Echocardiographic Abnormalities in Autosomal Dominant Polycystic Kidney Disease (ADPKD) Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Ethical Considerations
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Populations Demographic and Clinical Characteristics
3.2. Laboratory and Echocardiographic Findings
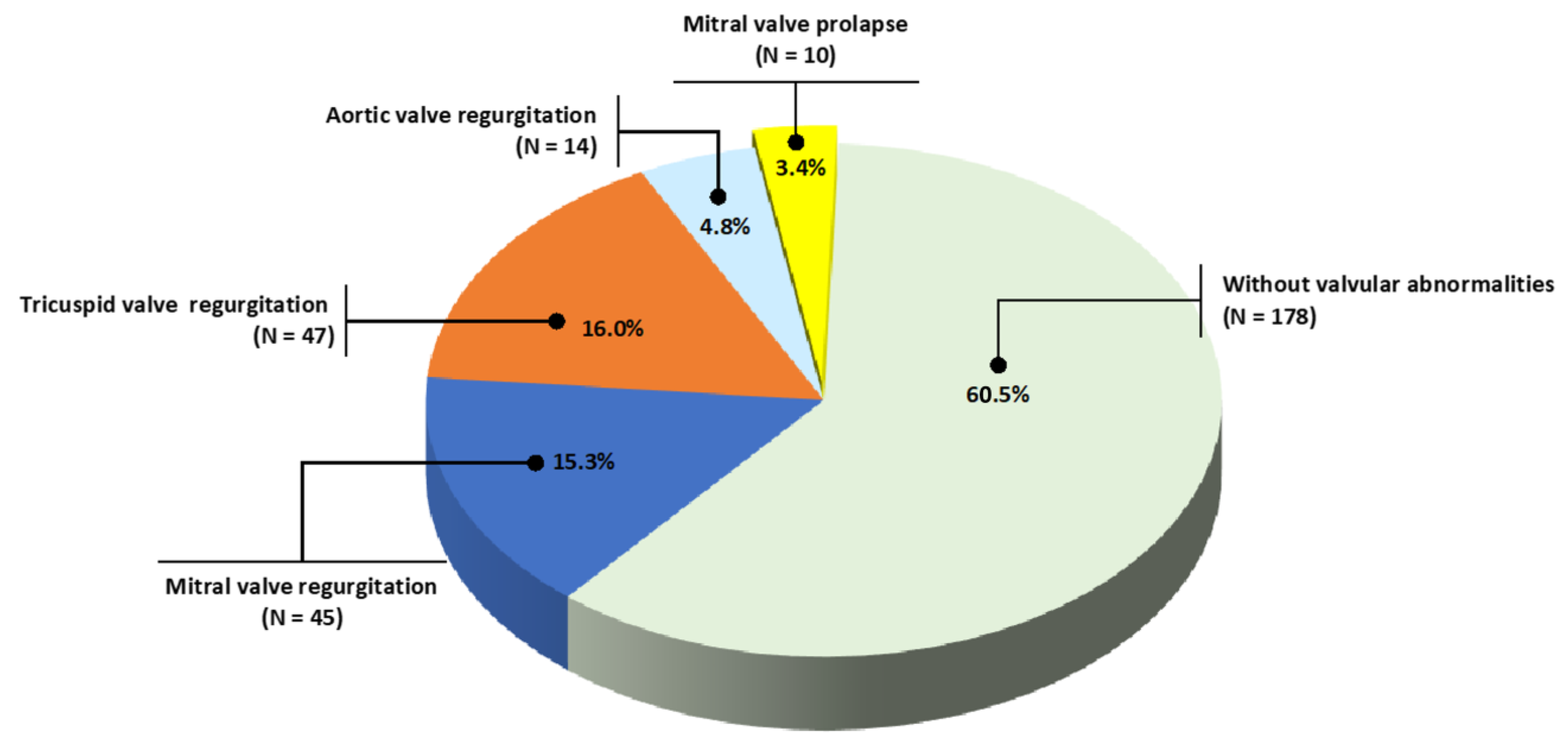
3.3. Valvar Abnormalities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lanktree, M.B.; Haghighi, A.; Guiard, E.; Iliuta, I.-A.; Song, X.; Harris, P.C.; Paterson, A.D.; Pei, Y. Prevalence Estimates of Polycystic Kidney and Liver Disease by Population Sequencing. J. Am. Soc. Nephrol. 2018, 29, 2593–2600. [Google Scholar] [CrossRef] [PubMed]
- Willey, C.J.; Blais, J.D.; Hall, A.K.; Krasa, H.B.; Makin, A.J.; Czerwiec, F.S. Prevalence of Autosomal Dominant Polycystic Kidney Disease in the European Union. Nephrol. Dial. Transplant. 2017, 32, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.S.L.; Shen, C.; Landsittel, D.P.; Grantham, J.J.; Cook, L.T.; Torres, V.E.; Chapman, A.B.; Bae, K.T.; Mrug, M.; Harris, P.C.; et al. Long-Term Trajectory of Kidney Function in Autosomal-Dominant Polycystic Kidney Disease. Kidney Int. 2019, 95, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Chebib, F.T.; Torres, V.E. Assessing Risk of Rapid Progression in Autosomal Dominant Polycystic Kidney Disease and Special Considerations for Disease-Modifying Therapy. Am. J. Kidney Dis. 2021, 78, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W. Renal Volume, Renin-Angiotensin-Aldosterone System, Hypertension, and Left Ventricular Hypertrophy in Patients with Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2009, 20, 1888–1893. [Google Scholar] [CrossRef]
- Klawitter, J.; Reed-Gitomer, B.Y.; McFann, K.; Pennington, A.; Klawitter, J.; Abebe, K.Z.; Klepacki, J.; Cadnapaphornchai, M.A.; Brosnahan, G.; Chonchol, M.; et al. Endothelial Dysfunction and Oxidative Stress in Polycystic Kidney Disease. Am. J. Physiol. Renal. Physiol. 2014, 307, F1198–F1206. [Google Scholar] [CrossRef]
- Reinecke, N.L.; Cunha, T.M.; Heilberg, I.P.; Higa, E.M.S.; Nishiura, J.L.; Neder, J.A.; Almeida, W.S.; Schor, N. Exercise Capacity in Polycystic Kidney Disease. Am. J. Kidney Dis. 2014, 64, 239–246. [Google Scholar] [CrossRef]
- Valero, F.A.; Martinez-Vea, A.; Bardají, A.; Gutierrez, C.; Garcia, C.; Richart, C.; Oliver, J.A. Ambulatory Blood Pressure and Left Ventricular Mass in Normotensive Patients with Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 1999, 10, 1020–1026. [Google Scholar] [CrossRef]
- Vendramini, L.C.; Dalboni, M.A.; de Carvalho, J.T.G., Jr.; Batista, M.C.; Nishiura, J.L.; Heilberg, I.P. Association of Vitamin D Levels with Kidney Volume in Autosomal Dominant Polycystic Kidney Disease (ADPKD). Front. Med. 2019, 6, 112. [Google Scholar] [CrossRef]
- Yu, A.S.L.; Shen, C.; Landsittel, D.P.; Harris, P.C.; Torres, V.E.; Mrug, M.; Bae, K.T.; Grantham, J.J.; Rahbari-Oskoui, F.F.; Flessner, M.F.; et al. Baseline Total Kidney Volume and the Rate of Kidney Growth Are Associated with Chronic Kidney Disease Progression in Autosomal Dominant Polycystic Kidney Disease. Kidney Int. 2018, 93, 691–699. [Google Scholar] [CrossRef]
- Rahbari-Oskoui, F.; Williams, O.; Chapman, A. Mechanisms and Management of Hypertension in Autosomal Dominant Polycystic Kidney Disease. Nephrol. Dial. Transplant. 2014, 29, 2194–2201. [Google Scholar] [CrossRef] [PubMed]
- Ecder, T.; Schrier, R.W. Cardiovascular Abnormalities in Autosomal-Dominant Polycystic Kidney Disease. Nat. Rev. Nephrol. 2009, 5, 221–228. [Google Scholar] [CrossRef]
- Kuo, I.Y.; Chapman, A.B. Polycystins, ADPKD, and Cardiovascular Disease. Kidney Int. Rep. 2020, 5, 396–406. [Google Scholar] [CrossRef]
- Alam, A.; Perrone, R.D. Left Ventricular Hypertrophy in ADPKD: Changing Demographics. Curr. Hypertens. Rev. 2013, 9, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Chebib, F.T.; Hogan, M.C.; El-Zoghby, Z.M.; Irazabal, M.V.; Senum, S.R.; Heyer, C.M.; Madsen, C.D.; Cornec-Le Gall, E.; Behfar, A.; Harris, P.C.; et al. Autosomal Dominant Polycystic Kidney Patients May Be Predisposed to Various Cardiomyopathies. Kidney Int. Rep. 2017, 2, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Oflaz, H.; Alisir, S.; Buyukaydin, B.; Kocaman, O.; Turgut, F.; Namli, S.; Pamukcu, B.; Oncul, A.; Ecder, T. Biventricular Diastolic Dysfunction in Patients with Autosomal-Dominant Polycystic Kidney Disease. Kidney Int. 2005, 68, 2244–2249. [Google Scholar] [CrossRef]
- Paavola, J.; Schliffke, S.; Rossetti, S.; Kuo, I.Y.-T.; Yuan, S.; Sun, Z.; Harris, P.C.; Torres, V.E.; Ehrlich, B.E. Polycystin-2 Mutations Lead to Impaired Calcium Cycling in the Heart and Predispose to Dilated Cardiomyopathy. J. Mol. Cell. Cardiol. 2013, 58, 199–208. [Google Scholar] [CrossRef]
- Wu, G.; Tian, X.; Nishimura, S.; Markowitz, G.S.; D’Agati, V.; Park, J.H.; Yao, L.; Li, L.; Geng, L.; Zhao, H.; et al. Trans-Heterozygous Pkd1 and Pkd2 Mutations Modify Expression of Polycystic Kidney Disease. Hum. Mol. Genet. 2002, 11, 1845–1854. [Google Scholar] [CrossRef]
- Boulter, C.; Mulroy, S.; Webb, S.; Fleming, S.; Brindle, K.; Sandford, R. Cardiovascular, Skeletal, and Renal Defects in Mice with a Targeted Disruption of the Pkd1 Gene. Proc. Natl. Acad. Sci. USA 2001, 98, 12174–12179. [Google Scholar] [CrossRef]
- Balbo, B.E.; Amaral, A.G.; Fonseca, J.M.; de Castro, I.; Salemi, V.M.; Souza, L.E.; dos Santos, F.; Irigoyen, M.C.; Qian, F.; Chammas, R.; et al. Cardiac Dysfunction in Pkd1-Deficient Mice with Phenotype Rescue by Galectin-3 Knockout. Kidney Int. 2016, 90, 580–597. [Google Scholar] [CrossRef]
- Pei, Y.; Obaji, J.; Dupuis, A.; Paterson, A.D.; Magistroni, R.; Dicks, E.; Parfrey, P.; Cramer, B.; Coto, E.; Torra, R.; et al. Unified Criteria for Ultrasonographic Diagnosis of ADPKD. J. Am. Soc. Nephrol. 2009, 20, 205–212. [Google Scholar] [CrossRef] [PubMed]
- James, P.A.; Oparil, S.; Carter, B.L.; Cushman, W.C.; Dennison-Himmelfarb, C.; Handler, J.; Lackland, D.T.; LeFevre, M.L.; MacKenzie, T.D.; Ogedegbe, O.; et al. 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults: Report from the Panel Members Appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014, 311, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Kraft, C.D.; Levine, R.A.; Nihoyannopoulos, P.; Otto, C.M.; Quinones, M.A.; Rakowski, H.; et al. American Society of Echocardiography Report Recommendations for Evaluation of the Severity of Native Valvular Regurgitation with Two-Dimensional and Doppler. J. Am. Soc. Echocardiogr. 2003, 16, 777–802. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Ase Guidelines and Standards Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Chapman, A.B.; Johnson, A.M.; Rainguet, S.; Hossack, K.; Gabow, P.; Schrier, R.W. Left Ventricular Hypertrophy in Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 1997, 8, 1292–1297. [Google Scholar] [CrossRef]
- de Almeida, E.A.F.; de Oliveira, E.I.; Lopes, J.A.; Almeida, A.G.; Lopes, M.G.; Prata, M.M. Diastolic Function in Several Stages of Chronic Kidney Disease in Patients with Autosomal Dominant Polycystic Kidney Disease: A Tissue Doppler Imaging Study. Kidney Blood Press. Res. 2007, 30, 234–239. [Google Scholar] [CrossRef]
- Perrone, R.D.; Ruthazer, R.; Terrin, N.C. Survival after End-Stage Renal Disease in Autosomal Dominant Polycystic Kidney Disease: Contribution of Extrarenal Complications to Mortality. Am. J. Kidney Dis. 2001, 38, 777–784. [Google Scholar] [CrossRef]
- Fick, G.M.; Johnson, A.M.; Hammond, W.S.; Gabow, P.A. Causes of Death in Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 1995, 5, 2048–2056. [Google Scholar] [CrossRef]
- Hickey, A.J.; Wolfers, J.; Wilcken, D.E. Mitral-Valve Prolapse: Prevalence in an Australian Population. Med. J. Aust. 1981, 1, 31–33. [Google Scholar] [CrossRef]
- Darsee, J.R.; Mikolich, J.R.; Nicoloff, N.B.; Lesser, L.E. Prevalence of Mitral Valve Prolapse in Presumably Healthy Young Men. Circulation 1979, 59, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Procacci, P.M.; Savran, S.V.; Schreiter, S.L.; Bryson, A.L. Prevalence of Clinical Mitral-Valve Prolapse in 1169 Young Women. N. Engl. J. Med. 1976, 294, 1086–1088. [Google Scholar] [CrossRef] [PubMed]
- Freed, L.A.; Levy, D.; Levine, R.A.; Larson, M.G.; Evans, J.C.; Fuller, D.L.; Lehman, B.; Benjamin, E.J. Prevalence and Clinical Outcome of Mitral-Valve Prolapse. N. Engl. J. Med. 1999, 341, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Devereux, R.B.; Brown, W.T.; Kramer-Fox, R.; Sachs, I. Inheritance of Mitral Valve Prolapse: Effect of Age and Sex on Gene Expression. Ann. Intern. Med. 1982, 97, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Lumiaho, A.; Ikäheimo, R.; Miettinen, R.; Niemitukia, L.; Laitinen, T.; Rantala, A.; Lampainen, E.; Laakso, M.; Hartikainen, J. Mitral Valve Prolapse and Mitral Regurgitation Are Common in Patients with Polycystic Kidney Disease Type 1. Am. J. Kidney Dis. 2001, 38, 1208–1216. [Google Scholar] [CrossRef]
- Hossack, K.F.; Leddy, C.L.; Johnson, A.M.; Schrier, R.W.; Gabow, P.A. Echocardiographic Findings in Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 1988, 319, 907–912. [Google Scholar] [CrossRef]
- Timio, M.; Monarca, C.; Pede, S.; Gentili, S.; Verdura, C.; Lolli, S. The Spectrum of Cardiovascular Abnormalities in Autosomal Dominant Polycystic Kidney Disease: A 10-Year Follow-up in a Five-Generation Kindred. Clin. Nephrol. 1992, 37, 245–251. [Google Scholar]
- Ivy, D.D.; Shaffer, E.M.; Johnson, A.M.; Kimberling, W.J.; Dobin, A.; Gabow, P.A. Cardiovascular Abnormalities in Children with Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 1995, 5, 2032–2036. [Google Scholar] [CrossRef]
- Leier, C.V.; Baker, P.B.; Kilman, J.W.; Wooley, C.F. Cardiovascular Abnormalities Associated with Adult Polycystic Kidney Disease. Ann. Intern. Med. 1984, 100, 683–688. [Google Scholar] [CrossRef]
- Harrap, S.B.; Davies, D.L.; Macnicol, A.M.; Dominiczak, A.F.; Fraser, R.; Wright, A.F.; Watson, M.L.; Briggs, J.D. Renal, Cardiovascular and Hormonal Characteristics of Young Adults with Autosomal Dominant Polycystic Kidney Disease. Kidney Int. 1991, 40, 501–508. [Google Scholar] [CrossRef]
- Saggar-Malik, A.K.; Missouris, C.G.; Gill, J.S.; Singer, D.R.J.; Markandu, N.D.; Macgregor, G.A. Left Ventricular Mass in Normotensive Subjects with Autosomal Dominant Polycystic Kidney Disease. BMJ 1994, 309, 1617. [Google Scholar] [CrossRef] [PubMed]
- Savis, A.; Simpson, J.M.; Kabir, S.; Peacock, K.; Beardsley, H.; Sinha, M.D. Prevalence of Cardiac Valvar Abnormalities in Children and Young People with Autosomal Dominant Polycystic Kidney Disease. Pediatr. Nephrol. 2022; 1–5, online ahead of print. [Google Scholar] [CrossRef]
- Levine, R.A.; Handschumacher, M.D.; Sanfilippo, A.J.; Hagege, A.A.; Harrigan, P.; Marshall, J.E.; Weyman, A.E. Three-Dimensional Echocardiographic Reconstruction of the Mitral Valve, with Implications for the Diagnosis of Mitral Valve Prolapse. Circulation 1989, 80, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.A.; Stathogiannis, E.; Newell, J.B.; Harrigan, P.; Weyman, A.E. Reconsideration of Echocardiographic Standards for Mitral Valve Prolapse: Lack of Association between Leaflet Displacement Isolated to the Apical Four Chamber View and Independent Echocardiographic Evidence of Abnormality. J. Am. Coll. Cardiol. 1988, 11, 1010–1019. [Google Scholar] [CrossRef]
- Sahn, D.J.; Wood, J.; Allen, H.D.; Peoples, W.; Goldberg, S.J. Echocardiographic Spectrum of Mitral Valve Motion in Children with and without Mitral Valve Prolapse: The Nature of False Positive Diagnosis. Am. J. Cardiol. 1977, 39, 422–431. [Google Scholar] [CrossRef]
- Markiewicz, W.; London, E.; Popp, R.L. Effect of Transducer Placement on Echocardiographic Mitral Valve Motion. Am. Heart J. 1978, 96, 555–556. [Google Scholar] [CrossRef]
- Abbasi, A.S.; Decristofaro, D.; Anabtawi, J.; Irwin, L. Mitral Valve Prolapse: Comparative Value of M-Mode, Two-Dimensional and Doppler Echocardiography. J. Am. Coll. Cardiol. 1983, 2, 1219–1223. [Google Scholar] [CrossRef]
- Choong, C.Y.; Abascal, V.M.; Weyman, J.; Levine, R.A.; Gentile, F.; Thomas, J.D.; Weyman, A.E. Prevalence of Valvular Regurgitation by Doppler Echocardiography in Patients with Structurally Normal Hearts by Two-Dimensional Echocardiography. Am. Heart J. 1989, 117, 636–642. [Google Scholar] [CrossRef]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L.; Bruder, O.; Cosyns, B.; et al. Recommendations for the Echocardiographic Assessment of Native Valvular Regurgitation: An Executive Summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef]
- Miyamoto, R.; Sekine, A.; Fujimaru, T.; Suwabe, T.; Mizuno, H.; Hasegawa, E.; Yamanouchi, M.; Chiga, M.; Mori, T.; Sohara, E.; et al. Echocardiographic Findings and Genotypes in Autosomal Dominant Polycystic Kidney Disease. Kidney Dis. 2021, 8, 246–252. [Google Scholar] [CrossRef]
- London, G.M.; Pannier, B.; Marchais, S.J.; Guerin, A.P. Calcification of the Aortic Valve in the Dialyzed Patient. J. Am. Soc. Nephrol. 2000, 11, 778–783. [Google Scholar] [CrossRef]
- Ureña-Torres, P.; D’Marco, L.; Raggi, P.; García–Moll, X.; Brandenburg, V.; Mazzaferro, S.; Lieber, A.; Guirado, L.; Bover, J. Valvular Heart Disease and Calcification in CKD: More Common than Appreciated. Nephrol. Dial. Transplant. 2019, 35, 2046–2053. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Baessler, A.; Hense, H.W.; Hengstenberg, C.; Muscholl, M.; Holmer, S.; Döring, A.; Broeckel, U.; Riegger, G.; Schunkert, H. Prevalence of Left Ventricular Diastolic Dysfunction in the Community: Results from a Doppler Echocardiographic-Based Survey of a Population Sample. Eur. Heart J. 2003, 24, 320–328. [Google Scholar] [CrossRef]
- Delling, F.N.; Vasan, R.S. Epidemiology and Pathophysiology of Mitral Valve Prolapse: New Insights into Disease Progression, Genetics, and Molecular Basis. Circulation 2014, 129, 2158–2170. [Google Scholar] [CrossRef] [PubMed]
- Ângelo, L.C.S.; Vieira, M.L.C.; Rodrigues, S.L.; Morelato, R.L.; Pereira, A.C.; Mill, J.G.; Krieger, J.E. Echocardiographic Reference Values in a Sample of Asymptomatic Adult Brazilian Population. Arq. Bras. Cardiol. 2007, 89, 184–190. [Google Scholar] [CrossRef]
- Bardaji, A.; Martinez Vea, A.; Gutierrez, C.; Ridao, C.; Richart, C.; Oliver, J.A. Left Ventricular Mass and Diastolic Function in Normotensive Young Adults with Autosomal Dominant Polycystic Kidney Disease. Am. J. Kidney Dis. 1998, 32, 970–975. [Google Scholar] [CrossRef]
- Cadnapaphornchai, M.A.; McFann, K.; Strain, J.D.; Masoumi, A.; Schrier, R.W. Increased Left Ventricular Mass in Children with Autosomal Dominant Polycystic Kidney Disease and Borderline Hypertension. Kidney Int. 2008, 74, 1192–1196. [Google Scholar] [CrossRef]
- de Almeida, E.A.F.; de Oliveira, E.I.; Lopes, J.A.; Almeida, A.G.; Prata, M.M. Tissue Doppler Imaging in the Evaluation of Left Ventricular Function in Young Adults with Autosomal Dominant Polycystic Kidney Disease. Am. J. Kidney Dis. 2006, 47, 587–592. [Google Scholar] [CrossRef]
- Martinez-Vea, A.; Bardají, A.; Gutierrez, C.; García, C.; Peralta, C.; Marcas, L.; Oliver, J.A. Exercise Blood Pressure, Cardiac Structure, and Diastolic Function in Young Normotensive Patients with Polycystic Kidney Disease: A Prehypertensive State. Am. J. Kidney Dis. 2004, 44, 216–223. [Google Scholar] [CrossRef]
- Chen, H.; Watnick, T.; Hong, S.N.; Daly, B.; Li, Y.; Seliger, S.L. Left Ventricular Hypertrophy in a Contemporary Cohort of Autosomal Dominant Polycystic Kidney Disease Patients. BMC Nephrol. 2019, 20, 386. [Google Scholar] [CrossRef]
- Schrier, R.W.; Abebe, K.Z.; Perrone, R.D.; Torres, V.E.; Braun, W.E.; Steinman, T.I.; Winklhofer, F.T.; Brosnahan, G.; Czarnecki, P.G.; Hogan, M.C.; et al. Blood Pressure in Early Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2014, 371, 2255–2266. [Google Scholar] [CrossRef]
- Perrone, R.D.; Abebe, K.Z.; Schrier, R.W.; Chapman, A.B.; Torres, V.E.; Bost, J.; Kaya, D.; Miskulin, D.C.; Steinman, T.I.; Braun, W.; et al. Cardiac Magnetic Resonance Assessment of Left Ventricular Mass in Autosomal Dominant Polycystic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 2508–2515. [Google Scholar] [CrossRef] [PubMed]
- Oto, O.A.; Edelstein, C.L. The Pathophysiology of Left Ventricular Hypertrophy, beyond Hypertension, in Autosomal Dominant Polycystic Kidney Disease. Nephron, 2022; 1–9, online ahead of print. [Google Scholar] [CrossRef]
- Lumiaho, A.; Pihlajamäki, J.; Hartikainen, J.; Ikäheimo, R.; Miettinen, R.; Niemitukia, L.; Lampainen, E.; Laakso, M. Insulin Resistance Is Related to Left Ventricular Hypertrophy in Patients with Polycystic Kidney Disease Type 1. Am. J. Kidney Dis. 2003, 41, 1219–1224. [Google Scholar] [CrossRef]
- Lash, J.P.; Go, A.S.; Appel, L.J.; He, J.; Ojo, A.; Rahman, M.; Townsend, R.R.; Xie, D.; Cifelli, D.; Cohan, J.; et al. Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline Characteristics and Associations with Kidney Function. Clin. J. Am. Soc. Nephrol. 2009, 4, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Hsu, C.-Y.; Li, Y.; Mishra, R.K.; Keane, M.; Rosas, S.E.; Dries, D.; Xie, D.; Chen, J.; He, J.; et al. Associations between Kidney Function and Subclinical Cardiac Abnormalities in CKD. J. Am. Soc. Nephrol. 2012, 23, 1725–1734. [Google Scholar] [CrossRef]
- Martinez-Vea, A.; Bardaji, A.; Gutierrez, C.; Garcia, C.; Peralta, C.; Aguilera, J.; Sanchez, P.; Vidiella, J.; Angelet, P.; Compte, T.; et al. Echocardiographic Evaluation in Patients with Autosomal Dominant Polycystic Kidney Disease and End-Stage Renal Disease. Am. J. Kidney Dis. 1999, 34, 264–272. [Google Scholar] [CrossRef]
- Otsuka, T.; Suzuki, M.; Yoshikawa, H.; Sugi, K. Left Ventricular Diastolic Dysfunction in the Early Stage of Chronic Kidney Disease. J. Cardiol. 2009, 54, 199–204. [Google Scholar] [CrossRef][Green Version]
- Dokainish, H. Left Ventricular Diastolic Function and Dysfunction: Central Role of Echocardiography. Glob. Cardiol. Sci. Pract. 2015, 2015, 3. [Google Scholar] [CrossRef]
| Total (n = 294) | Hypertensive (n = 199) | Normotensive (n = 95) | p Value | |
|---|---|---|---|---|
| Sex (Male) | 120 (40.8) | 79 (39.7) | 41 (43.2) | 0.33 |
| Age (years) | 41.0 ± 13.8 | 46.0 ± 12.1 | 30.0 ± 10.9 | <0.01 |
| BMI (kg/m2) | 25.4 ± 4.6 | 26.5 ± 4.6 | 23.3 ± 3.6 | <0.01 |
| Race | ||||
| Caucasian | 181 (61.6) | 121 (60.8) | 60 (63.1) | 0.87 |
| Non-caucasian | 113 (38.4) | 78 (39.2) | 35 (36.7) | |
| Dyslipidemia | 46 (15.6) | 42 (21.1) | 4 (4.2) | <0.01 |
| Diabetes mellitus | 9 (3.0) | 9 (4.5) | 0 (0) | 0.03 |
| AMI/CAD | 1 (0.3) | 1 (0.5) | 0 (0) | 0.67 |
| Stroke | 5 (1.7) | 5 (2.5) | 0 (0) | 0.14 |
| CKD stages | ||||
| CKD I | 105 (35.7) | 33 (16.6) | 72 (75.8) | <0.001 |
| CKD II | 79 (26.9) | 60 (30.1) | 19 (20.0) | |
| CKD III | 70 (23.8) | 66 (33.2) | 4 (4.2) | |
| CKD IV | 32 (10.9) | 32 (16.1) | 0 (0) | |
| CKD V | 8 (2.7) | 8 (4.0) | 0 (0) | |
| TKV (mL) * | 476.6 (236.2–915.6) | 679.2 (371.1–1124.7) | 237.6 (171.3–375.8) | <0.01 |
| Kidney Length (cm) * | 16.9 (13.4–23.3) | 20.3 (15.6–25.6) | 13.6 (12.0–16.1) | <0.01 |
| Tobacco use | 40 (13.6) | 31 (15.6) | 9 (9.4) | 0.11 |
| Antihypertensive drugs | ||||
| ACEi/ARB | 177 (88.9) | 177 (88.9) | - | - |
| Diuretics | 107 (53.8) | 107 (53.8) | - | - |
| Calcium channel blockers | 54 (27.1) | 54 (27.1) | - | - |
| Beta-blockers | 44 (22.1) | 44 (22.1) | - | - |
| Total (n = 294) | Hypertensive (n = 199) | Normotensive (n = 95) | p Value | |
|---|---|---|---|---|
| sCreatinine (mg/dL) | 1.08 (0.86–1.57) | 1.29 (0.98–1.92) | 0.89 (0.76–1.00) | <0.01 |
| eGFR (mL/min/1.73 m2) | 75.5 (44.5–101.0) | 56.5 (34.1–81.6) | 105.2 (90.1–115.7) | <0.01 |
| sNa (mEq/L) | 140.0 (138.0–142.8) | 141.0 (138.8–143.0) | 139.0 (137.0–142.0) | <0.01 |
| sK (mEq/L) | 4.4 (4.1–4.7) | 4.4 (4.1–4.8) | 4.25 (4.0–4.6) | 0.02 |
| sUric Acid (mg/dL) | 5.6 (4.6–7.1) | 6.2 (5.0–7.6) | 4.6 (3.9–5.5) | <0.01 |
| sGlucose (mg/dL) | 92.0 (87.0–98.0) | 94.0 (88.0–100.0) | 88.0 (84.5–95.0) | <0.01 |
| HDL (mg/dL) | 49.0 (41.0–57.0) | 48.5 (39.8–55.3) | 50.0 (42.0–60.5) | 0.14 |
| LDL (mg/dL) | 108.0 (88.0–128.0) | 111.0 (93.0–135.0) | 95.5 (75.8–116.0) | <0.01 |
| Triglycerides (mg/dL) | 104.0 (75.0–151.0) | 123.5 (86.8–166.3) | 82.0 (62.0–104.5) | <0.01 |
| Echocardiographic parameters | ||||
| AORTA (mm) | 31.0 (30.0–34.0) | 32.0 (30.0–35.0) | 30.0 (28.0–32.0) | <0.01 |
| LA (mm) | 35.0 (32.0–37.0) | 36.0 (34.0–38.0) | 34.0 (30.0–36.0) | <0.01 |
| IVS (mm) | 9.0 (8.0–10.0) | 9.0 (9.0–10.0) | 8.0 (8.0–9.0) | <0.01 |
| LVPW (mm) | 9.0 (8.0–9.0) | 9.0 (8.0–10.0) | 8.0 (8.0–9.0) | <0.01 |
| LVMI (g/m2) | 78.8 (69.7–90.5) | 81.8 (72.3–91.9) | 75.8 (64.4–84.9) | <0.01 |
| Increased LVMI—n (%) | 6 (2.0) | 6 (2.0) | 0 (0) | |
| EF (%) | 68.0 (65.0–71.0) | 68.0 (66.0–71.0) | 67.5 (64.0–70.0) | 0.23 |
| Impaired LV relaxation—n (%) | 76 (25.8) | 70 (35.2) | 6 (6.3) | <0.001 |
| LVMI | ||||
|---|---|---|---|---|
| Independent Variables | Univariate | Multivariate * | ||
| β | p | β | p | |
| Age (years) | 0.25 | <0.01 | 0.22 | <0.01 |
| Sex, (M) | 0.15 | 0.02 | 0.19 | <0.01 |
| BMI (kg/m2) | 0.16 | 0.01 | - | - |
| Tobacco use (yes) | 0.15 | 0.02 | - | - |
| Hypertension (yes) | 0.23 | <0.01 | 0.12 | 0.09 |
| Dyslipidemia (yes) | 0.07 | 0.26 | - | - |
| eGFR (mL/min/1.73 m2) | −0.18 | <0.01 | - | - |
| Uric Acid (mg/dL) | 0.11 | 0.14 | - | - |
| LDL (mg/dL) | 0.02 | 0.74 | - | - |
| Triglycerides (mg/dL) | 0.18 | 0.01 | - | - |
| ACEi/ARB (yes) | 0.04 | 0.58 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfeferman, M.B.; Rocha, D.R.d.; Rodrigues, F.G.; Pfeferman, E.; Heilberg, I.P. Echocardiographic Abnormalities in Autosomal Dominant Polycystic Kidney Disease (ADPKD) Patients. J. Clin. Med. 2022, 11, 5982. https://doi.org/10.3390/jcm11205982
Pfeferman MB, Rocha DRd, Rodrigues FG, Pfeferman E, Heilberg IP. Echocardiographic Abnormalities in Autosomal Dominant Polycystic Kidney Disease (ADPKD) Patients. Journal of Clinical Medicine. 2022; 11(20):5982. https://doi.org/10.3390/jcm11205982
Chicago/Turabian StylePfeferman, Mariana Becker, Daniel Ribeiro da Rocha, Fernanda Guedes Rodrigues, Elcio Pfeferman, and Ita Pfeferman Heilberg. 2022. "Echocardiographic Abnormalities in Autosomal Dominant Polycystic Kidney Disease (ADPKD) Patients" Journal of Clinical Medicine 11, no. 20: 5982. https://doi.org/10.3390/jcm11205982
APA StylePfeferman, M. B., Rocha, D. R. d., Rodrigues, F. G., Pfeferman, E., & Heilberg, I. P. (2022). Echocardiographic Abnormalities in Autosomal Dominant Polycystic Kidney Disease (ADPKD) Patients. Journal of Clinical Medicine, 11(20), 5982. https://doi.org/10.3390/jcm11205982






