Outcomes and Complications of Pelvic Chondrosarcomas Treated Using Navigation Guidance and Multidisciplinary Approach: Is the Tumor Volume a Prognostic Factor?
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of the Study
2.2. Surgical Technique
2.3. Statistical Analysis
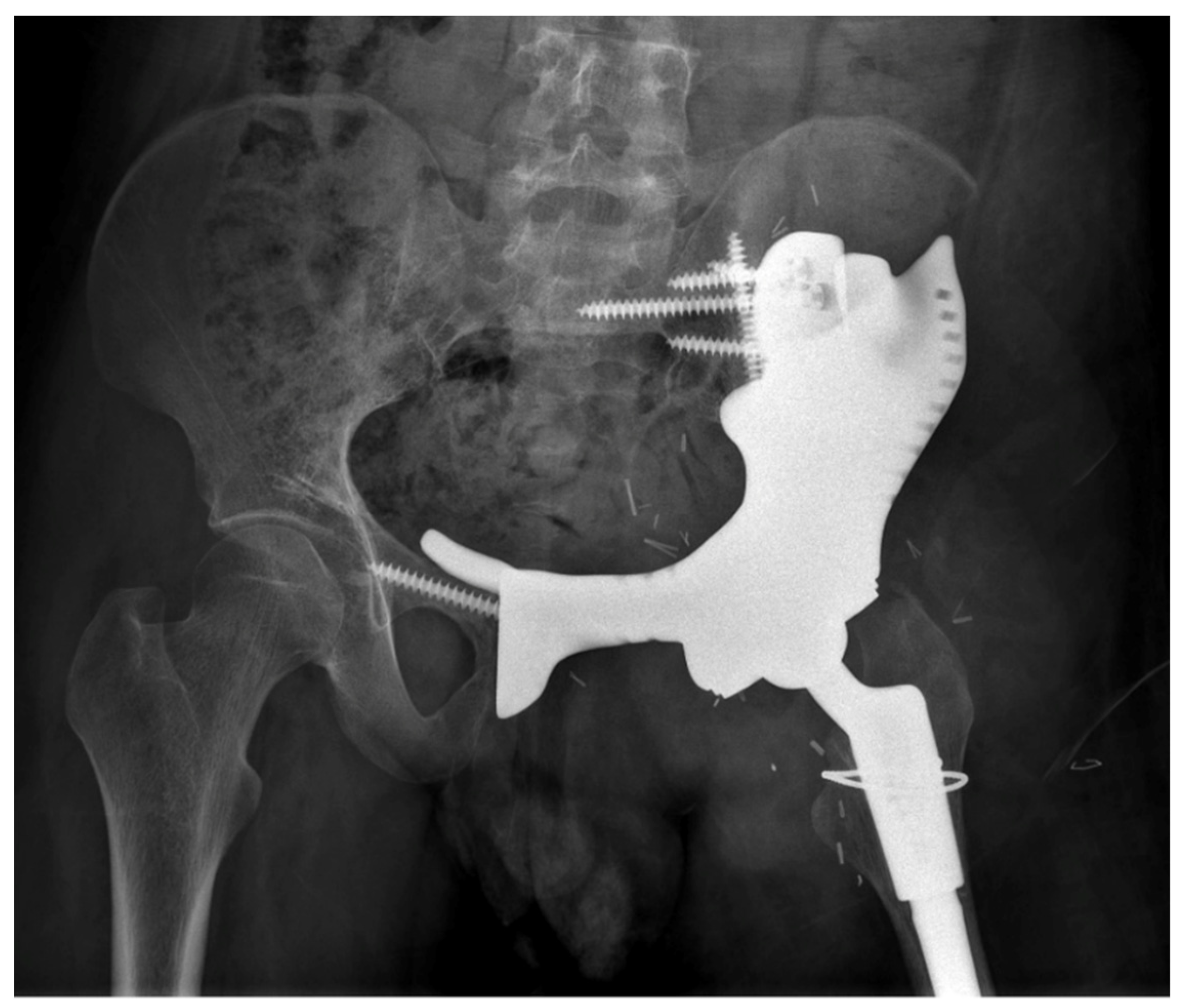
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The WHO Classification of Tumours Editorial Board. WHO Classification of Tumours Soft Tissue and Bone Tumours, 5th ed.; IARC Press: Lyon, France, 2020. [Google Scholar]
- Gatta, G.; Capocaccia, R.; Botta, L.; Mallone, S.; De Angelis, R.; Ardanaz, E.; Comber, H.; Dimitrova, N.; Leinonen, M.K.; Siesling, S.; et al. Burden and centralised treatment in Europe of rare tumours: Results of RARECAREnet—A population-based study. Lancet Oncol. 2017, 18, 1022–1039. [Google Scholar] [CrossRef] [PubMed]
- Weinschenk, R.C.; Wang, W.-L.; Lewis, V.O. Chondrosarcoma. J. Am. Acad. Orthop. Surg. 2021, 29, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, A.Y.; Burgueno, J.E.; Koniaris, L.G.; Gutierrez, J.C.; Duncan, R.; Scully, S.P. Chondrosarcoma in the United States (1973 to 2003): An Analysis of 2890 Cases from the SEER Database. J. Bone Jt. Surg. 2009, 91, 1063–1072. [Google Scholar] [CrossRef]
- Lawrenz, J.M.; Curtis, G.L.; Styron, J.F.; George, J.; Anderson, P.M.; Zahler, S.; Shepard, D.R.; Rubin, B.P.; Nystrom, L.M.; Mesko, N.W. Adult Primary Bone Sarcoma and Time to Treatment Initiation: An Analysis of the National Cancer Database. Sarcoma 2018, 2018, 1728302. [Google Scholar] [CrossRef] [PubMed]
- Amadeo, B.; Penel, N.; Coindre, J.-M.; Ray-Coquard, I.; Ligier, K.; Delafosse, P.; Bouvier, A.-M.; Plouvier, S.; Gallet, J.; Lacourt, A.; et al. Incidence and time trends of sarcoma (2000–2013): Results from the French network of cancer registries (FRANCIM). BMC Cancer 2020, 20, 190. [Google Scholar] [CrossRef]
- Guo, W.; Li, D.; Tang, X.; Ji, T. Surgical treatment of pelvic chondrosarcoma involving periacetabulum. J. Surg. Oncol. 2010, 101, 160–165. [Google Scholar] [CrossRef]
- Unni, K.K. Cartilaginous lesions of bone. J. Orthop. Sci. 2001, 6, 457–472. [Google Scholar] [CrossRef]
- Angelini, A.; Guerra, G.; Mavrogenis, A.F.; Pala, E.; Picci, P.; Ruggieri, P. Clinical outcome of central conventional chondrosarcoma. J. Surg. Oncol. 2012, 106, 929–937. [Google Scholar] [CrossRef]
- Strauss, S.J.; Frezza, A.M.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; Bonvalot, S.; et al. Bone sarcomas: ESMO–EURACAN–GENTURIS–ERN PaedCan Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 1520–1536. [Google Scholar] [CrossRef]
- Donati, D.; Ghoneimy AEl Bertoni, F.; Di Bella, C.; Mercuri, M. Surgical treatment and outcome of conventional pelvic chondrosarcoma. J. Bone Jt. Surg. Br. 2005, 87, 1527–1530. [Google Scholar] [CrossRef]
- Normand, A.N.; Cannon, C.P.; Lewis, V.O.; Lin, P.P.; Yasko, A.W. Curettage of Biopsy-diagnosed Grade 1 Periacetabular Chondrosarcoma. Clin. Orthop. Relat. Res. 2007, 459, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Biermann, J.S.; Chow, W.; Reed, D.; Lucas, D.; Adkins, D.R.; Agulnik, M.; Benjamin, R.S.; Brigman, B.; Budd, G.T.; Curry, W.T.; et al. NCCN Guidelines Insights: Bone Cancer, Version 2.2017. J. Natl. Compr. Cancer Netw. 2017, 15, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, H.; Hogendoorn, P.C.; Dijkstra, S.D.; Van Rijswijk, C.S.; Krol, A.D.; Taminiau, A.H.; Bovee, J.V. The Clinical Approach Towards Chondrosarcoma. Oncologist 2008, 13, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Geirnaerdt, M.J.; Hermans, J.; Bloem, J.L.; Kroon, H.M.; Pope, T.L.; Taminiau, A.H.; Hogendoorn, P.C. Usefulness of radiography in differentiating enchondroma from central grade 1 chondrosarcoma. Am. J. Roentgenol. 1997, 169, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Frezza, A.M.; Cesari, M.; Baumhoer, D.; Biau, D.; Bielack, S.; Campanacci, D.A.; Casanova, J.; Esler, C.; Ferrari, S.; Funovics, P.T.; et al. Mesenchymal chondrosarcoma: Prognostic factors and outcome in 113 patients. A European Musculoskeletal Oncology Society study. Eur. J. Cancer 2015, 51, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Italiano, A.; Mir, O.; Cioffi, A.; Palmerini, E.; Piperno-Neumann, S.; Perrin, C.; Chaigneau, L.; Penel, N.; Duffaud, F.; Kurtz, J.; et al. Advanced chondrosarcomas: Role of chemotherapy and survival. Ann. Oncol. 2013, 24, 2916–2922. [Google Scholar] [CrossRef]
- Fromm, J.; Klein, A.; Baur-Melnyk, A.; Knösel, T.; Lindner, L.; Birkenmaier, C.; Roeder, F.; Jansson, V.; Dürr, H.R. Survival and prognostic factors in conventional central chondrosarcoma. BMC Cancer 2018, 18, 849. [Google Scholar] [CrossRef]
- Laitinen, M.K.; Evans, S.; Stevenson, J.; Sumathi, V.; Kask, G.; Jeys, L.M.; Parry, M.C. Clinical differences between central and peripheral chondrosarcomas. Bone Jt. J. 2021, 103, 984–990. [Google Scholar] [CrossRef]
- Thorkildsen, J.; Taksdal, I.; Bjerkehagen, B.; Norum, O.; Myklebust, T.A.; Zaikova, O. Risk stratification for central conventional chondrosarcoma of bone: A novel system predicting risk of metastasis and death in the Cancer Registry of Norway cohort. J. Surg. Oncol. 2020, 121, 1115–1125. [Google Scholar] [CrossRef]
- Lee, F.Y.; Mankin, H.J.; Fondren, G.; Gebhardt, M.C.; Springfield, D.S.; Rosenberg, A.E.; Jennings, L.C. Chondrosarcoma of Bone. J. Bone Jt. Surg. 1999, 81, 326–338. [Google Scholar] [CrossRef]
- Björnsson, J.; McLeod, R.A.; Unni, K.K.; Ilstrup, D.M.; Pritchard, D.J. Primary chondrosarcoma of long bones and limb girdles. Cancer 1998, 83, 2105–2119. [Google Scholar] [CrossRef]
- Sheth, D.S.; Yasko, A.W.; Johnson, M.E.; Ayala, A.G.; Murray, J.A.; Romsdahl, M.M. Chondrosarcoma of the pelvis: Prognostic factors for 67 patients treated with definitive surgery. Cancer 1996, 78, 745–750. [Google Scholar] [CrossRef]
- Rizzo, M.; Ghert, M.A.; Harrelson, J.M.; Scully, S.P. Chondrosarcoma of Bone. Clin. Orthop. Relat. Res. 2001, 391, 224–233. [Google Scholar] [CrossRef]
- Kreicbergs, A.; Boquist, L.; Borssén, B.; Larsson, S.-E. Prognostic factors in chondrosarcoma. A comparative study of cellular DNA content and clinicopathologic features. Cancer 1982, 50, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, F.; Abudu, A.; Grimer, R.J.; Carter, S.R.; Tillman, R.M.; Ayoub, K.; Mangham, D.C.; Davies, A.M. Risk factors for survival and local control in chondrosarcoma of bone. J. Bone Jt. Surg. Br. 2002, 84, 93–99. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Angelini, A.; Drago, G.; Merlino, B.; Ruggieri, P. Survival analysis of patients with chondrosarcomas of the pelvis. J. Surg. Oncol. 2013, 108, 19–27. [Google Scholar] [CrossRef]
- Wong, K.C.; Kumta, S.M. Computer-assisted tumor surgery in malignant bone tumors. Clin. Orthop. Relat. Res. 2013, 471, 750–761. [Google Scholar] [CrossRef]
- Cheong, D.; Letson, G.D. Computer-assisted navigation and musculoskeletal sarcoma surgery. Cancer Control 2011, 18, 171–176. [Google Scholar] [CrossRef]
- Gerbers, J.G.; Stevens, M.; Ploegmakers, J.J.; Bulstra, S.K.; Jutte, P.C. Computer-assisted surgery in orthopedic oncology: Technique, indications, and a descriptive study of 130 cases. Acta Orthop. 2014, 85, 663–669. [Google Scholar] [CrossRef]
- Ieguchi, M.; Hoshi, M.; Takada, J.; Hidaka, N.; Nakamura, H. Navigation-assisted surgery for bone and soft tissue tumors with bony extension. Clin. Orthop. Relat. Res. 2012, 470, 275–283. [Google Scholar] [CrossRef]
- Young, P.S.; Bell, S.W.; Mahendra, A. The evolving role of computer-assisted navigation in musculoskeletal oncology. Bone Jt. J. 2015, 97, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Joyce, D.M. Navigation in Pelvic Surgery. In Surgery of Pelvic Bone Tumors; Springer International Publishing: Cham, Switzerland, 2021; pp. 135–153. [Google Scholar]
- Angelini, A.; Crimì, A.; Pala, E.; Ruggieri, P. Surgical Approaches in Pelvic Bone Tumors. In Surgery of Pelvic Bone Tumor; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–13. [Google Scholar]
- Bertrand, T.E.; Cruz, A.; Binitie, O.; Cheong, D.; Letson, D.G. Do Surgical Margins Affect Local Recurrence and Survival in Extremity, Nonmetastatic, High-grade Osteosarcoma? Clin. Orthop. Relat. Res. 2016, 474, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Kaneuchi, Y.; Stevenson, J.; Parry, M.; Kurisunkal, V.; Clark, R.; Tsuda, Y.; Laitinen, M.; Grimer, R.; Jeys, L. Navigation-assisted pelvic resections and reconstructions for periacetabular chondrosarcomas. Eur. J. Surg. Oncol. 2021, 47, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Skeletal Lesions Interobserver Correlation among Expert Diagnosticians (SLICED) Study Group. Reliability of histopathologic and radiologic grading of cartilaginous neoplasms in long bones. J. Bone Jt. Surg. Am. 2007, 89, 2113–2123. [Google Scholar] [CrossRef]
- Eefting, D.; Schrage, Y.M.; Geirnaerdt, M.J.; Le Cessie, S.; Taminiau, A.H.; Bovée, J.V.; Hogendoorn, P.C. Assessment of interobserver variability and histologic parameters to improve reliability in classification and grading of central cartilaginous tumors. Am. J. Surg. Pathol. 2009, 33, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Bovée, J.V.; Cleton-Jansen, A.M.; Taminiau, A.H.; Hogendoorn, P.C. Emerging pathways in the development of chondrosarcoma of bone and implications for targeted treatment. Lancet Oncol. 2005, 6, 599–607. [Google Scholar] [CrossRef]
- Rosenthal, D.I.; Schiller, A.L.; Mankin, H.J. Chondrosarcoma: Correlation of radiological and histological grade. Radiology 1984, 150, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Rakoczy, K.; Hart, J.; Jones, K.B.; Groundland, J.S. Presenting features and overall survival of chondrosarcoma of the pelvis. Cancer Treat. Res. Commun. 2022, 30, 100510. [Google Scholar] [CrossRef]
- Kurisunkal, V.; Laitinen, M.K.; Kaneuchi, Y.; Kapanci, B.; Stevenson, J.; Parry, M.C.; Reito, A.; Fujiwara, T.; Jeys, L.M. Is 2 mm a wide margin in high-grade conventional chondrosarcomas of the pelvis? Bone Jt. J. 2021, 103, 1150–1154. [Google Scholar] [CrossRef]
- Kyoo-Ho, S.; Rougraff, B.T.; Simon, M.A. Oncologic outcome of primary bone sarcoma of the pelvis. Clin Orthop. 1994, 304, 207–217. [Google Scholar]
- Ozaki, T.; Lindner, N.; Hillmann, A.; Rödl, R.; Blasius, S.; Winkelmann, W. Influence of intralesional surgery on treatment outcome of chondrosarcoma. Cancer Interdiscip. Int. J. Am. Cancer Soc. 1996, 77, 1292–1297. [Google Scholar] [CrossRef]
- Jeys, L.; Matharu, G.S.; Nandra, R.S.; Grimer, R.J. Can computer navigation-assisted surgery reduce the risk of an intralesional margin and reduce the rate of local recurrence in patients with a tumour of the pelvis or sacrum? Bone Jt. J. 2013, 95, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Nandra, R.; Matharu, G.; Stevenson, J.; Parry, M.; Grimer, R.; Jeys, L. Long-term outcomes after an initial experience of computer-navigated resection of primary pelvic and sacral bone tumours: Soft-tissue margins must be adequate to reduce local recurrences. Bone Jt. J. 2019, 101, 484–490. [Google Scholar] [CrossRef] [PubMed]



| Features | Classification | Value |
|---|---|---|
| Sex | M (n, %) F (n, %) | 21, 60% 14, 40% |
| Age | Median, (IQR) | 54 years (41–65) |
| Grade | High (n, %) Low (n, %) | 30, 86% 5, 14% |
| Oncologic outcome | NED AWD DWD D other D | 18 patients 3 patients 10 patients 4 patients |
| MSTS score | (Median, Range) | 20 (10–28) |
| Volume of the tumor | (Median, IQR) | 683 cc (149–1151) |
| Local Recurrence Rate | % | 37% |
| Metastasis rate | % | 31% |
| Infection rate | % | 16% |
| Dislocation rate | % | 8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crimì, A.; Binitie, O.T.; Crimì, F.; Letson, G.D.; Joyce, D.M. Outcomes and Complications of Pelvic Chondrosarcomas Treated Using Navigation Guidance and Multidisciplinary Approach: Is the Tumor Volume a Prognostic Factor? J. Clin. Med. 2022, 11, 7111. https://doi.org/10.3390/jcm11237111
Crimì A, Binitie OT, Crimì F, Letson GD, Joyce DM. Outcomes and Complications of Pelvic Chondrosarcomas Treated Using Navigation Guidance and Multidisciplinary Approach: Is the Tumor Volume a Prognostic Factor? Journal of Clinical Medicine. 2022; 11(23):7111. https://doi.org/10.3390/jcm11237111
Chicago/Turabian StyleCrimì, Alberto, Odion T. Binitie, Filippo Crimì, G. Douglas Letson, and David M. Joyce. 2022. "Outcomes and Complications of Pelvic Chondrosarcomas Treated Using Navigation Guidance and Multidisciplinary Approach: Is the Tumor Volume a Prognostic Factor?" Journal of Clinical Medicine 11, no. 23: 7111. https://doi.org/10.3390/jcm11237111
APA StyleCrimì, A., Binitie, O. T., Crimì, F., Letson, G. D., & Joyce, D. M. (2022). Outcomes and Complications of Pelvic Chondrosarcomas Treated Using Navigation Guidance and Multidisciplinary Approach: Is the Tumor Volume a Prognostic Factor? Journal of Clinical Medicine, 11(23), 7111. https://doi.org/10.3390/jcm11237111






