Particularities of Catheter Ablation in Women with Atrial Fibrillation and Associated Ischemic Heart Disease
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ghuran, A.V.; Camm, A.J. Ischaemic heart disease presenting as arrhythmias. Br. Med. Bull. 2001, 59, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Stirbys, P. Myocardial Ischemia as a Genuine Cause Responsible for the Organization and “Fertilization” of Conflictogenic Atrial Fibrillation: New Conceptual Insights into Arrhythmogenicity. J. Atr. Fibrillation 2013, 5, 797. [Google Scholar] [CrossRef] [PubMed]
- Meschia, J.F.; Merrill, P.; Soliman, E.Z.; Howard, V.J.; Barrett, K.M.; Zakai, N.A.; Kleindorfer, D.; Safford, M.; Howard, G. Racial disparities in awareness and treatment of atrial fibrillation: The REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke 2010, 41, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Mohanty, P.; Di Biase, L.; Sanchez, J.E.; Shaheen, M.H.; Burkhardt, J.D.; Bassouni, M.; Cummings, J.; Wang, Y.; Lewis, W.R.; et al. Outcomes and complications of catheter ablation for atrial fibrillation in females. Heart Rhythm 2010, 7, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498, Erratum in Eur. Heart J. 2021, 42, 507; Erratum in Eur. Heart J. 2021, 42, 546–547; Erratum in Eur. Heart J. 2021, 42, 4194. [Google Scholar] [CrossRef] [PubMed]
- Boudi, F.B.; Kalayeh, N.; Movahed, M.R. High-Density Lipoprotein Cholesterol (HDL-C) Levels Independently Correlates with Cardiac Arrhythmias and Atrial Fibrillation. J. Intensive Care Med. 2020, 35, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Trieb, M.; Kornej, J.; Knuplez, E.; Hindricks, G.; Thiele, H.; Sommer, P.; Scharnagl, H.; Dagres, N.; Dinov, B.; Bollmann, A.; et al. Atrial fibrillation is associated with alterations in HDL function, metabolism, and particle number. Basic Res. Cardiol. 2019, 114, 27. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, M.; Hisatome, I. Gender Difference in the Association Between Uric Acid and Atrial Fibrillation. Circ. J. 2018, 83, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.H.; Appelman, Y.E. Gender differences in coronary heart disease. Neth. Heart J. 2010, 18, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.T.; Xu, Y.J.; Yang, Y.Q. Gender Differences in Arrhythmias: Focused on Atrial Fibrillation. J. Cardiovasc. Transl. Res. 2020, 13, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Salton, C.J.; Chuang, M.L.; O’Donnell, C.J.; Kupka, M.J.; Larson, M.; Kissinger, K.V.; Edelman, R.R.; Levy, D.; Manning, W.J. Gender differences and normal left ventricular anatomy in an adult population free of hypertension. A cardiovascular magnetic resonance study of the Framingham Heart Study Offspring cohort. J. Am. Coll. Cardiol. 2002, 39, 1055–1060. [Google Scholar] [CrossRef]
- Cheruiyot, I.; Munguti, J.; Olabu, B.; Gichangi, P. A meta-analysis of the relationship between anatomical variations of pulmonary veins and atrial fibrillation. Acta Cardiol. 2020, 75, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alegret, J.M.; Viñolas, X.; Martínez-Rubio, A.; Pedrote, A.; Beiras, X.; García-Sacristán, J.F.; Crespo-Mancebo, F.; Ruiz-Mateas, F. Gender Differences in Patients with Atrial Fibrillation Undergoing Electrical Cardioversion. J. Womens Health 2015, 24, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Rienstra, M.; Van Veldhuisen, D.J.; Hagens, V.E.; Ranchor, A.V.; Veeger, N.J.; Crijns, H.J.; Van Gelder, I.C. RACE Investigators. Gender-related differences in rhythm control treatment in persistent atrial fibrillation: Data of the Rate Control Versus Electrical Cardioversion (RACE) study. J. Am. Coll. Cardiol. 2005, 46, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Bansal, M.; Kasliwal, R.R. Echocardiography for left atrial appendage structure and function. Indian Heart J. 2012, 64, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Beck, H.; Curtis, A.B. Sex Differences In Outcomes of Ablation of Atrial Fibrillation. J. Atr. Fibrillation 2014, 6, 1024. [Google Scholar] [CrossRef] [PubMed]
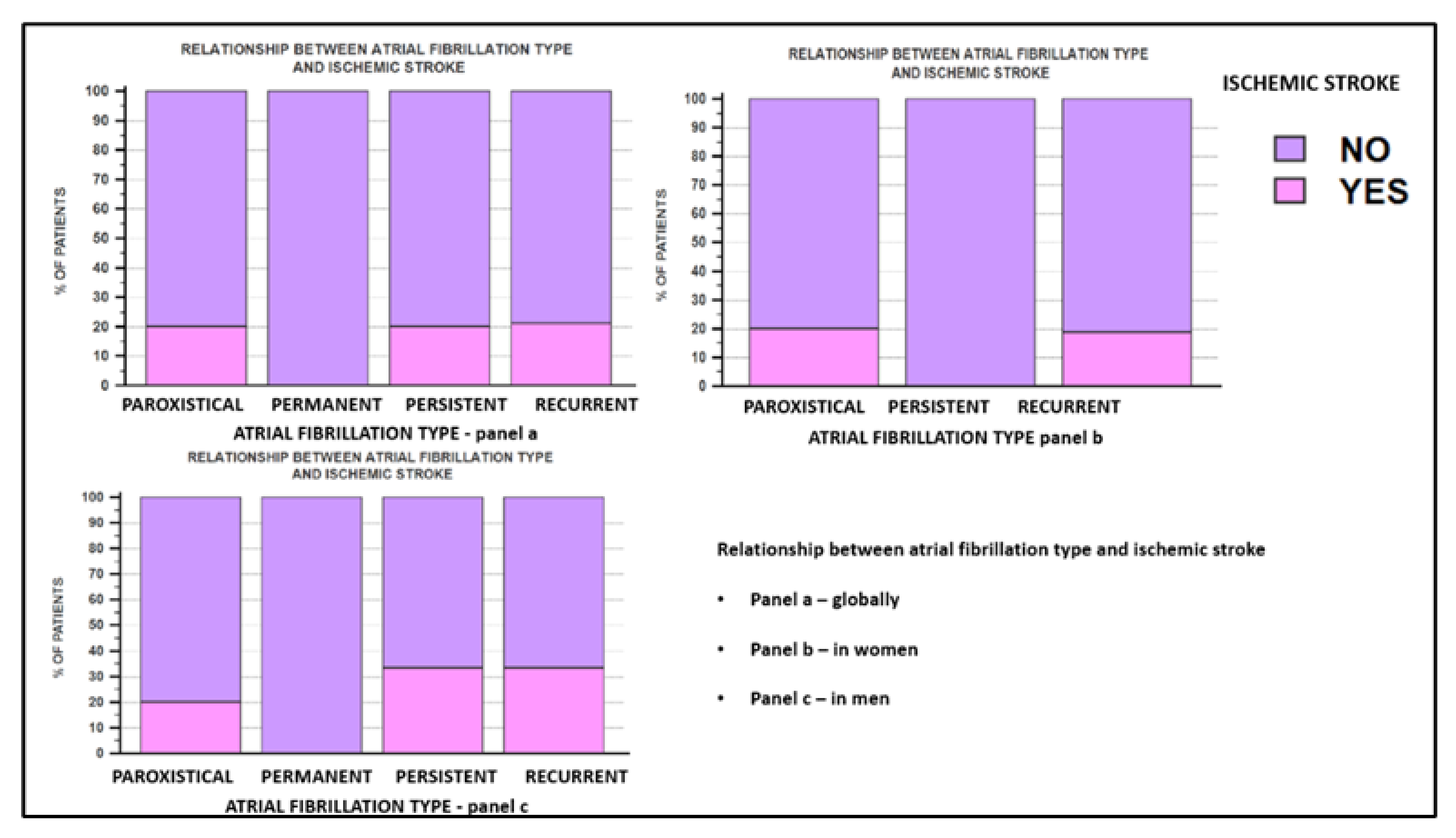
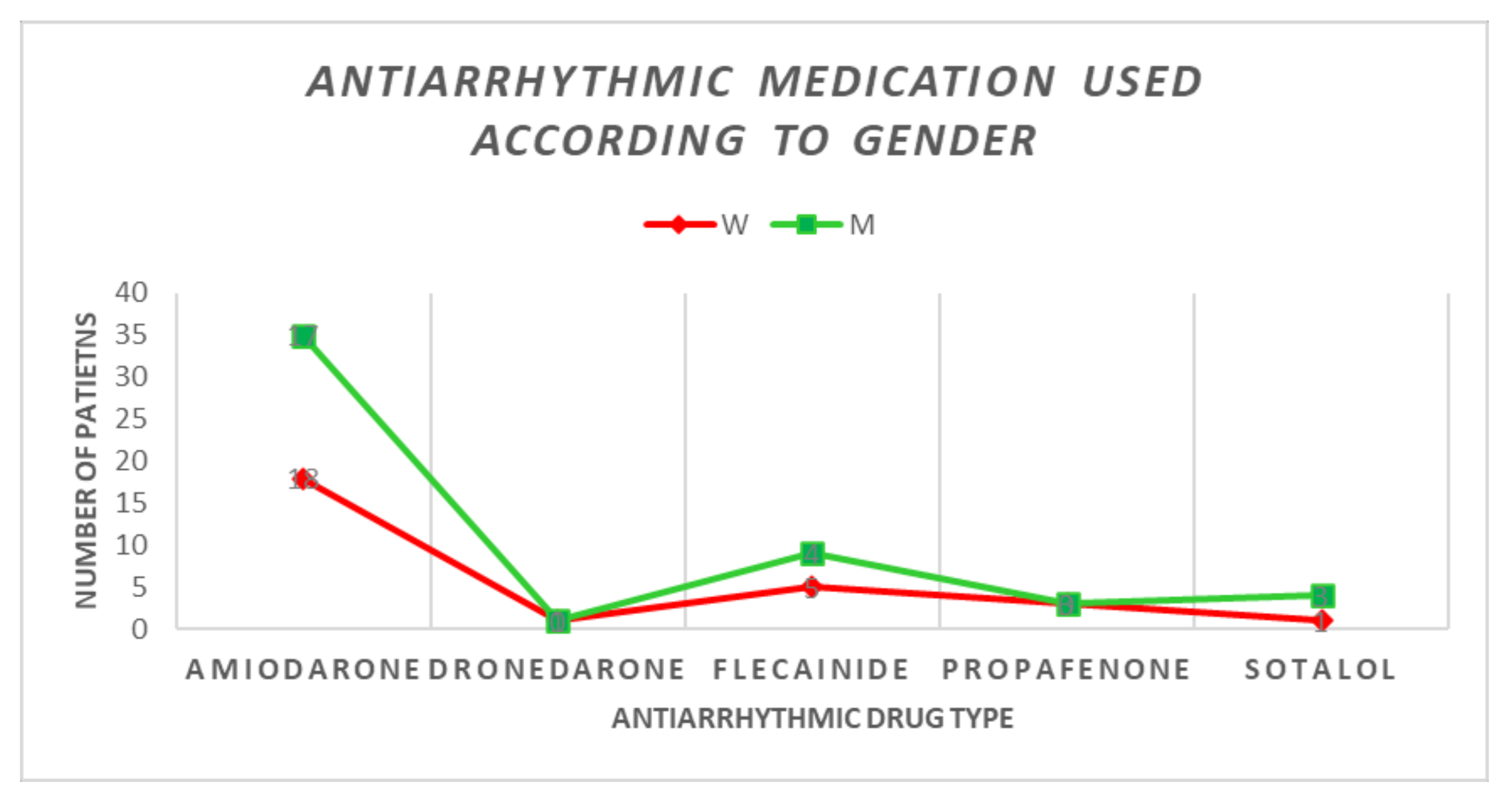
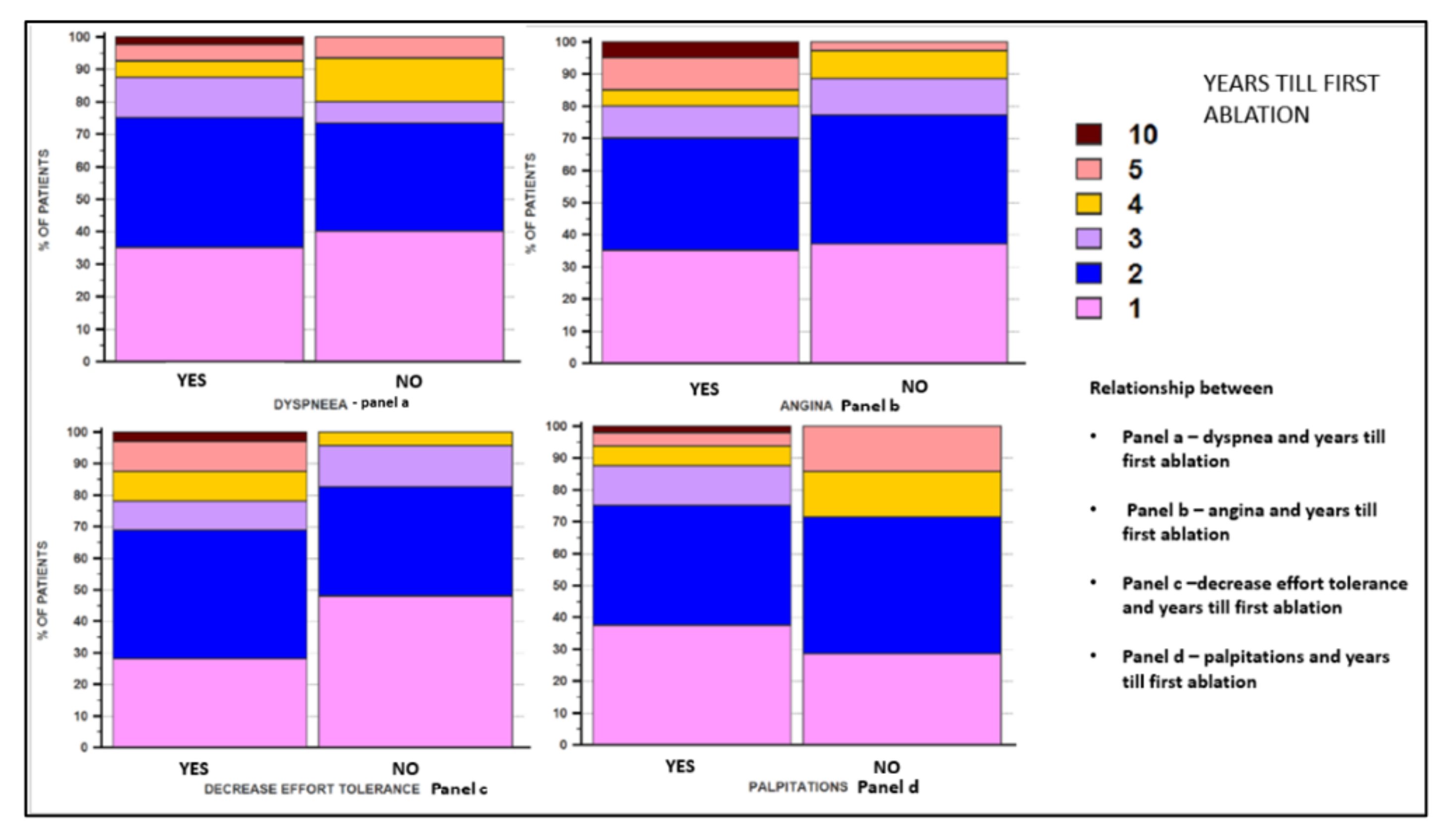
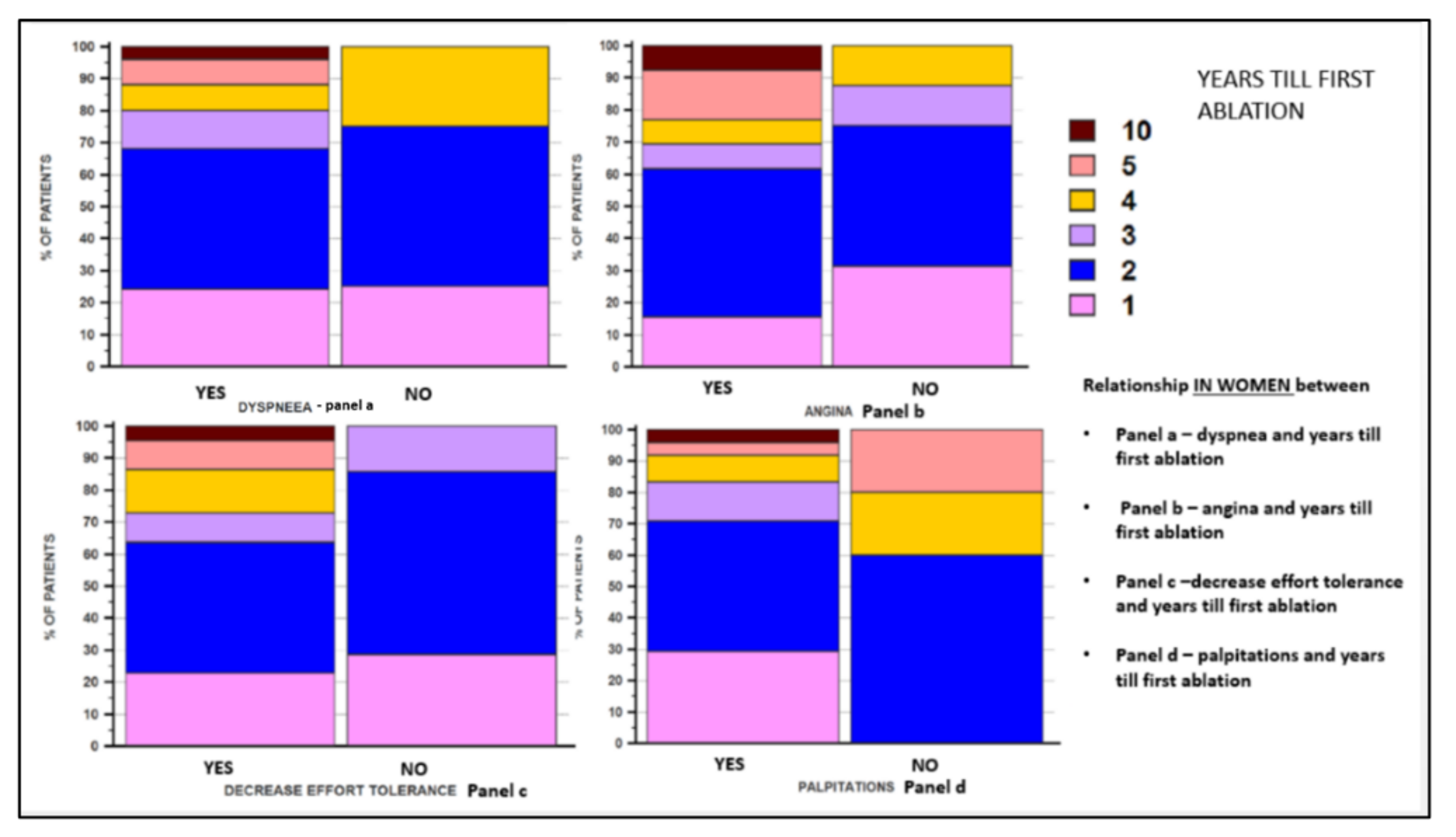
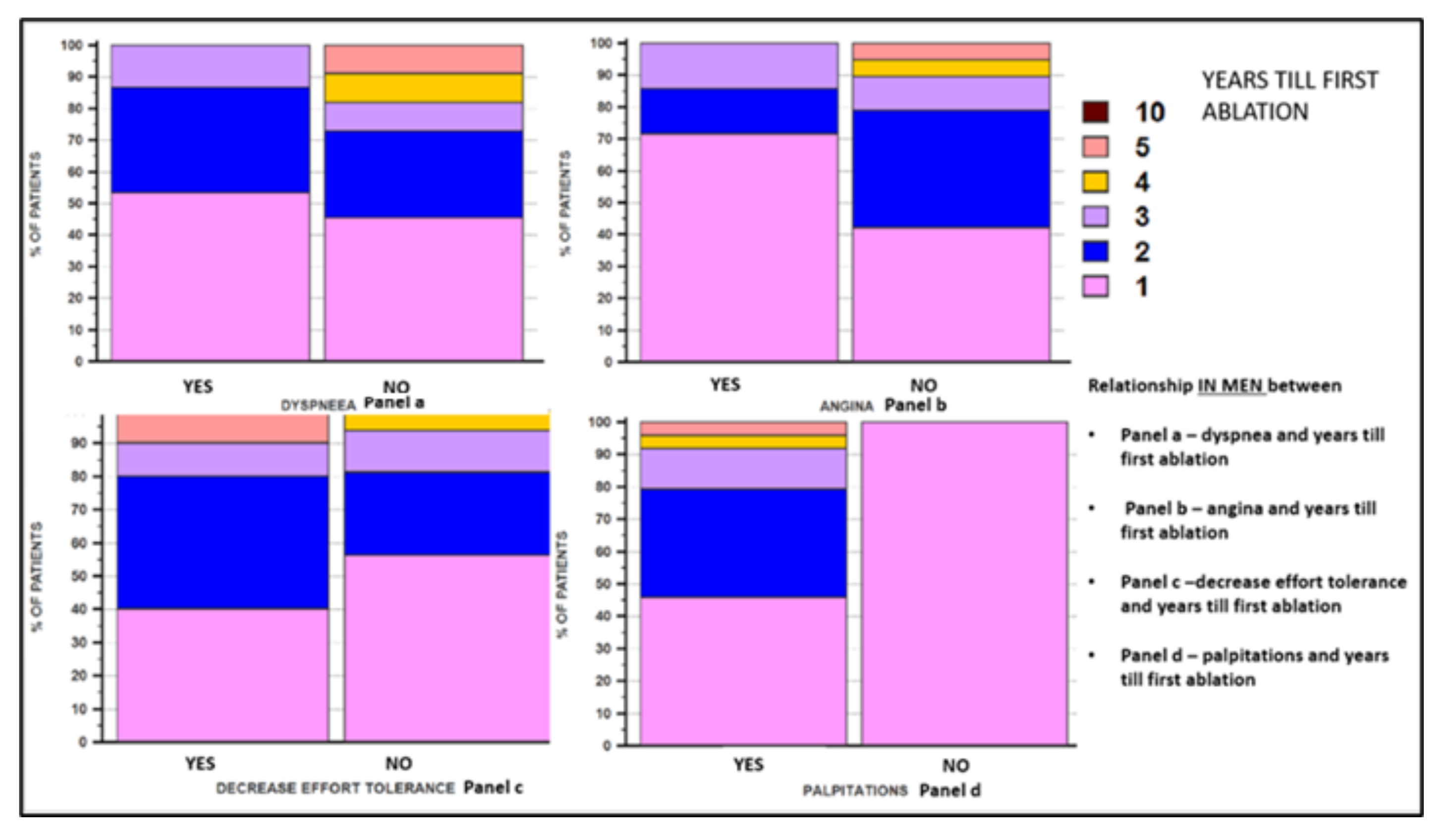
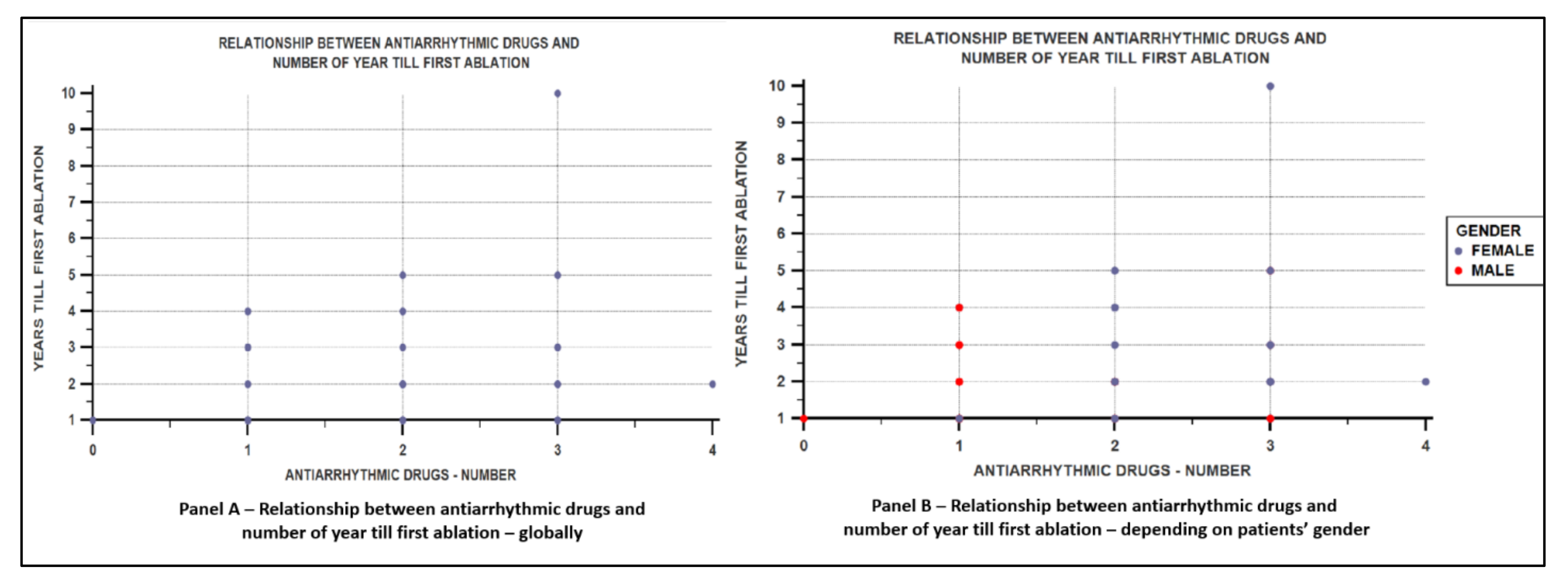

| Parameters | Female Sex | Male Sex | p Value | |
|---|---|---|---|---|
| Number of patients | 29 (52.7%) | 26 (47.3%) | ||
| Age | Mean ± SD | 59.34 ± 8.99 | 61.69 ± 6.89 | p = NS |
| Smoking | Yes | 3 (10.34) | 22 (84.61) | p < 0.0001 |
| No | 26 (89.65) | 4 (15.38) | ||
| Alcohol | Yes | 0 (0) | 5 (19.23) | p = 0.044 |
| No | 29 (100) | 21 (80.76) | ||
| Palpitation | Yes | 24 (82.75) | 24 (92.30) | p = NS |
| No | 5 (17.24) | 2 (7.69) | ||
| Decreased effort tolerance | Yes | 22 (75.86) | 10 (38.46) | p = 0.011 |
| No | 7 (24.13) | 16 (61.530 | ||
| Dyspnea | Yes | 25 (86.20) | 15 (57.69) | p = 0.038 |
| No | 4 (13.79) | 11 (42.30) | ||
| Angina | Yes | 13 (44.82) | 7 (26.92) | p = NS |
| No | 16 (55.17) | 19 (73.07) | ||
| Mean HR * | Mean ± SD | 77.34 ± 20.47 | 84.65 ± 22.40 | p = NS |
| DPB * | Mean ± SD (Median) | 80.17 ± 11.60 (80) | 78.46 ± 10.07 (80) | p = NS |
| SBP * | Mean ± SD | 135.17 ± 15.08 | 134.23 ± 17.18 | p = NS |
| Total cholesterol | Mean ± SD | 198 ± 59.48 | 173.73 ± 47.73 | p = NS |
| LDL * | Mean ± SD (Median) | 124.63 ± 54.08 (106) | 104.57 ± 39.30 (100.5) | p = NS |
| HDL* | Mean ± SD | 48.31 ± 11.10 | 42.69 ± 8.67 | p = 0.043 |
| Triglyceride | Mean ± SD | 124.31 ± 52.19 | 154.30 ± 73.78 | p = NS |
| Glucose level * | Mean ± SD (Median) | 106.55 ± 25.51 (97) | 106.53 ± 28.28 (99.5) | p = NS |
| Uric acid | Mean ± SD | 5.88 ± 2.38 | 7.32 ± 1.9 | p = 0.017 |
| ESR * | Mean ± SD (Median) | 16.20 ± 13.23 (12) | 8.07 ± 4.84 (8) | p = 0.01 |
| TSH * | Mean ± SD | 2.89 ± 2.61 | 2.16 ± 0.97 | p = NS |
| AF at admission | Yes | 9 (31.03) | 14 (53.84) | p = NS |
| No | 20 (68.96) | 12 (46.15) | ||
| Paroxysmal | 5 (17.24) | 10 (38.46) | p = 0.006 | |
| Persistent | 8 (27.58) | 12 (46.15) | ||
| Recurrent | 16 (55.17) | 3 (11.53) | ||
| Permanent | 0 (0) | 1 (3.84) | ||
| Onset of AF (number of years) * | Mean ± SD (Median) | 6.55 ± 2.65 (6) | 5 ± 1.32 (5) | p = 0.014 |
| Obesity | Yes | 22 (75.86) | 13 (50) | p = NS |
| No | 7 (24.13) | 13 (50) | ||
| Arterial Hypertension | Yes | 28 (96.55) | 24 (92.30) | p = NS |
| No | 1 (3.44) | 2 (7.69) | ||
| Microvascular IHD * | Yes | 22 (75.86) | 12 (46.15) | p = 0.047 |
| No | 7 (24.13) | 14 (53.84) | ||
| Macrovascular IHD * | Yes | 7 (24.13) | 14 (53.84) | p = 0.047 |
| No | 22 (75.86) | 12 (46.15) | ||
| Number of coronary lesions | One vessel | 7 (100) | 10 (71.42) | p = NS |
| Two vessels | 0 (0) | 3 (21.42) | ||
| Multivessel | 0 (0) | 1 (7.14) | ||
| Old MI * | Yes | 1 (3.44) | 7 (26.92) | p = 0.037 |
| No | 28 (96.55) | 19 (73.07) | ||
| Diabetes mellitus | Yes | 8 (27.58) | 5 (19.23) | p = NS |
| No | 21 (72.41) | 21 (80.76) | ||
| HF * | Yes | 11 (37.93) | 7 (26.92) | p = NS |
| No | 18 (62.06) | 19 (73.07) | ||
| NYHA * CLASS | No HF | 18 (62.06) | 19 (73.07) | p = 0.046 |
| NYHA II | 11 (37.93) | 4 (15.38) | ||
| NYHA III | 0 (0) | 3 (11.53) | ||
| NYHA IV | - | - | ||
| DCM * | Yes | 1 (3.44) | 4 (15.38) | p = NS |
| No | 28 (96.55) | 22 (84.61) | ||
| Thyroid pathology | Yes | 14 (49.27) | 9 (34.61) | p = NS |
| No | 15 (51.72) | 17 (65.38) | ||
| Other arrhythmias | Yes | 16 (55.17) | 13 (50) | p = NS |
| No | 13 (44.82) | 13 (50) | ||
| Ischemic stroke | Yes | 4 (13.79) | 7 (26.92) | p = NS |
| No | 25 (86.20) | 19 (73.07) |
| Parameters | Global | Female Sex | Male Sex | p Value | |
|---|---|---|---|---|---|
| Transthoracic echocardiography | |||||
| LA diameter | Mean ± SD (Median) | 43.21 ± 6.10 (43) | 41.75 ± 6.47 (41) | 44.84 ± 5.31 (44) | p = 0.05 |
| LVEDD | Mean ± SD (Median) | 50.36 ± 6.21 (50) | 48.13 ± 4.8 (46) | 52.84 ± 6.73 (50) | p = 0.005 |
| LVESD | Mean ± SD (Median) | 37.7 ± 27.26 (34) | 39.62 ± 36.96 (34) | 35.61 ± 8.16 (35) | p = 0.288 |
| LVEF% | Mean ± SD (Median) | 50.34 ± 5.99 (50) | 51.51 ± 5.34 (50) | 49.03 ± 6.50 (50) | p = 0.174 |
| Diastolic dysfunction | Yes | 51 (92.7%) | 27 (93.10%) | 25 (96.15%) | p = 0.148 |
| No | 4 (7.3%) | 2 (6.9%) | 1 (3.85%) | ||
| Grade I | 45 (88.2%) | p = 0.548 | |||
| Grade II | 5 (9.8%) | ||||
| Grade III | 1 (2%) | ||||
| Mitral insufficiency | Yes | 52 (94.5%) | 27 (93.10%) | 25 (96.15%) | p = 0.922 |
| No | 3 (5.5%) | 2 (6.9%) | 1 (3.85%) | ||
| Mild | 45 (88.2%) | 23 (92%) | 22 (84.61%) | p = 0.548 | |
| Moderate | 5 (9.8%) | 2 (8%) | 3 (11.53%) | ||
| Severe | 1 (2%) | 0 (0%) | 1 (3.84%) | ||
| Pulmonary hypertension | Yes | 20 (36.4%) | 11 (37.9%) | 9 (34.61%) | p = 0.979 |
| No | 35 (63.6%) | 18 (62.1%) | 17 (65.4%) | ||
| Transesophageal echocardiography | |||||
| LAA diameter | Mean ± SD (Median) | 1.75 ± 0.47 (1.8) | 1.45 ± 0.34 (1.4) | 2.08 ± 0.37 (2.1) | p < 0.0001 |
| LAA velocity | Mean ± SD (Median) | 0.66 ± 0.23 (0.66) | 0.59 ± 0.16 (0.6) | 0.74 ± 0.28 (0.75) | p = 0.0263 |
| Spontaneous echo contrast LAA | Yes | 8 (14.5 %) | 3 (10.33%) | 5 (19.23%) | p = 0.5822 |
| No | 47 (85.5%) | 26 (89.65%) | 21 (80.76%) | ||
| LAA thrombus in the past | Yes | 3 (5.5%) | 1 (3.44%) | 2 (7.69%) | p = 0.922 |
| No | 52 (94.5%) | 28 (96.56%) | 24 (92.31%) | ||
| PFO | Yes | 9 (16.4%) | 8 (27.58%) | 1 (3.89%) | p = 0.044 |
| No | 46 (83.6%) | 21 (72.41%) | 25 (96.15%) | ||
| Cardiac and pulmonary vein CT angiogram | |||||
| Indication for CT | 54 (98.2%) | 28 (96.55%) | 26 (100%) | p = 0.956 | |
| 4 PV | Yes | 54 (98.2%) | 28 (96.55%) | 26 (100%) | p = 0.960 |
| No | 1 (1.8%) | 1 (3.45%) | 0 (0%) | ||
| PV anomaly | Yes | 15 (27.8%) | 10 (38.46%) | 5 (19.23%) | p = 0.378 |
| No | 40 (72.2%) | 19 (61.53%) | 21 (80.77%) | ||
| APVD | 1 (7.1%) | 1 (3.44%) | 0 (0%) | p = 0.956 | |
| Common left-sided trunk | 13 (92.9%) | 10 (34.4%) | 3 (11.53%) | p = 0.045 | |
| Female Sex | Male Sex | p Value | ||
|---|---|---|---|---|
| CHA2DS2-VASc Score | Median | 3 | 3 | p = 0.166 |
| HAS-BLED Score | Median | 1 | 1 | p = 0.946 |
| Anticoagulant | Yes | 89.66% (26/29) | 100% (26/26) | p = 0.274 |
| No | 10.34% (3/29) | 0% (0/26) | ||
| DOACs | 46.15% (12/26) | 57.7% (15/26) | p = 0.578 | |
| VKA | 53.85% (14/26) | 42.3% (11/26) | ||
| Antiplatelet medication | Yes | 6.9 % (2/29) | 15.38 % (4/26) | p = 0.565 |
| No | 93.1% (27/29) | 84.62% (22/26) | ||
| Aspirin | 50% (1/2) | 50% (2/4) | p = 0.386 | |
| Clopidogrel | 50% (1/2) | 50% (2/4) |
| Drug Class | Global | Female Sex | Male Sex | p Value | |
|---|---|---|---|---|---|
| Beta-blockers | 46/55 (83.63%) | 25/29 (86.2%) | 21/26 (80.7%) | p = 0.898 | |
| Beta-blocker type | Bisoprolol | 15 (32.6%) | 5 (33.3%) | 10 (66.66%) | p = 0.05 |
| Metoprolol | 26 (56.52%) | 19 (73.07%) | 7 (26.93%) | ||
| Propranolol | 2 (4.34%) | 1 (50%) | 1 (50%) | ||
| Nebivolol | 2 (4.34%) | 0 (0%) | 2 (100%) | ||
| Carvedilol | 1 (2.17%) | 1 (100%) | 0 (0%) | ||
| Non-dihydropyridinic CCBs | 0 (0%) | 0 (0%) | 0 (0%) | p = 0.979 | |
| Digoxin | 5 (9.1%) | 3 (60%) | 2 (40%) | p = 0.922 | |
| Global | Female Sex | Male Sex | p Value | ||
|---|---|---|---|---|---|
| Pharmacological cardioversion | Yes | 22 (40.0%) | 13 (44.82) | 9 (34.61) | p = 0.619 |
| No | 33 (60.0%) | 16 (55.17) | 17 (65.38) | ||
| Electrical cardioversion (EC) | Yes | 23 (41.8%) | 8 (27.58) | 15 (57.69) | p = 0.047 |
| No | 32 (58.2%) | 21 (72.41) | 11 (42.30) | ||
| Number of EC * | Median (MIN–MAX) | 0 (0–4) | 0 (0–4) | 1 (0–3) | p = 0.113 |
| Years until the first ablation * | Mean ± SD (Median) | 2.2 ± 1.55 (2) | 2.55 ± 1.84 (2) | 1.80 ± 1.05 (1.50) | p = 0.05 |
| Indication for ablation | Paroxysmal | 15 (27.3%) | 5 (17.24) | 10 (38.46) | p = 0.006 |
| Recurrent | 19 (34.5%) | 16 (55.17) | 3 (11.53) | ||
| Persistent | 20 (36.4%) | 8 (27.58) | 12 (46.15) | ||
| Permanent | 1 (1.8%) | 0 (0) | 1 (3.84) | ||
| Type of ablation | Radiofrequency | 49 (89.1%) | 26 (89.65) | 23 (88.46) | p = 0.770 |
| Cryoablation | 6 (10.9%) | 3 (10.34) | 3 (11.53) | ||
| EC during ablation | Yes | 21 (38.2%) | 11 (38.93) | 10 (38.46) | p = 0.812 |
| No | 34 (61.8%) | 18 (62.06) | 16 (61.53) | ||
| Number of electrical shocks during the ablation * | 0 | 34 (61.8%) | 18 (62.06) | 16 (61.53) | p = 0.212 |
| 1 | 17 (30.9%) | 7 (24.13) | 10 (38.46) | ||
| 2 | 2 (3.6%) | 2 (6.89) | 0 (0) | ||
| 3 | 2 (3.6%) | 2 (6.89) | 0 (0) | ||
| Complication | Yes | 5 (9.1%) | 2 (6.9%) | 3 (11.54%) | p = 0.328 |
| No | 50 (90.9%) | 27 (93.1%) | 23 (88.46%) | ||
| Femoral hematoma | 2 (40%) | 0 (0%) | 2 (7.69%) | ||
| Small pericardial effusion | 2 (40%) | 2 (6.89%) | 0 (0%) | ||
| Tamponade | 1 (20%) | 0 (0%) | 1 (3.84%) |
| Relapse of AF | Women | Men | Global | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sex | Yes | 17 (58.6%) | - | 18 (69.2%) | - | 35 (63.6%) | |||
| No | 12 (41.4%) | 8 (30.8%) | 20 (36.4%) | ||||||
| Age | Yes | 57.47 ± 10.39 | p = 0.186 | 62.22 ± 5.47 | p = 0.650 | 59.91 ± 8.46 | p = 0.517 | ||
| No | 62 ± 5.98 | 60.50 ± 9.73 | 61.40 ± 7.50 | ||||||
| LDL-cholesterol | Yes | 124.76 ± 48.46 | p = 0.984 | 97.72 ± 29.39 | p = 0.187 | 110.85 ± 41.53 | p = 0.371 | ||
| No | 125.16 ± 63.47 | 120 ± 55.02 | 123.10 ± 58.77 | ||||||
| Uric Acid | Yes | 6.32 ± 2.75 | p = 0.244 | 6.92 ± 1.60 | p = 0.104 | 6.63 ± 2.22 | p = 0.781 | ||
| No | 5.26 ± 1.64 | 8.23 ± 2.30 | 6.45 ± 2.39 | ||||||
| LAA velocity | Yes | 0.58 ± 0.15 | p = 0.738 | 0.67 ± 0.26 | p = 0.05 | 0.62 ± 0.22 | p = 0.160 | ||
| No | 0.60 ± 0.17 | 0.89 ± 0.27 | 0.72 ± 0.25 | ||||||
| AF type | Paroxysmal | Yes | 2 (11.76%) | p = 0.441 | 5 (27.77%) | p = 0.295 | 7 (20%) | p = 0.35 | |
| No | 3 (25%) | 5 (62.5%) | 8 (40%) | ||||||
| Recurrent | Yes | 11 (64.7%) | 3 (16.66%) | 14 (40%) | |||||
| No | 5 (41.66%) | 0 (0%) | 5 5(25%) | ||||||
| Persistent | Yes | 4 (23.52%) | 9 (50%) | 13 (37.14%) | |||||
| No | 4 (33.33%) | 3 (37.5) | 7 (35) | ||||||
| Permanent | Yes | 0 (0%) | 1 (5.55%) | 1 (2.85%) | |||||
| No | 0 (0%) | 0 (0%) | 0 (0%) | ||||||
| IHD type | Microvascular | Yes | 50% | p = 0.218 | 58.33% | p = 0.491 | 52.94% | p = 0.007 | |
| No | 50% | 41.67% | 47.1% | ||||||
| Macrovascular | Yes | 85.71% | 78.58% | 80.95% | |||||
| No | 14.29% | 21.42% | 19.05% | ||||||
| Thyroid pathology | With | Yes | 10 (58.82%) | p = 0.329 | 8 (44.44%) | p = 0.256 | 18 (51.42%) | p = 0.103 | |
| No | 4 (33.33%) | 1 (12.5%) | 5 (25%) | ||||||
| Without | Yes | 7 (41.17%) | 10 (55.55%) | 17 (48.57%) | |||||
| No | 8 (66.6%) | 7 (87.5%) | 15 (75%) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irimie, D.A.; Sitar-Tăut, A.V.; Caloian, B.; Frîngu, F.; Cismaru, G.; Roşu, R.; Puiu, M.; Minciună, I.A.; Simu, G.; Zdrenghea, D.; et al. Particularities of Catheter Ablation in Women with Atrial Fibrillation and Associated Ischemic Heart Disease. J. Clin. Med. 2022, 11, 5568. https://doi.org/10.3390/jcm11195568
Irimie DA, Sitar-Tăut AV, Caloian B, Frîngu F, Cismaru G, Roşu R, Puiu M, Minciună IA, Simu G, Zdrenghea D, et al. Particularities of Catheter Ablation in Women with Atrial Fibrillation and Associated Ischemic Heart Disease. Journal of Clinical Medicine. 2022; 11(19):5568. https://doi.org/10.3390/jcm11195568
Chicago/Turabian StyleIrimie, Diana Andrada, Adela Viviana Sitar-Tăut, Bogdan Caloian, Florina Frîngu, Gabriel Cismaru, Radu Roşu, Mihai Puiu, Ioan Alexandru Minciună, Gelu Simu, Dumitru Zdrenghea, and et al. 2022. "Particularities of Catheter Ablation in Women with Atrial Fibrillation and Associated Ischemic Heart Disease" Journal of Clinical Medicine 11, no. 19: 5568. https://doi.org/10.3390/jcm11195568
APA StyleIrimie, D. A., Sitar-Tăut, A. V., Caloian, B., Frîngu, F., Cismaru, G., Roşu, R., Puiu, M., Minciună, I. A., Simu, G., Zdrenghea, D., & Pop, D. (2022). Particularities of Catheter Ablation in Women with Atrial Fibrillation and Associated Ischemic Heart Disease. Journal of Clinical Medicine, 11(19), 5568. https://doi.org/10.3390/jcm11195568







