C.; Domingos, V.B.; Sole, D. Frequency of rhinitis and orofacial disorders in patients with dental malocclusion. Rev. Paul. Pediatr. 2016, 34, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Corey, J.P. Acoustic rhinometry: Should we be using it? Curr. Opin. Otolaryngol. Head Neck Surg. 2006, 14, 29–34. [Google Scholar] [CrossRef] [PubMed]
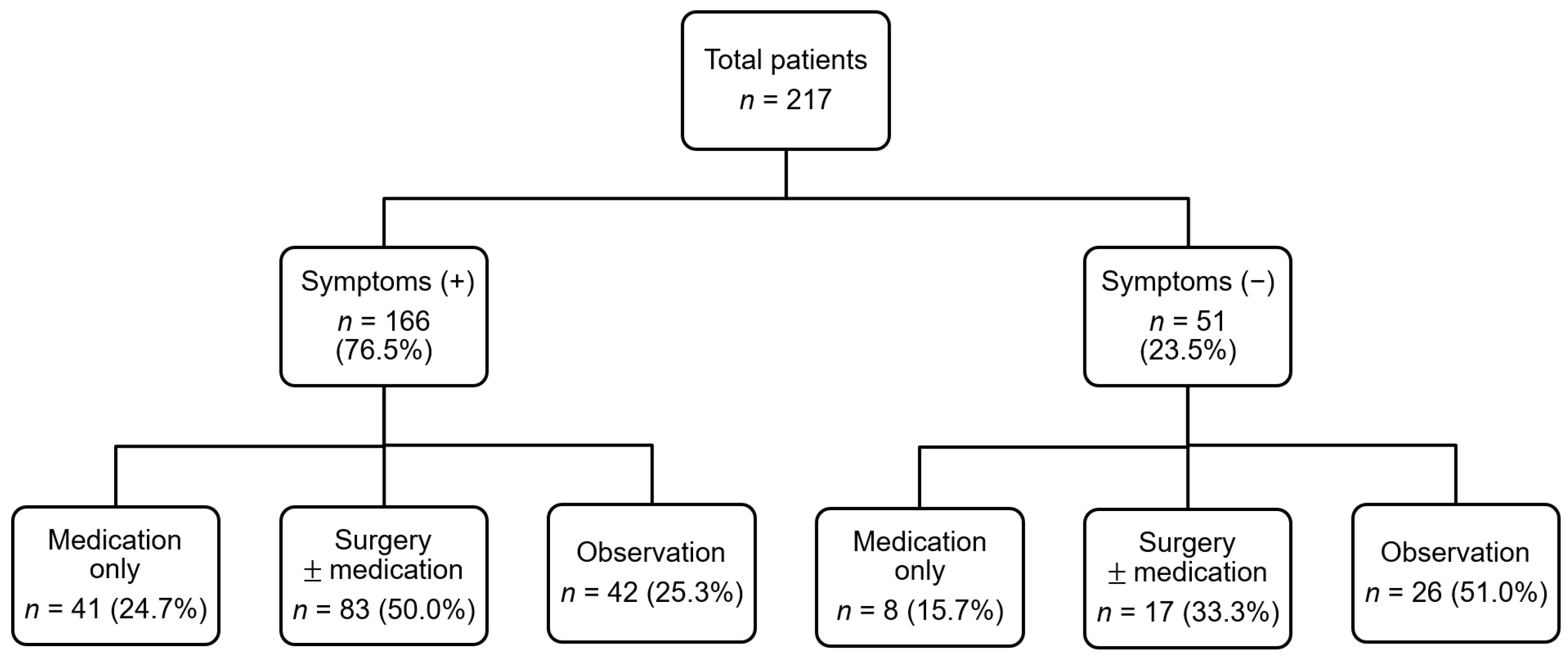
| Variable | Total Orthodontic Patients (n = 217) |
|---|---|
| Median age (range), y | 13.8 (5–29) |
| Sex, n (%) | |
| Male | 102 (47.0) |
| Female | 115 (53.0) |
| Symptoms, n (%) | 166 (76.5) |
| Nasal obstruction | 125 (57.6) |
| Rhinorrhea | 104 (47.9) |
| Sneezing | 82 (37.8) |
| Itching | 60 (27.6) |
| Sleep disturbance | 15 (6.9) |
| Abnormal PE findings, n (%) | 202 (93.1) |
| IT hypertrophy | 178 (82.0) |
| T&A hypertrophy | 69 (31.8) |
| Deviated nasal septum | 16 (7.4) |
| Treatment, n (%) | 149 (68.7) |
| Medical treatment | 49 (22.6) |
| Surgical treatment | 100 (46.1) |
| Adenotonsillectomy | 21 (9.7) |
| Turbinoplasty | 30 (13.8) |
| Adenotonsillectomy with turbinoplasty | 49 (22.6) |
| Variable | Treatment, n (%) | No Treatment | pa | Odds Ratio | |
|---|---|---|---|---|---|
| Medication | Surgery | (95% CI) | |||
| Nasal obstruction (+), (n = 125) | 22 (17.6) | 93 (74.4) | 10 (8.0) | <0.001 | 3.7 (2.7–5.1) |
| Nasal obstruction (–), (n = 92) | 27 (29.3) | 7 (7.6) | 58 (63.1) | ||
| Variable | Total Orthodontic Patients (n = 217) | pa | Odds Ratio a (95% CI) | |
|---|---|---|---|---|
| SPT (+) | SPT (–) | |||
| Total, n (%) | 173 (79.7) | 44 (20.3) | ||
| Rhinologic Sx, n (%) | 136 (78.6) | 30 (68.2) | 0.145 | 1.7 (0.8–3.6) |
| Nasal obstruction | 101 (58.4) | 24 (54.5) | 0.646 | 1.2 (0.6–2.3) |
| Rhinorrhea | 94 (54.3) | 10 (22.7) | <0.001 | 4.0 (1.9–8.7) |
| Sneezing | 77 (44.5) | 5 (11.4) | <0.001 | 6.3 (2.4–16.6) |
| Itching | 52 (30.1) | 8 (18.2) | 0.116 | 1.9 (0.8–4.4) |
| Abnormal PE findings, n (%) | ||||
| IT hypertrophy | 148 (85.5) | 30 (68.2) | 0.007 | 2.8 (1.3–5.9) |
| T&A hypertrophy | 60 (34.7) | 9 (20.5) | 0.07 | 2.1 (0.9–4.6) |
| Deviated nasal septum | 13 (7.5) | 3 (6.8) | 0.875 | 1.1 (0.3–4.1) |
| Treatment, n (%) | 123 (71.1) | 26 (59.1) | 0.125 | 0.6 (0.3–1.2) |
| Medical treatment | 41 (33.7) | 8 (18.2) | 0.434 | 1.4 (0.6–3.2) |
| Surgical treatment | 82 (35.8) | 18 (40.9) | 0.441 | 1.3 (0.7–2.5) |
| Adenotonsillectomy | 15 (8.7) | 6 (13.6) | 0.320 | 0.6 (0.2–1.7) |
| Turbinoplasty | 26 (15.2) | 4 (9.1) | 0.308 | 1.8 (0.6–5.4) |
| Adenotonsillectomy with turbinoplasty | 41 (30.4) | 8 (18.2) | 0.434 | 1.4 (0.6–3.2) |
| Variable | Orthodontic Group | Control Group | p Value a | Odds Ratio a (95% CI) |
|---|---|---|---|---|
| Rhinologic Sx, n (%) | 136 (78.6) | 173 (100) | ||
| Nasal obstruction | 101 (58.4) | 149 (86.1) | <0.001 | 4.4 (2.6–7.5) |
| Rhinorrhea | 94 (54.3) | 122 (70.5) | 0.002 | 2.0 (1.3–3.1) |
| Sneezing | 77 (44.5) | 117 (67.6) | <0.001 a | 2.6 (1.7–4.0) |
| Itching | 52 (30.1) | 61 (35.3) | 0.302 | 0.8 (0.5–1.2) |
| Abnormal PE findings, n (%) | ||||
| IT hypertrophy | 148 (85.5) | 158 (91.3) | 0.093 | 0.6 (0.3–1.1) |
| T&A hypertrophy | 60 (34.7) | 60 (34.7) | 1.000 | 1.0 (0.6–1.6) |
| Deviated nasal septum | 13 (7.5) | 22 (12.7) | 0.109 | 0.6 (0.3–1.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, S.H.; Choi, J.H.; Mo, J.-H. Clinical Characteristics of Patients with Dental Malocclusion: An Otolaryngologic Perspective. J. Clin. Med. 2022, 11, 6318. https://doi.org/10.3390/jcm11216318
Yoo SH, Choi JH, Mo J-H. Clinical Characteristics of Patients with Dental Malocclusion: An Otolaryngologic Perspective. Journal of Clinical Medicine. 2022; 11(21):6318. https://doi.org/10.3390/jcm11216318
Chicago/Turabian StyleYoo, Shin Hyuk, Ji Hyeok Choi, and Ji-Hun Mo. 2022. "Clinical Characteristics of Patients with Dental Malocclusion: An Otolaryngologic Perspective" Journal of Clinical Medicine 11, no. 21: 6318. https://doi.org/10.3390/jcm11216318
APA StyleYoo, S. H., Choi, J. H., & Mo, J.-H. (2022). Clinical Characteristics of Patients with Dental Malocclusion: An Otolaryngologic Perspective. Journal of Clinical Medicine, 11(21), 6318. https://doi.org/10.3390/jcm11216318






