Chondral/Desmal Osteogenesis in 3D Spheroids Sensitized by Psychostimulants
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Substances
2.3. xCELLigence Real Time Monitoring of Cell Proliferation
2.4. Establishment of HMSC- and MG63-Derived Spheroids
2.5. Spheroid Size Evaluation
2.6. Chondrogenic Spheroid 3D Culture
2.7. Osteogenic Spheroid 3D Culture
2.8. RNA Isolation and Quantitative RT-PCR
2.9. Staining of Cryosectioned Spheroids
2.10. Live/Dead Vitality Staining
2.11. Software and Statistical Analysis
3. Results
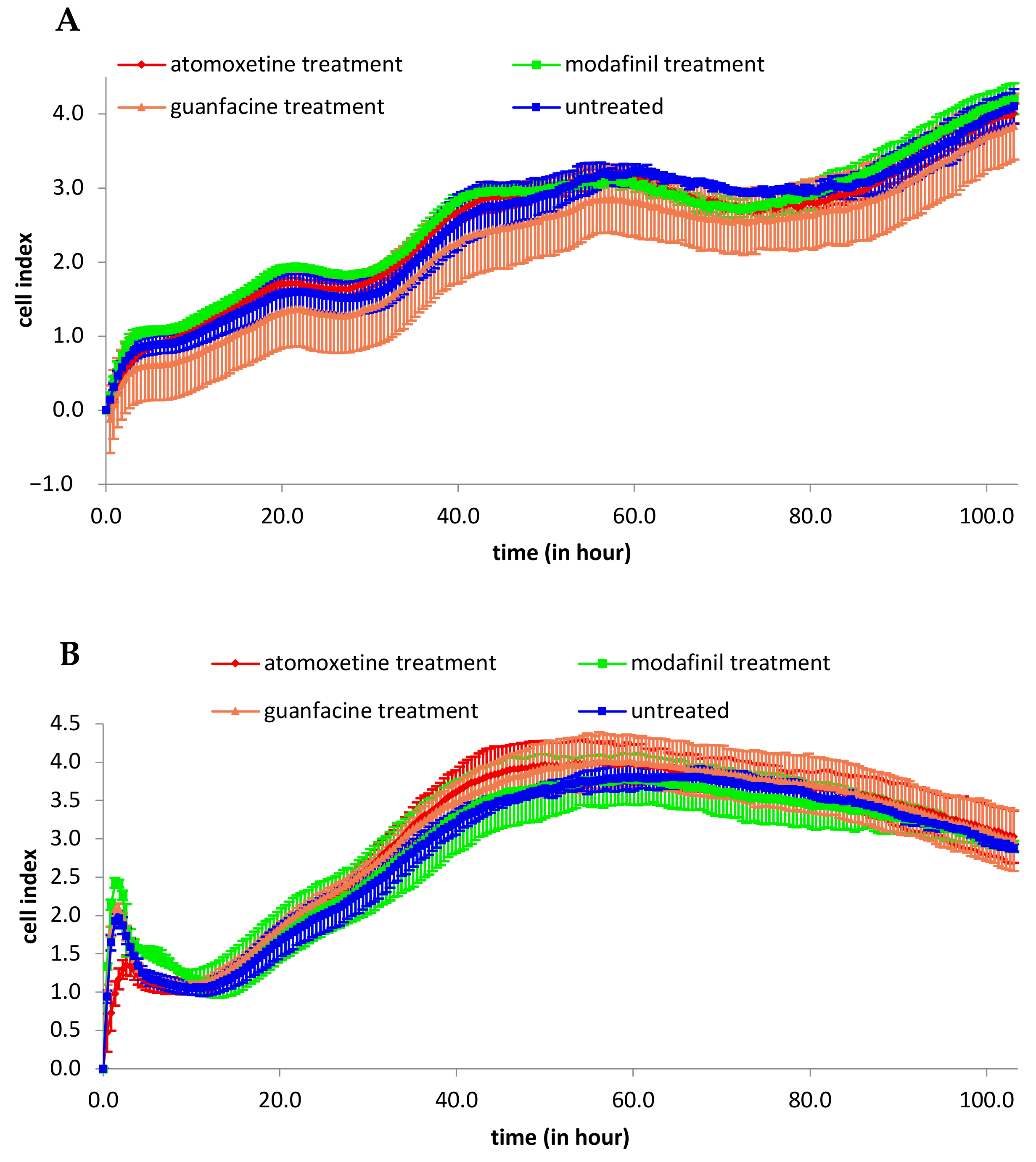
3.1. Analysis of 2D Cell Proliferation in Modafinil, Atomoxetine and Guanfacine Treated MG63 and hMSC
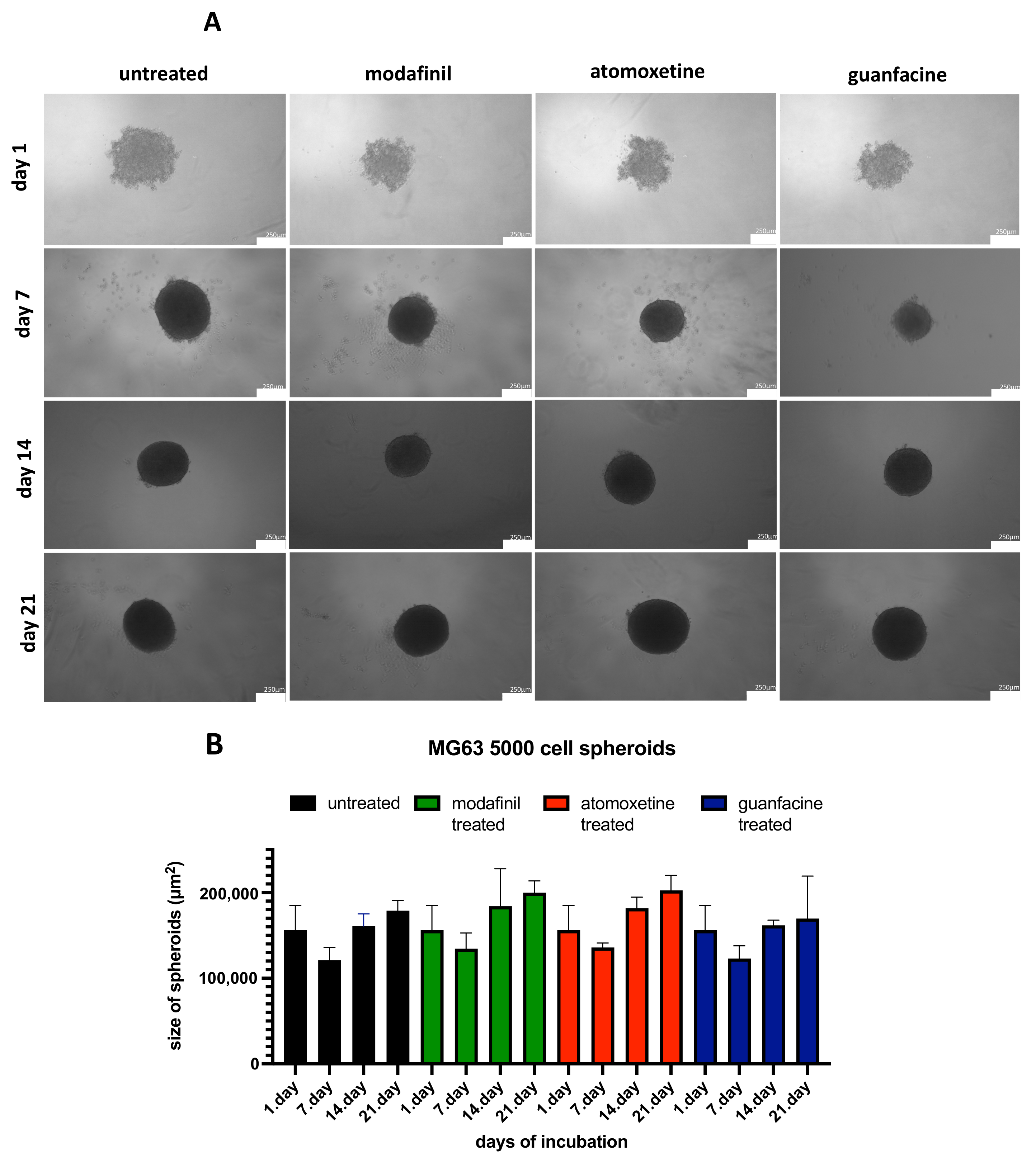
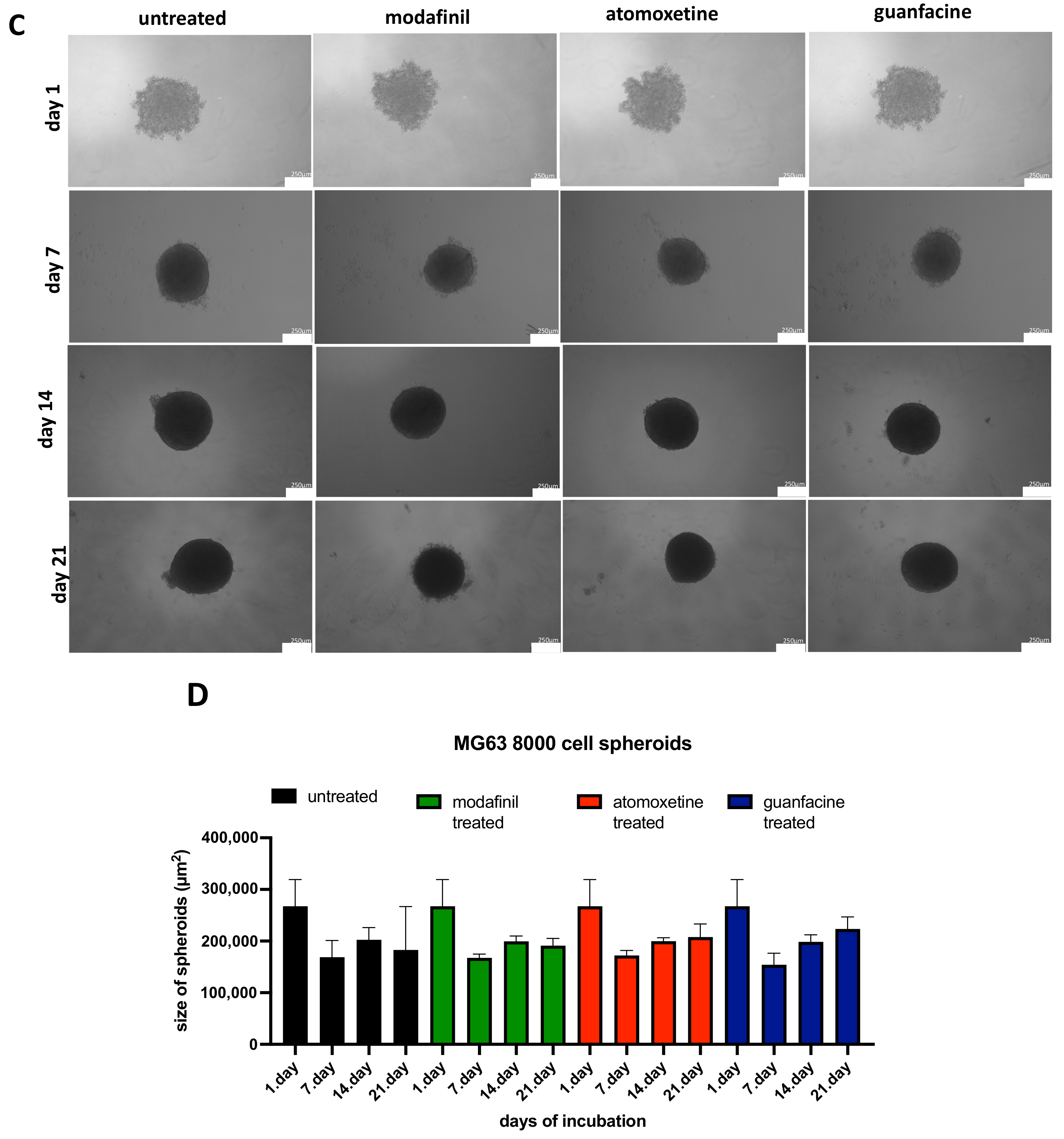
3.2. Analysis of the Spheroid Size of 5000/8000-Cell MG63 Spheroids
3.3. Analysis of the Spheroid Size of 5000/8000-cell hMSC Spheroids
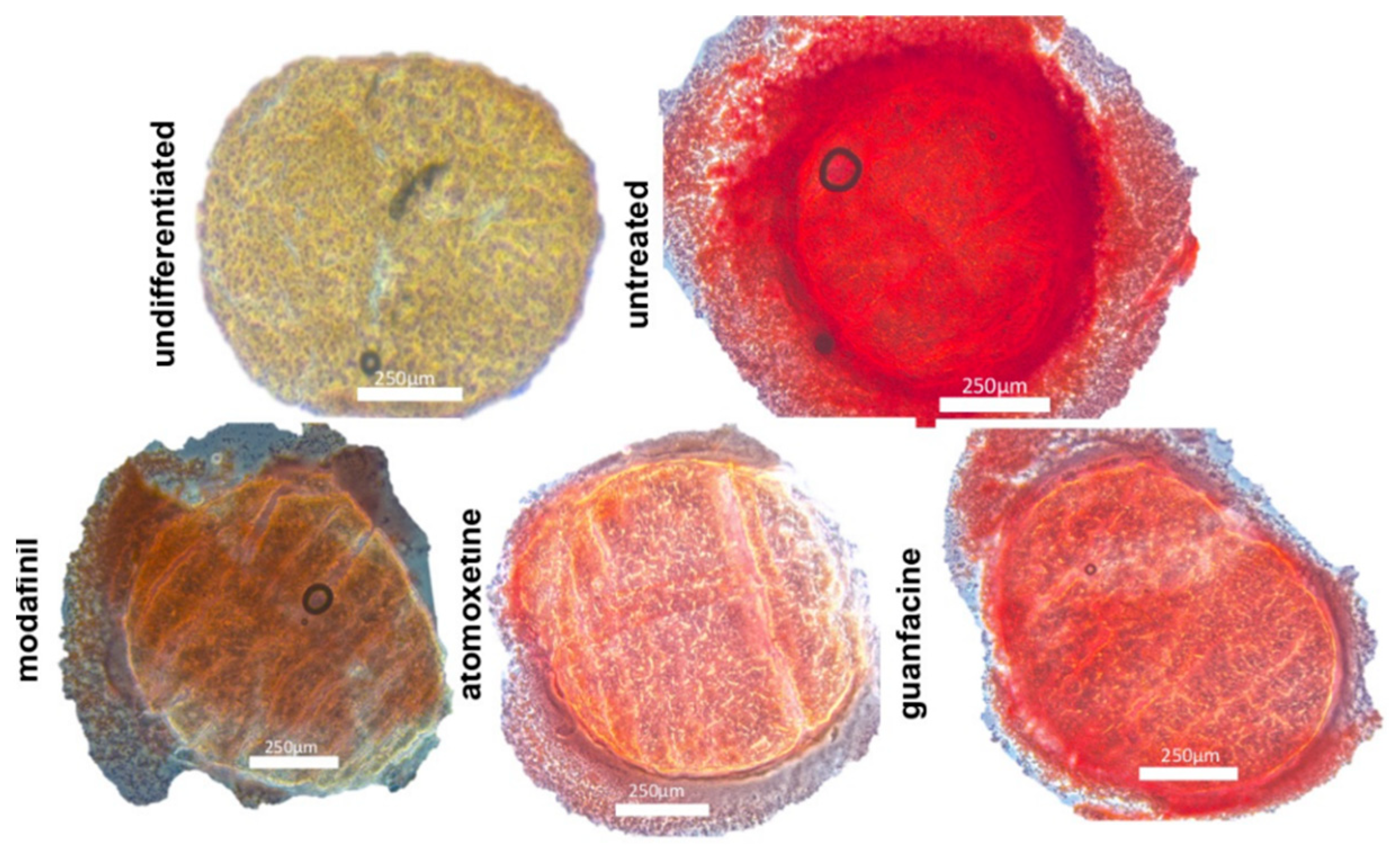
3.4. Histological Spheroid Sections of Chondrogenic Spheroids after the Administration of Modafinil, Atomoxetine and Guanfacine
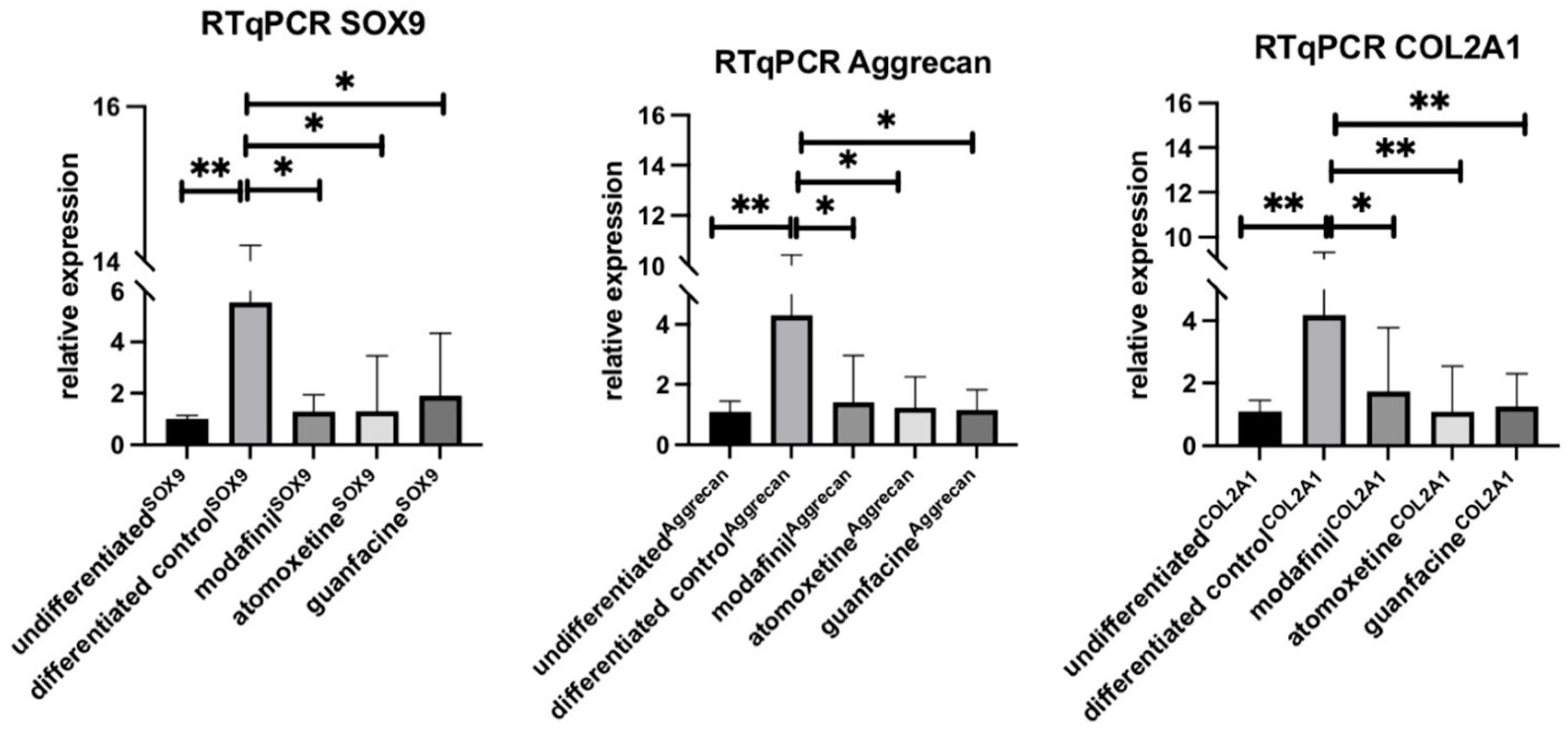
3.5. Influence of Modafinil, Atomoxetine and Guanfacine on the Expression of SOX9, Aggrecan and COL2A1
3.6. Histological Spheroid Sections of Osteogenic Spheroids after Administration of Modafinil, Atomoxetine and Guanfacine
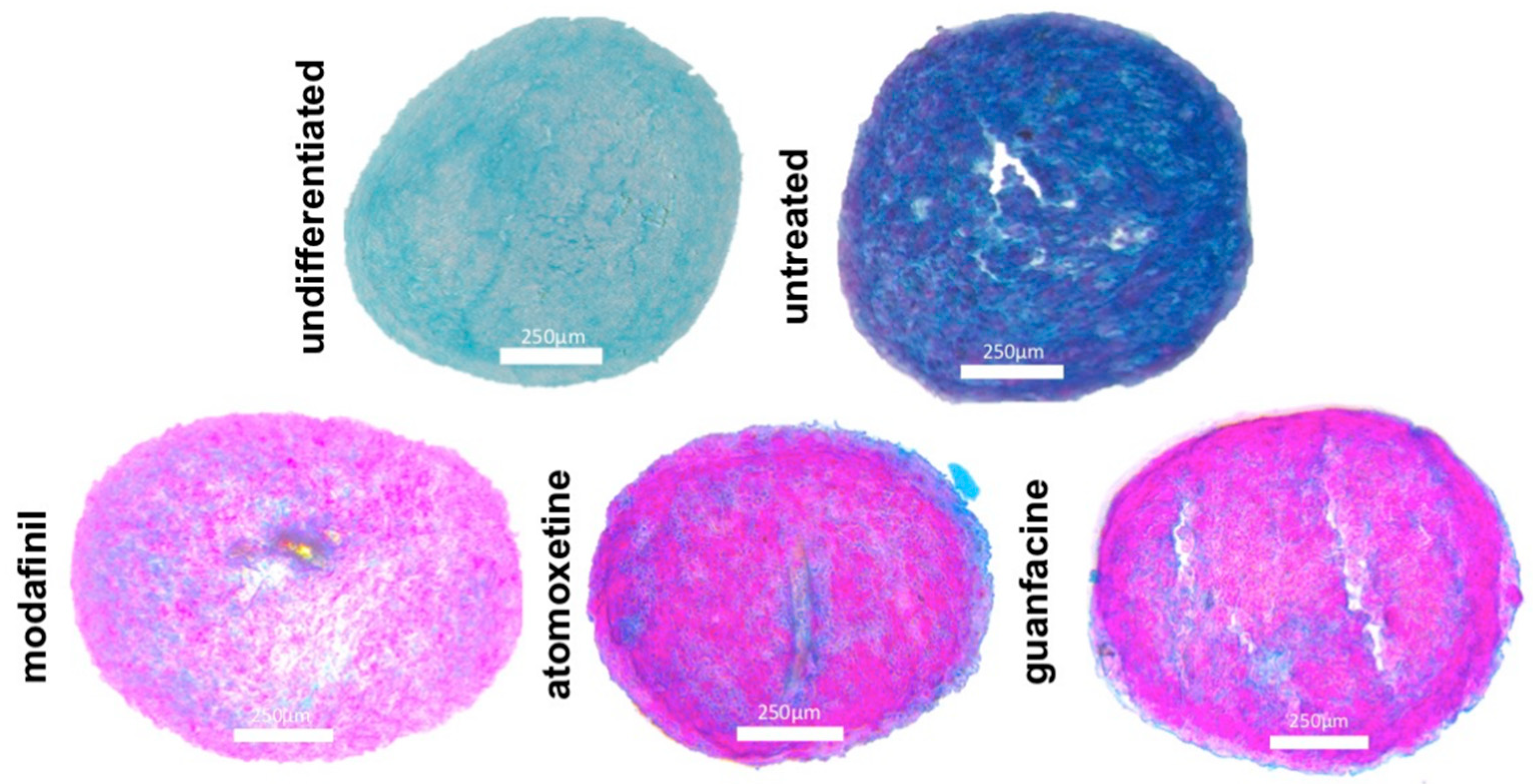
3.7. Histological Spheroid Sections of MG63 Spheroid after Administration of Modafinil, Atomoxetine and Guanfacine
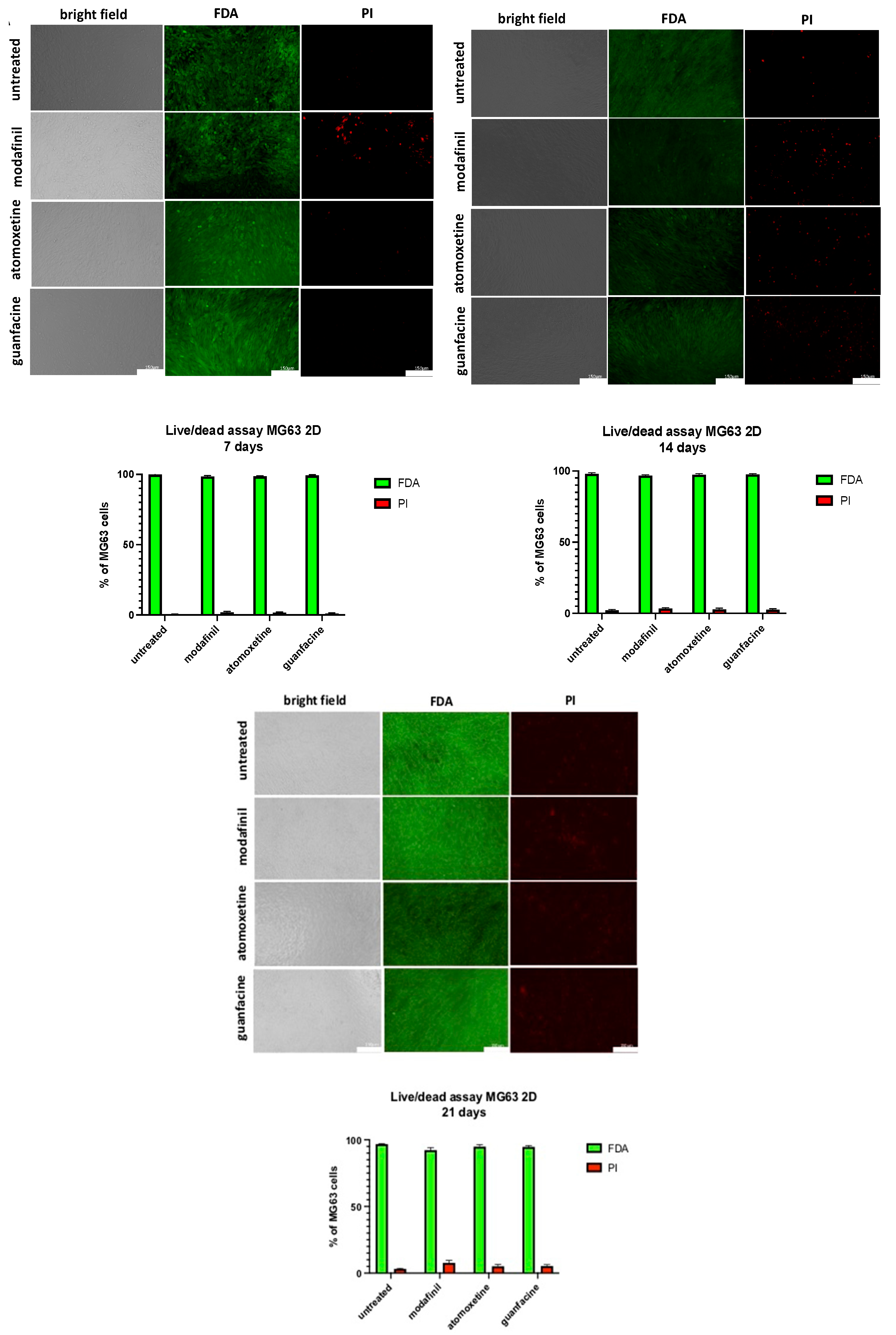
3.8. Live/Dead Staining of MG63-Monolayer after Administration of Modafinil, Atomoxetine and Guanfacine
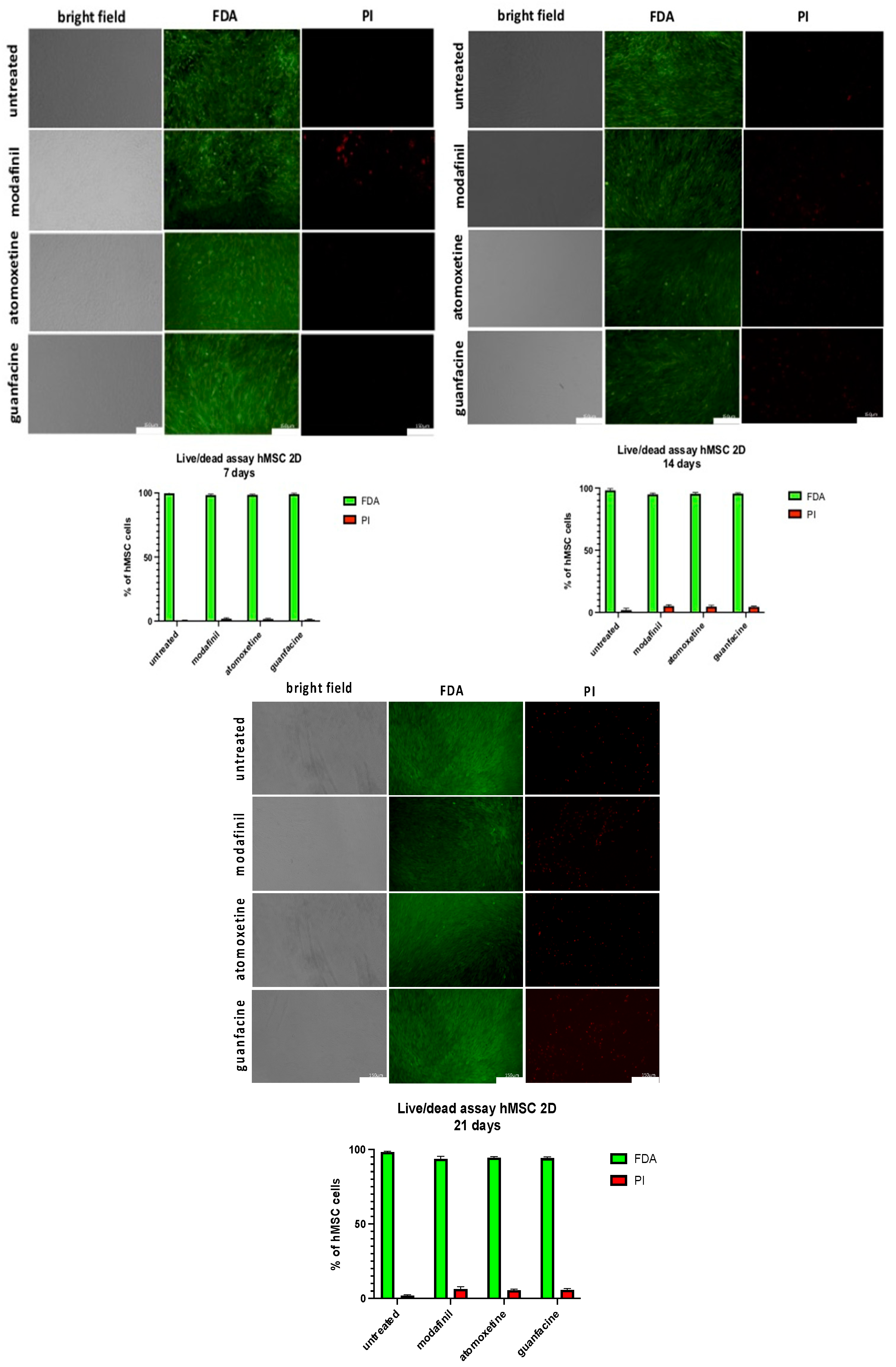
3.9. Live/dead Staining of hMSC- Monolayer after Administration of Modafinil, Atomoxetine and Guanfacine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilens, T.E.; Spencer, T.J. Understanding attention-deficit/hyperactivity disorder from childhood to adulthood. Postgrad Med. 2010, 122, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Ge, Q.; Fang, L.; Yu, H.; Zou, Z.; Zhang, P.; Lv, S.; Tong, P.; Xiao, L.; Chen, D.; et al. TGF-β/Smad2 signalling regulates enchondral bone formation of Gli1+ periosteal cells during fracture healing. Cell Prolif. 2020, 53, 12904. [Google Scholar] [CrossRef] [PubMed]
- Science Direct: Endochondral Ossification. Available online: https://www.sciencedirect.com/topics/veterinary-science-and-veterinary-medicine/endochondral-ossification (accessed on 16 June 2022).
- Wu, Z.; Korntner, S.H.; Mullen, A.M.; Zeugolis, D.I. Collagen type II: From biosynthesis to advanced biomaterials for cartilage engineering. Biomater. Biosyst. 2021, 4, 100030. [Google Scholar] [CrossRef]
- Hallett, S.A.; Ono, W.; Ono, N. Growth Plate Chondrocytes: Skeletal Development, Growth and Beyond. Int. J. Mol. Sci. 2019, 20, 6009. [Google Scholar] [CrossRef]
- Sivaraj, K.K.; Majev, P.G.; Jeong, H.W.; Dharmalingam, B.; Zeuschner, D.; Schröder, S.; Bixel, M.G.; Timmen, M.; Stange, R.; Adams, R.H. Mesenchymal stromal cell-derived septoclasts resorb cartilage during developmental ossification and fracture healing. Nat. Commun. 2022, 71, 1–16. [Google Scholar]
- Ben-Ami, I.S.; Ankory, R.; Kadar, A.; Rotman, D.; Snir, N.; Schermann, H. The Effect of Previous Methylphenidate Use on Incidence of Stress Fractures in Military Recruits: A Retrospective Cohort. J. Bone Jt. Surg. Am. Vol. 2018, 100, 30–935. [Google Scholar] [CrossRef]
- Schermann, H.; Ben-Ami, I.S.; Tudor, A.; Amar, E.; Rath, E.; Yanovich, R. Past Methylphenidate Exposure and Stress Fractures in Combat Soldiers: A Case-Control Study. Am. J. Sports Med. 2018, 46, 728–733. [Google Scholar] [CrossRef]
- Chou, I.C.; Lin, C.C.; Sung, F.C.; Kao, C.H. Attention-deficit-hyperactivity disorder increases risk of bone fracture: A population-based cohort study. Dev. Med. Child Neurol. 2014, 56, 1111–1116. [Google Scholar] [CrossRef]
- Lefebvre, V.; Dvir-Ginzberg, M. SOX9 and the many facets of its regulation in the chondrocyte lineage. Connect. Tissue Res. 2017, 58, 2–14. [Google Scholar] [CrossRef]
- Hayes, A.J.; Melrose, J. Aggrecan, the Primary Weight-Bearing Cartilage Proteoglycan, Has Context-Dependent, Cell-Directive Properties in Embryonic Development and Neurogenesis: Aggrecan Glycan Side Chain Modifications Convey Interactive Biodiversity. Biomolecules 2020, 10, 1244. [Google Scholar] [CrossRef]
- Annamalai, R.T.; Mertz, D.R.; Daley, E.L.; Stegemann, J.P. Collagen Type II enhances chondrogenic differentiation in agarose-based modular microtissues. Cytotherapy 2016, 18, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Gumustas, F.; Yilmaz, I.; Sirin, D.Y.; Gumustas, S.A.; Batmaz, A.G.; Isyar, M.; Akkaya, S.; Mahirogullari, M. Chondrocyte proliferation, viability and differentiation is declined following administration of methylphenidate utilized for the treatment of attention-deficit/hyperactivity disorder. Hum. Exp. Toxicol. 2017, 36, 981–992. [Google Scholar] [CrossRef]
- Wagener, N.; Lehmann, W.; Weiser, L.; Jäckle, K.; Di Fazio, P.; Schilling, A.F.; Böker, K.O. Psychostimulants Modafinil, Atomoxetine and Guanfacine Impair Bone Cell Differentiation and MSC Migration. Int. J. Mol. Sci. 2022, 23, 10257. [Google Scholar] [CrossRef]
- Darwish, M.; Kirby, M.; D’Andrea, D.M.; Yang, R.; Hellriegel, E.T.; Robertson, P., Jr. Pharmacokinetics of armodafinil and modafinil after single and multiple doses in patients with excessive sleepiness associated with treated obstructive sleep apnea: A randomized, open-label, crossover study. Clin. Ther. 2010, 32, 2074–2087. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Li, G.F.; Markowitz, J.S. Atomoxetine: A Review of Its Pharmacokinetics and Pharmacogenomics Relative to Drug Disposition. J. Child Adolesc. Psychopharmacol. 2016, 26, 314–326. [Google Scholar] [CrossRef]
- Boellner, S.W.; Pennick, M.; Fiske, K.; Lyne, A.; Shojaei, A. Pharmacokinetics of a guanfacine extended-release formulation in children and adolescents with attention-deficit-hyperactivity disorder. Pharmacotherapy 2007, 27, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Spandidos, A.; Wang, X.; Wang, H.; Seed, B. PrimerBank: A resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 2010, 38, 792–799. [Google Scholar] [CrossRef]
- Spandidos, A.; Wang, X.; Wang, H.; Dragnev, S.; Thurber, T.; Seed, B. A comprehensive collection of experimentally validated primers for Polymerase Chain Reaction quantitation of murine transcript abundance. BMC Genom. 2008, 9, 633. [Google Scholar] [CrossRef]
- Wang, X.; Seed, B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 2003, 31, 1–8. [Google Scholar] [CrossRef]
- Song, H.; Park, K.H. Regulation and function of SOX9 during cartilage development and regeneration. Semin. Cancer Biol. 2020, 67, 12–23. [Google Scholar] [CrossRef]
- Kiani, C.; Chen, L.; Wu, Y.J.; Yee, A.J.; Yang, B.B. Structure and function of aggrecan. Cell Res. 2002, 12, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Tamaddon, M.; Walton, R.S.; Brand, D.D.; Czernuszka, J.T. Characterisation of freeze-dried type II collagen and chondroitin sulfate scaffolds. J. Mater. Sci. Mater. Med. 2013, 24, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Bastian, O.W.; Kuijer, A.; Koenderman, L.; Stellato, R.K.; van Solinge, W.W.; Leenen, L.P.; Blokhuis, T.J. Impaired bone healing in multitrauma patients is associated with altered leukocyte kinetics after major trauma. J. Inflamm. Res. 2016, 9, 69–78. [Google Scholar] [CrossRef]
- Pei, M.; Chen, D.; Li, J.; Wei, L. Histone deacetylase 4 promotes TGF-beta1-induced synovium-derived stem cell chondrogenesis but inhibits chondrogenically differentiated stem cell hypertrophy. Differentiation 2009, 78, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.C.; Tsaryk, R.; Gonçalves, R.M.; Pereira, C.L.; Landes, C.; Brochhausen, C.; Ghanaati, S.; Barbosa, M.A.; Kirkpatrick, C.J. Poly(γ-Glutamic Acid) as an Exogenous Promoter of Chondrogenic Differentiation of Human Mesenchymal Stem/Stromal Cells. Tissue Eng. Part A 2015, 21, 1869–1885. [Google Scholar] [CrossRef]
- Dickinson, L.C.; Lee, J.; Ringdahl, I.C.; Schedewie, H.K.; Kilgore, B.S.; Elders, M.J. Impaired growth in hyperkinetic children receiving pemoline. J. Pediatr. 1979, 94, 538–541. [Google Scholar] [CrossRef]
- Sopher, A.B.; Fennoy, I.; Oberfield, S.E. An update on childhood bone health: Mineral accrual, assessment and treatment. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 35–40. [Google Scholar] [CrossRef]
- Grünblatt, E.; Bartl, J.; Walitza, S. Methylphenidate enhances neuronal differentiation and reduces proliferation concomitant to activation of Wnt signal transduction pathways. Transl. Psychiatry 2018, 8, 51. [Google Scholar] [CrossRef]
- Kong, N.; Bao, Y.; Zhao, H.; Kang, X.; Tai, X.; Shen, Y. Methylphenidate causes cytotoxicity on photoreceptor cells via autophagy. Hum. Exp. Toxicol. 2021, 40, 71–80. [Google Scholar] [CrossRef]
- Howard, J.T.; Walick, K.S.; Rivera, J.C. Preliminary Evidence of an Association Between ADHD Medications and Diminished Bone Health in Children and Adolescents. J. Pediatr. Orthop. 2017, 37, 348–354. [Google Scholar] [CrossRef]
- Kaya, Y.E.; Karaarslan, N.; Sirin, D.Y.; Ozbek, H.; Kaplan, N.; Yilmaz, I. Investigation of the Effects of Methylphenidate, an Amphetamine Derivative, on Intervertebral Disc Tissue Cell Cultures and Matrix Structures. Turk. Neurosurg. 2019, 29, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Lawson, M.J.; Beltran, T.A.; Padilla, C.R.; Berry-Cabán, C.S.; Choi, Y.S. Attention deficit hyperactivity disorder medications and bone mineral density of adults in the United States. Bone Rep. 2022, 16, 101570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Du, M.; Zhuang, S. Impact of long-term treatment of methylphenidate on height and weight of school age children with ADHD. Neuropediatrics 2010, 41, 55–59. [Google Scholar] [CrossRef] [PubMed]



| Component Volume/Concentration Company |
|---|
| DMEM (low glucose) 500 mL, Sigma Aldrich, Taufkirchen, Germany |
| Fetal calf serum (FCS)50 mL (10%), PAN Biotech, Aidenbach, Germany |
| Penicillin/streptomycin5 mL (1%), PAN Biotech, Aidenbach, Germany |
| sodium pyruvate 100 mM, PAN Biotech, Aidenbach, Germany |
| dexamethasone 100µM, Carl Roth, Karlsruhe, Germany |
| hTGF-b10.1 mg/mL, PAN Biotech, Aidenbach, Germany |
| L-proline 4 g PAN Biotech, Aidenbach, Germany |
| ITS1 mL PAN Biotech, Aidenbach, Germany |
| ascorbic acid-2 phosphate 2895 mg Cayman Chemical Company, Ann Arbor, MI, USA |
| Component Volume/Concentration Company |
|---|
| DMEM (low glucose) 500 mL, Sigma Aldrich, Taufkirchen, Germany |
| Fetal calf serum (FCS) 50 mL (10%), PAN Biotech, Aidenbach, Germany |
| Penicillin/streptomycin 5 mL (1%), PAN Biotech, Aidenbach, Germany |
| ascorbic acid-2 phosphate 200 µM, Cayman chemical company, Ann Arbor, MI, USA |
| ß-glycerolphosphate10 Mm, Carl Roth, Karlsruhe, Germany |
| dexamethasone 0.1 µM, Carl Roth, Karlsruhe, Germany |
| Gene Primer Sequences |
|---|
| GAPDHFor: 5′- AGGTCGGAGTCAACGGAT-3′ |
| Rev:5′-TCCTGGAAGATGGTGATG-3′ |
| SOX9 For: 5′-AGGAAGCTCGCGGACCAGTAC-3′ |
| Rev:5′-GGTGGTCCTTCTTGTGCTGCAC-3′ |
| AggrecanFor: 5′- GAAAGGCATCGTGTTCCATT-3′ |
| Rev: 5′- ACGTCCTCACACCAGGAAAC- 3′ |
| COL2A1 For: 5′- CCTGGCAAAGATGGTGAGACAG-3′ |
| Rev: 3′-CCTGGTTTTCCACCTTCACCTG-5′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagener, N.; Lehmann, W.; Böker, K.O.; Röhner, E.; Di Fazio, P. Chondral/Desmal Osteogenesis in 3D Spheroids Sensitized by Psychostimulants. J. Clin. Med. 2022, 11, 6218. https://doi.org/10.3390/jcm11206218
Wagener N, Lehmann W, Böker KO, Röhner E, Di Fazio P. Chondral/Desmal Osteogenesis in 3D Spheroids Sensitized by Psychostimulants. Journal of Clinical Medicine. 2022; 11(20):6218. https://doi.org/10.3390/jcm11206218
Chicago/Turabian StyleWagener, Nele, Wolfgang Lehmann, Kai O. Böker, Eric Röhner, and Pietro Di Fazio. 2022. "Chondral/Desmal Osteogenesis in 3D Spheroids Sensitized by Psychostimulants" Journal of Clinical Medicine 11, no. 20: 6218. https://doi.org/10.3390/jcm11206218
APA StyleWagener, N., Lehmann, W., Böker, K. O., Röhner, E., & Di Fazio, P. (2022). Chondral/Desmal Osteogenesis in 3D Spheroids Sensitized by Psychostimulants. Journal of Clinical Medicine, 11(20), 6218. https://doi.org/10.3390/jcm11206218





