An Evaluation of Phase Analysis to Interpret Atrial Activation Patterns during Persistent Atrial Fibrillation for Targeted Ablation
Abstract
1. Introduction
2. Materials and Methods
- (a)
- Manual Measurement of Atrial Activation Times
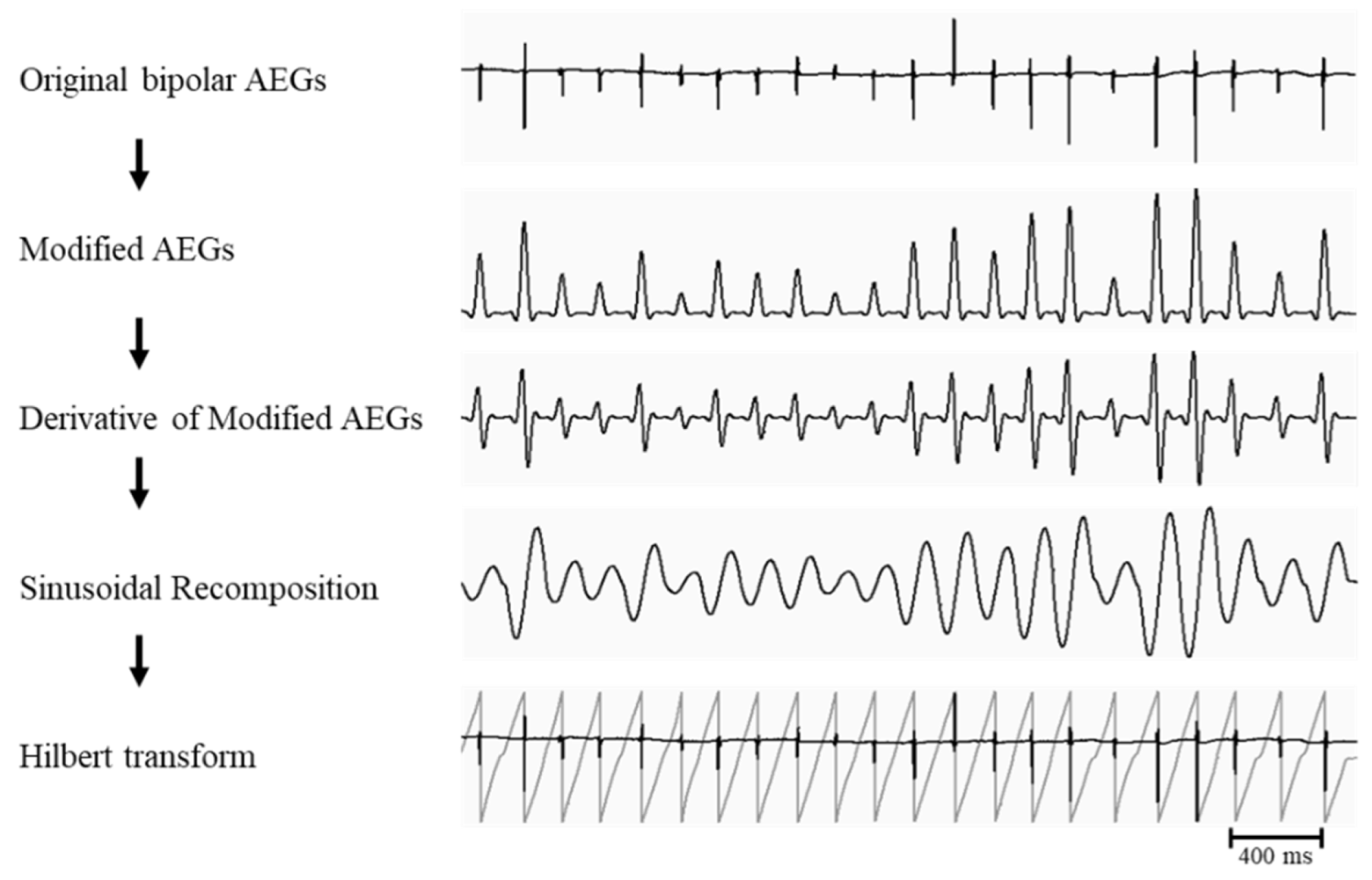
- (b) Phase Analysis
- (c) Evaluation of the phase analysis
3. Results
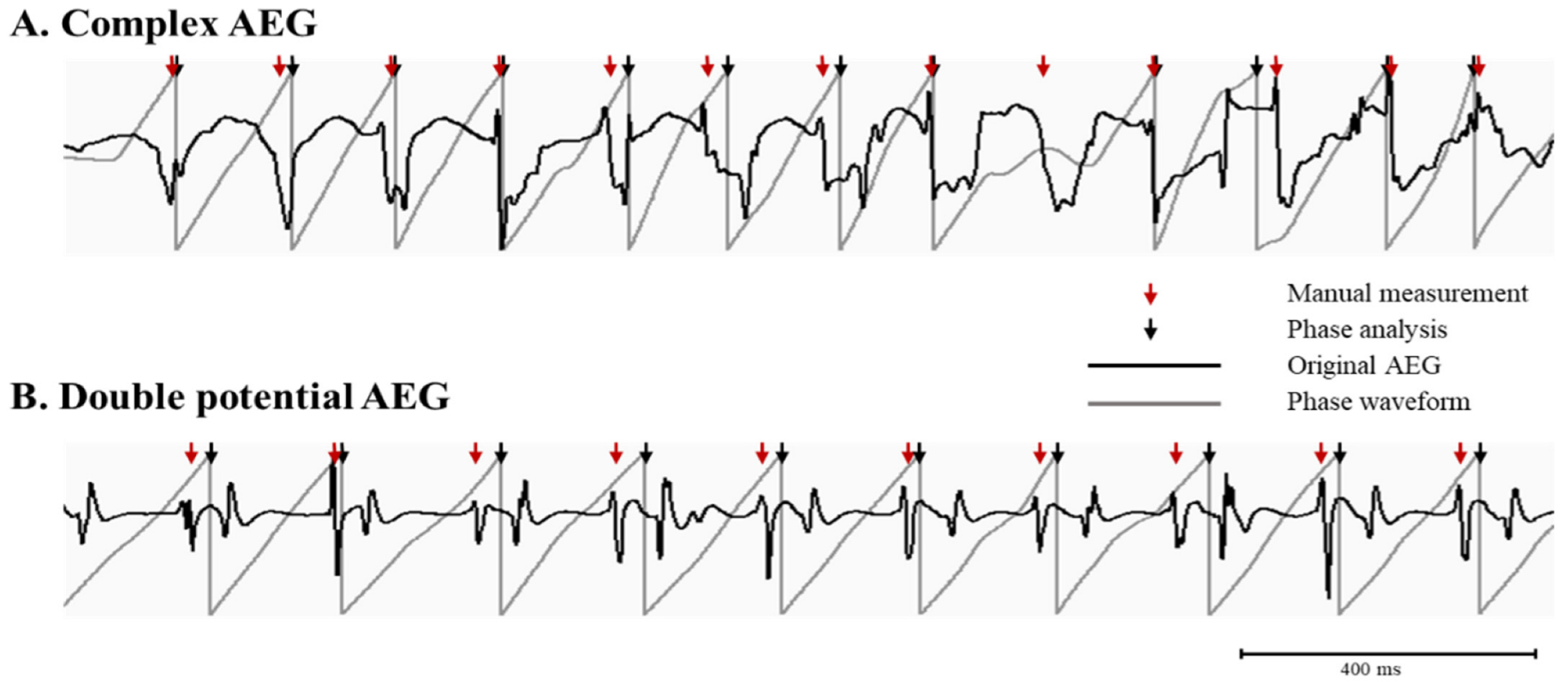
3.1. The Phase Analysis Compared to Manual Measurement
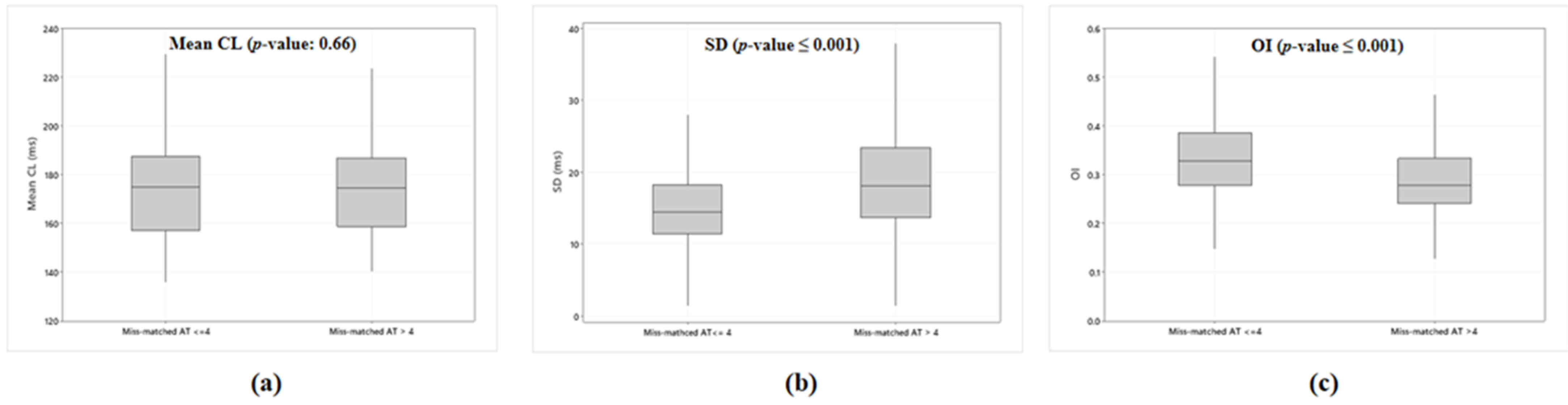
3.2. Accuracy of the Phase Analysis Dependent on AEG Characteristics
4. Discussion
4.1. Major Finding
4.2. Targeted Ablation in Persistent and LSP AF Using the Signal Processing Algorithms
4.3. Implications
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haissaguerre, M.; Hocini, M.; Denis, A.; Shah, A.J.; Komatsu, Y.; Yamashita, S.; Daly, M.; Amraoui, S.; Zellerhoff, S.; Picat, M.Q.; et al. Driver domains in persistent atrial fibrillation. Circulation 2014, 130, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Narayan, S.M.; Krummen, D.E.; Clopton, P.; Shivkumar, K.; Miller, J.M. Direct or coincidental elimination of stable rotors or focal sources may explain successful atrial fibrillation ablation: On-treatment analysis of the CONFIRM trial (Conventional ablation for AF with or without focal impulse and rotor modulation). J. Am. Coll. Cardiol. 2013, 62, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.M.; Kalra, V.; Das, M.K.; Jain, R.; Garlie, J.B.; Brewster, J.A.; Dandamudi, G. Clinical Benefit of Ablating Localized Sources for Human Atrial Fibrillation: The Indiana University FIRM Registry. J. Am. Coll. Cardiol. 2017, 69, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Zaman, J.A.B.; Sauer, W.H.; Alhusseini, M.I.; Baykaner, T.; Borne, R.T.; Kowalewski, C.A.B.; Busch, S.; Zei, P.C.; Park, S.; Viswanathan, M.N.; et al. Identification and Characterization of Sites Where Persistent Atrial Fibrillation Is Terminated by Localized Ablation. Circ. Arrhythm. Electrophysiol. 2018, 11, e005258. [Google Scholar] [CrossRef]
- Podziemski, P.; Zeemering, S.; Kuklik, P.; van Hunnik, A.; Maesen, B.; Maessen, J.; Crijns, H.J.; Verheule, S.; Schotten, U. Rotors Detected by Phase Analysis of Filtered, Epicardial Atrial Fibrillation Electrograms Colocalize with Regions of Conduction Block. Circ. Arrhythm. Electrophysiol. 2018, 11, e005858. [Google Scholar] [CrossRef]
- Vijayakumar, R.; Vasireddi, S.K.; Cuculich, P.S.; Faddis, M.N.; Rudy, Y. Methodology Considerations in Phase Mapping of Human Cardiac Arrhythmias. Circ. Arrhythm. Electrophysiol. 2016, 9, e004409. [Google Scholar] [CrossRef]
- Lee, S.; Khrestian, C.M.; Sahadevan, J.; Markowitz, A.; Waldo, A.L. New Insights into Understanding Rotor Versus Focal Activation in Patients with Persistent Atrial Fibrillation. JACC Clin. Electrophysiol. 2021, 7, 909–919. [Google Scholar] [CrossRef]
- Lee, S.; Sahadevan, J.; Khrestian, C.M.; Cakulev, I.; Markowitz, A.; Waldo, A.L. Simultaneous Biatrial High-Density (510–512 Electrodes) Epicardial Mapping of Persistent and Long-Standing Persistent Atrial Fibrillation in Patients: New Insights into the Mechanism of Its Maintenance. Circulation 2015, 132, 2108–2117. [Google Scholar] [CrossRef]
- Lee, S.; Sahadevan, J.; Khrestian, C.M.; Markowitz, A.; Waldo, A.L. Characterization of Foci and Breakthrough Sites during Persistent and Long-Standing Persistent Atrial Fibrillation in Patients: Studies Using High-Density (510––512 Electrodes) Biatrial Epicardial Mapping. J. Am. Heart Assoc. 2017, 6, e005274. [Google Scholar] [CrossRef]
- de Sa, D.D.C.; Thompson, N.; Stinnett-Donnelly, J.; Znojkiewicz, P.; Habel, N.; Muller, J.G.; Bates, J.H.; Buzas, J.S.; Spector, P.S. Electrogram fractionation: The relationship between spatiotemporal variation of tissue excitation and electrode spatial resolution. Circ. Arrhythm. Electrophysiol. 2011, 4, 909–916. [Google Scholar] [CrossRef]
- Lee, S.; Ryu, K.; Waldo, A.L.; Khrestian, C.M.; Durand, D.M.; Sahadevan, J. An algorithm to measure beat-to-beat cycle lengths for assessment of atrial electrogram rate and regularity during atrial fibrillation. J. Cardiovasc. Electrophysiol. 2013, 24, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Kuklik, P.; Zeemering, S.; van Hunnik, A.; Maesen, B.; Pison, L.; Lau, D.H.; Maessen, J.; Podziemski, P.; Meyer, C.; Schaffer, B.; et al. Identification of Rotors during Human Atrial Fibrillation Using Contact Mapping and Phase Singularity Detection: Technical Considerations. IEEE Trans. Biomed. Eng. 2017, 64, 310–318. [Google Scholar] [CrossRef]
- Botteron, G.W.; Smith, J.M. A technique for measurement of the extent of spatial organization of atrial activation during atrial fibrillation in the intact human heart. IEEE Trans. Biomed. Eng. 1995, 42, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, V.; Bartolini, P.; Calcagnini, G.; Morelli, S.; Michelucci, A.; Gensini, G. Automated classification of human atrial fibrillation from intraatrial electrograms. Pacing Clin. Electrophysiol. 2000, 23, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Everett, T.H.T.; Moorman, J.R.; Kok, L.C.; Akar, J.G.; Haines, D.E. Assessment of global atrial fibrillation organization to optimize timing of atrial defibrillation. Circulation 2001, 103, 2857–2861. [Google Scholar] [CrossRef]
- Nademanee, K.; McKenzie, J.; Kosar, E.; Schwab, M.; Sunsaneewitayakul, B.; Vasavakul, T.; Khunnawat, C.; Ngarmukos, T. A new approach for catheter ablation of atrial fibrillation: Mapping of the electrophysiologic substrate. J. Am. Coll. Cardiol. 2004, 43, 2044–2053. [Google Scholar] [CrossRef]
- Atienza, F.; Almendral, J.; Jalife, J.; Zlochiver, S.; Ploutz-Snyder, R.; Torrecilla, E.G.; Arenal, A.; Kalifa, J.; Fernandez-Aviles, F.; Berenfeld, O. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm 2009, 6, 33–40. [Google Scholar] [CrossRef]
- Ng, J.; Gordon, D.; Passman, R.S.; Knight, B.P.; Arora, R.; Goldberger, J.J. Electrogram morphology recurrence patterns during atrial fibrillation. Heart Rhythm 2014, 11, 2027–2034. [Google Scholar] [CrossRef]
- Jadidi, A.S.; Lehrmann, H.; Keyl, C.; Sorrel, J.; Markstein, V.; Minners, J.; Park, C.I.; Denis, A.; Jais, P.; Hocini, M.; et al. Ablation of Persistent Atrial Fibrillation Targeting Low-Voltage Areas with Selective Activation Characteristics. Circ. Arrhythm. Electrophysiol. 2016, 9, e002962. [Google Scholar] [CrossRef]
- Lin, Y.J.; Lo, M.T.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; Chao, T.F.; Chung, F.P.; Liao, J.N.; Lin, C.Y.; Kuo, H.Y.; et al. Benefits of Atrial Substrate Modification Guided by Electrogram Similarity and Phase Mapping Techniques to Eliminate Rotors and Focal Sources Versus Conventional Defragmentation in Persistent Atrial Fibrillation. JACC Clin. Electrophysiol. 2016, 2, 667–678. [Google Scholar] [CrossRef]
- Roney, C.H.; Ng, F.S.; Debney, M.T.; Eichhorn, C.; Nachiappan, A.; Chowdhury, R.A.; Qureshi, N.A.; Cantwell, C.D.; Tweedy, J.H.; Niederer, S.A.; et al. Determinants of new wavefront locations in cholinergic atrial fibrillation. Europace 2018, 20, iii3–iii15. [Google Scholar] [CrossRef] [PubMed]
- Gizurarson, S.; Dalvi, R.; Das, M.; Ha, A.C.T.; Suszko, A.; Chauhan, V.S. Hierarchical Schema for Identifying Focal Electrical Sources during Human Atrial Fibrillation: Implications for Catheter-Based Atrial Substrate Ablation. JACC Clin. Electrophysiol. 2016, 2, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.; Sehgal, V.; Ng, J.K.; Gordon, D.; Goldberger, J.J. Iterative method to detect atrial activations and measure cycle length from electrograms during atrial fibrillation. IEEE Trans. Biomed. Eng. 2014, 61, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Houben, R.P.; de Groot, N.M.; Lindemans, F.W.; Allessie, M.A. Automatic mapping of human atrial fibrillation by template matching. Heart Rhythm 2006, 3, 1221–1228. [Google Scholar] [CrossRef]
- Jadidi, A.S.; Cochet, H.; Shah, A.J.; Kim, S.J.; Duncan, E.; Miyazaki, S.; Sermesant, M.; Lehrmann, H.; Lederlin, M.; Linton, N.; et al. Inverse relationship between fractionated electrograms and atrial fibrosis in persistent atrial fibrillation: Combined magnetic resonance imaging and high-density mapping. J. Am. Coll. Cardiol. 2013, 62, 802–812. [Google Scholar] [CrossRef]
- Narayan, S.M.; Shivkumar, K.; Krummen, D.E.; Miller, J.M.; Rappel, W.J. Panoramic electrophysiological mapping but not electrogram morphology identifies stable sources for human atrial fibrillation: Stable atrial fibrillation rotors and focal sources relate poorly to fractionated electrograms. Circ. Arrhythm. Electrophysiol. 2013, 6, 58–67. [Google Scholar] [CrossRef]
- Kochhauser, S.; Verma, A.; Dalvi, R.; Suszko, A.; Alipour, P.; Sanders, P.; Champagne, J.; Macle, L.; Nair, G.M.; Calkins, H.; et al. Spatial Relationships of Complex Fractionated Atrial Electrograms and Continuous Electrical Activity to Focal Electrical Sources: Implications for Substrate Ablation in Human Atrial Fibrillation. JACC Clin. Electrophysiol. 2017, 3, 1220–1228. [Google Scholar] [CrossRef]
- Benharash, P.; Buch, E.; Frank, P.; Share, M.; Tung, R.; Shivkumar, K.; Mandapati, R. Quantitative Analysis of Localized Sources Identified by Focal Impulse and Rotor Modulation Mapping in Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2015, 8, 554–561. [Google Scholar] [CrossRef]
- Roney, C.H.; Cantwell, C.D.; Bayer, J.D.; Qureshi, N.A.; Lim, P.B.; Tweedy, J.H.; Kanagaratnam, P.; Peters, N.S.; Vigmond, E.J.; Ng, F.S. Spatial Resolution Requirements for Accurate Identification of Drivers of Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2017, 10, e004899. [Google Scholar] [CrossRef]
- King, B.; Porta-Sánchez, A.; Massé, S.; Zamiri, N.; Balasundaram, K.; Kusha, M.; Jackson, N.; Haldar, S.; Umapathy, K.; Nanthakumar, K. Effect of spatial resolution and filtering on mapping cardiac fibrillation. Heart Rhythm 2017, 14, 608–615. [Google Scholar] [CrossRef]
- Walters, T.E.; Lee, G.; Spence, S.; Kalman, J.M. The effect of electrode density on the interpretation of atrial activation patterns in epicardial mapping of human persistent atrial fibrillation. Heart Rhythm 2016, 13, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Jiang, C.Y.; Betts, T.R.; Chen, J.; Deisenhofer, I.; Mantovan, R.; Macle, L.; Morillo, C.A.; Haverkamp, W.; Weerasooriya, R.; et al. Approaches to catheter ablation for persistent atrial fibrillation. N. Engl. J. Med. 2015, 372, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zheng, L.; Jiang, C.; Fan, J.; Liu, X.; Zhan, X.; Li, J.; Wang, L.; Yang, H.; Zhu, W.; et al. Circumferential Pulmonary Vein Isolation Plus Low-Voltage Area Modification in Persistent Atrial Fibrillation. JACC Clin. Electrophysiol. 2022, 8, 882–891. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Wazni, O.; McGann, C.; Greene, T.; Dean, J.M.; Dagher, L.; Kholmovski, E.; Mansour, M.; Marchlinski, F.; Wilber, D.; et al. Effect of MRI-Guided Fibrosis Ablation vs Conventional Catheter Ablation on Atrial Arrhythmia Recurrence in Patients with Persistent Atrial Fibrillation: The DECAAF II Randomized Clinical Trial. JAMA 2022, 327, 2296–2305. [Google Scholar] [CrossRef] [PubMed]
- Pathik, B.; Kalman, J.M.; Walters, T.; Kuklik, P.; Zhao, J.; Madry, A.; Prabhu, S.; Nalliah, C.; Kistler, P.; Lee, G. Transient Rotor Activity during Prolonged 3-Dimensional Phase Mapping in Human Persistent Atrial Fibrillation. JACC Clin. Electrophysiol. 2018, 4, 72–83. [Google Scholar] [CrossRef]
- Kuklik, P.; Zeemering, S.; Maesen, B.; Maessen, J.; Crijns, H.J.; Verheule, S.; Ganesan, A.N.; Schotten, U. Reconstruction of instantaneous phase of unipolar atrial contact electrogram using a concept of sinusoidal recomposition and Hilbert transform. IEEE Trans. Biomed. Eng. 2015, 62, 296–302. [Google Scholar] [CrossRef]
- Schotten, U.; Lee, S.; Zeemering, S.; Waldo, A.L. Paradigm shifts in electrophysiological mechanisms of atrial fibrillation. Europace 2021, 23, ii9–ii13. [Google Scholar] [CrossRef]
- de Bakker, J.M.; van Capelle, F.J.; Janse, M.J.; Tasseron, S.; Vermeulen, J.T.; de Jonge, N.; Lahpor, J.R. Fractionated electrograms in dilated cardiomyopathy: Origin and relation to abnormal conduction. J. Am. Coll. Cardiol. 1996, 27, 1071–1078. [Google Scholar] [CrossRef]
- Tung, R.; Nakahara, S.; Ramirez, R.; Lai, C.; Fishbein, M.C.; Shivkumar, K. Distinguishing epicardial fat from scar: Analysis of electrograms using high-density electroanatomic mapping in a novel porcine infarct model. Heart Rhythm 2010, 7, 389–395. [Google Scholar] [CrossRef]

| Patient No. | Age | Gender | AF Duration | Valvular Disease | CAD | Heart Failure |
|---|---|---|---|---|---|---|
| 1 | 60 | M | >1 year | MR | − | + |
| 2 | 57 | M | >1 year | MS | − | + |
| 3 | 67 | M | 1 month | MR | − | + |
| 4 | 70 | M | 9 years | AS, TR | − | + |
| 5 | 70 | F | 8.5 years | AS | − | + |
| 6 | 80 | F | 2.5 years | TR | − | + |
| 7 | 63 | M | >1 year | MR, TR | + | + |
| SD Cutoff | Accuracy of Phase Analysis |
|---|---|
| SD < 10 ms | 98% |
| SD < 15 ms | 94% |
| SD < 20 ms | 88% |
| SD < 30 ms | 83% |
| Overall | 82% |
| AEG Characteristics | ||||
|---|---|---|---|---|
| Complex AEG | Double Potential | Ventricular Complex | Variable Amplitude | Others |
| 61% | 16% | 12% | 8% | 3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Khrestian, C.M.; Sahadevan, J.; Waldo, A.L. An Evaluation of Phase Analysis to Interpret Atrial Activation Patterns during Persistent Atrial Fibrillation for Targeted Ablation. J. Clin. Med. 2022, 11, 5807. https://doi.org/10.3390/jcm11195807
Lee S, Khrestian CM, Sahadevan J, Waldo AL. An Evaluation of Phase Analysis to Interpret Atrial Activation Patterns during Persistent Atrial Fibrillation for Targeted Ablation. Journal of Clinical Medicine. 2022; 11(19):5807. https://doi.org/10.3390/jcm11195807
Chicago/Turabian StyleLee, Seungyup, Celeen M. Khrestian, Jayakumar Sahadevan, and Albert L. Waldo. 2022. "An Evaluation of Phase Analysis to Interpret Atrial Activation Patterns during Persistent Atrial Fibrillation for Targeted Ablation" Journal of Clinical Medicine 11, no. 19: 5807. https://doi.org/10.3390/jcm11195807
APA StyleLee, S., Khrestian, C. M., Sahadevan, J., & Waldo, A. L. (2022). An Evaluation of Phase Analysis to Interpret Atrial Activation Patterns during Persistent Atrial Fibrillation for Targeted Ablation. Journal of Clinical Medicine, 11(19), 5807. https://doi.org/10.3390/jcm11195807






