PD-L1-Positive High-Grade Triple-Negative Breast Cancer Patients Respond Better to Standard Neoadjuvant Treatment—A Retrospective Study of PD-L1 Expression in Relation to Different Clinicopathological Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
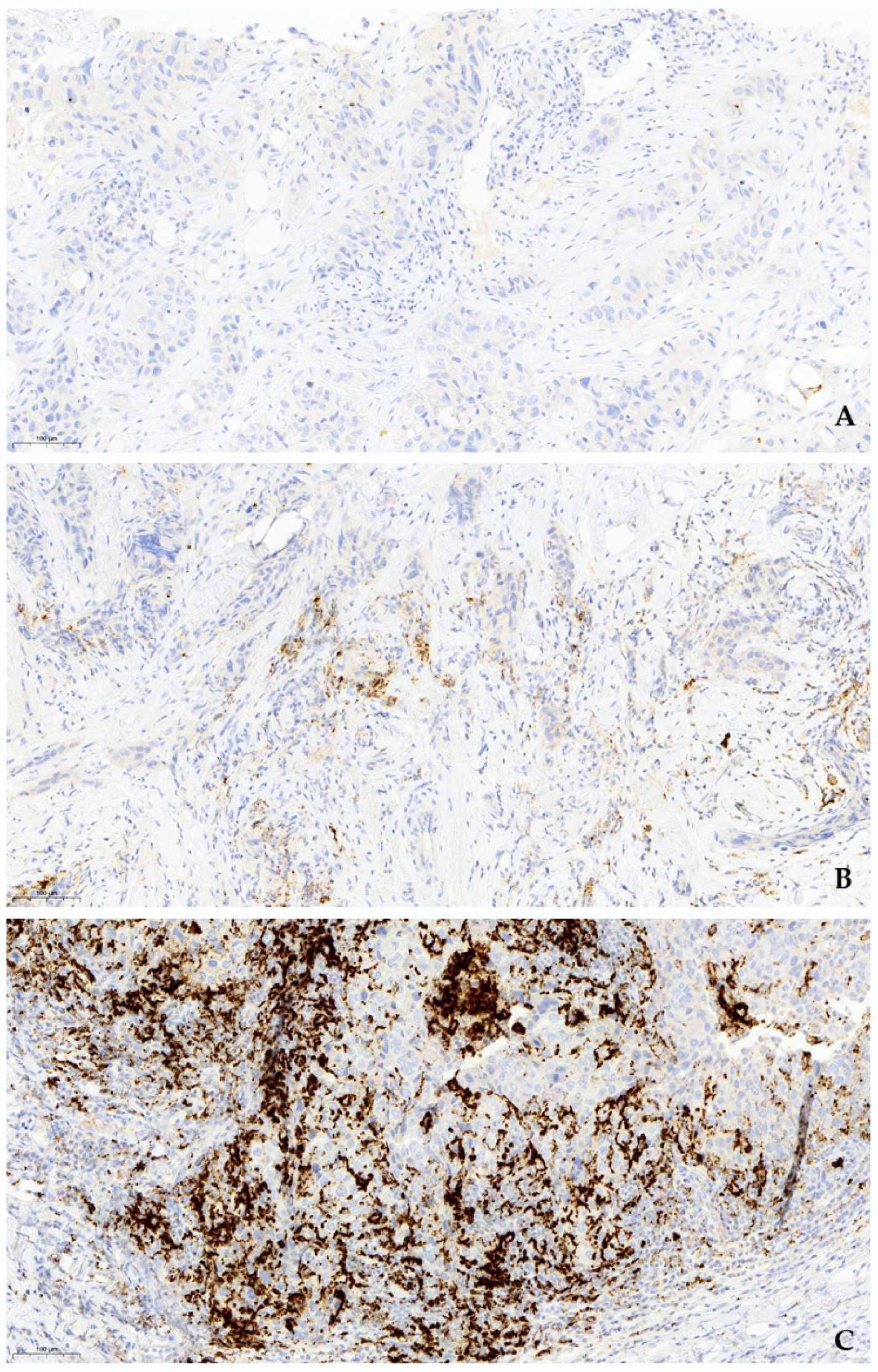
2.2. Immunohistochemical Analysis
2.3. Molecular Analysis
2.4. Pathological Response to Neoadjuvant Treatment
2.5. Statistical Analysis
3. Results
3.1. Histological Findings
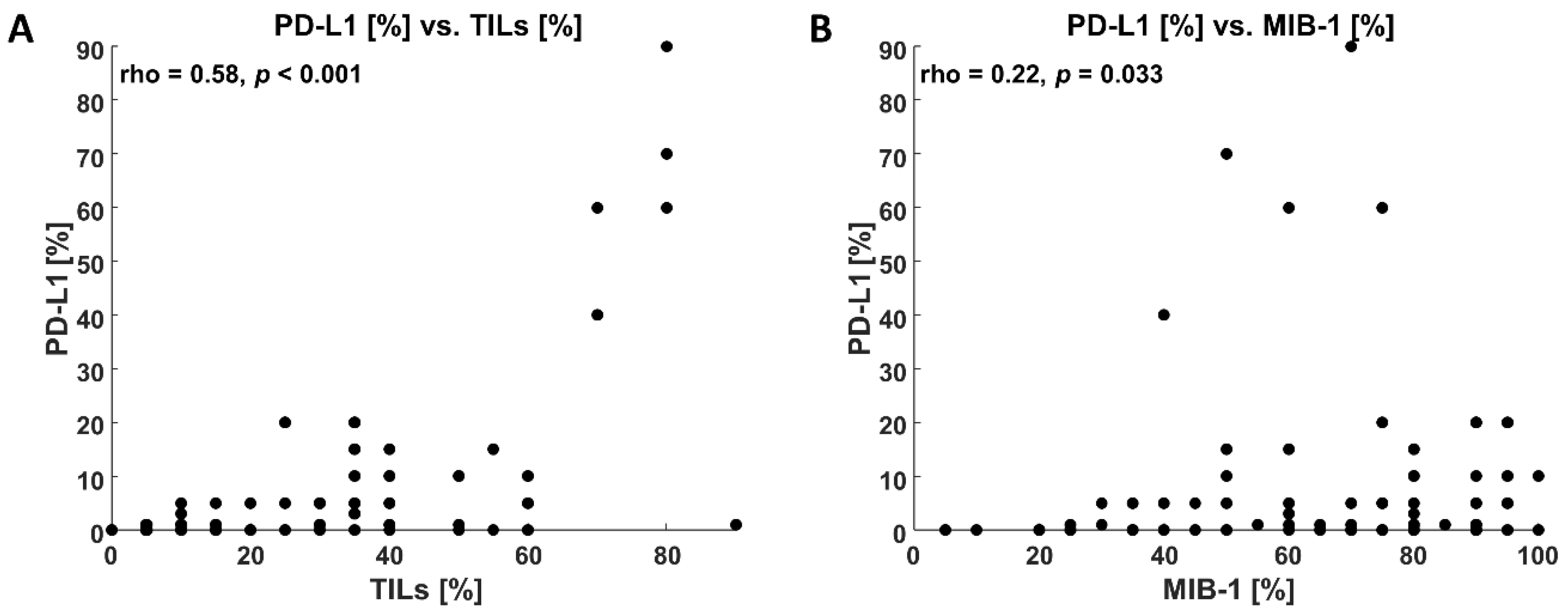
3.2. Immunohistochemical Findings
3.3. Molecular Findings
3.4. Clinicopathological Findings
3.5. Response to Neoadjuvant Treatment in Different Clinical Stage Groups with Regard to PD-L-1 Status and Chemotherapy Regimen (Standard AC-Taxol Protocol vs. Enhanced with Carboplatin or/and AC Dense-Dosed, Table 2)
| IIA | IIIA | IIIB | IIIC | |||||
|---|---|---|---|---|---|---|---|---|
| Total no of cases with nCHTH | 23 | 24 | 4 | 24 | ||||
| PD-L1+ | PD-L1- | PD-L1+ | PD-L1- | PD-L1+ | PD-L1- | PD-L1+ | PD-L1- | |
| pCR/RCB 0 | 13 | 6 | 6 | 4 | 8 | 2 | ||
| pPR/RCB I | 1 | 1 | 1 | 2 | 1 | 3 | ||
| RCB II | 1 | 3 | 4 | 1 | 1 | 1 | ||
| RCB III | 1 | 2 | 2 | 2 | 3 | 6 | ||
3.6. Response to Neoadjuvant Treatment in BRCA-Mutated Patients with Regard to PD-L-1 Status and Chemotherapy Regimen (Standard AC-Taxol Protocol vs. Enhanced with Carboplatin or AC Dense-Dosed, Figure 4)

3.7. PD-L1 Status and Clinical Outcome
3.8. Multivariable Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Sikov, W.M.; Polley, M.-Y.; Twohy, E.; Perou, C.M.; Singh, B.; Berry, D.A.; Tolaney, S.M.; Somlo, G.; Port, E.R.; Ma, H.; et al. CALGB (Alliance) 40603: Long-term outcomes (LTOs) after neoadjuvant chemotherapy (NACT) +/− carboplatin (Cb) and bevacizumab (Bev) in triple-negative breast cancer (TNBC). Cancer J. Clin. Oncol. 2022, 40, 1323–1334. [Google Scholar] [CrossRef]
- Zhang, X.; Ge, X.; Jiang, T.; Yang, R.; Li, S. Research progress on immunotherapy in triple-negative breast cancer (Review). Int. J. Oncol. 2022, 61, 95. [Google Scholar] [CrossRef]
- Aggarwal, C.; Abreu, D.R.; Felip, E.; Carcereny, E.; Gottfried, M.; Wehler, T.; Ahn, M.-J.; Dolled-Filhart, M.; Zhang, J.; Shentu, Y.; et al. Prevalence of PD-L1 expression in patients with non-small cell lung cancer screened for enrollment in KEYNOTE-001, -010, and -024. Ann. Oncol. 2016, 27 (Suppl. S6), vi363. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, H.; Zhou, Y.; Mao, F.; Lin, Y.; Pan, B.; Zhang, X.; Xu, Q.; Huang, X.; Sun, Q. Prognostic Value of PD-L1 in Breast Cancer: A Meta-Analysis. Breast J. 2017, 23, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.Z.; Sarian, L.O.; Derchain, S.F.M.; Pinto, G.A.; Vassallo, J. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum. Pathol. 2016, 47, 78–84. [Google Scholar] [CrossRef]
- Ohaegbulam, K.C.; Assal, A.; Lazar-Molnar, E.; Yao, Y.; Zang, X. Human cancer immunotherapy with antibodies to the PD-1 andPD-L1 pathway. Trends Mol. Med. 2015, 21, 24–33. [Google Scholar] [CrossRef]
- Allison, J.P. Immune checkpoint blockade in cancer therapy the 2015 Lasker-Debakey clinical medical research award. JAMA 2015, 314, 1113–1114. [Google Scholar] [CrossRef]
- Luen, S.; Virassamy, B.; Savas, P.; Salgado, R.; Loi, S. The Genomic Landscape of Breast Cancer and Its Interaction with HostImmunity. Breast 2016, 29, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-Negative Breast Cancer: Challenges and Opportunities of a Heterogeneous Disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef] [PubMed]
- Solinas, C.; Carbognin, L.; De Silva, P.; Criscitiello, C.; Lambertini, M. Tumor-Infiltrating Lymphocytes in Breast Cancer According to Tumor Subtype: Current State of the Art. Breast 2017, 35, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Agostinetto, E.; Eiger, D.; Punie, K.; de Azambuja, E. Emerging Therapeutics for Patients with Triple-Negative Breast Cancer. Curr. Oncol. Rep. 2021, 23, 57. [Google Scholar] [CrossRef] [PubMed]
- Sobral-Leite, M.; Van de Vijver, K.; Michaut, M.; van der Linden, R.; Hooijer, G.; Horlings, H.M.; Severson, T.M.; Mulligan, A.M.; Weerasooriya, N.; Sanders, J.; et al. Assessment of PD-L1 expression across breast cancer molecular subtypes, in relation to mutation rate, BRCA1-like status, tumor-infiltrating immune cells and survival. Oncoimmunology 2018, 7, e1509820. [Google Scholar] [CrossRef]
- Glodzik, D.; Bosch, A.; Hartman, J.; Aine, M.; Vallon-Christersson, J.; Reuterswärd, C.; Karlsson, A.; Mitra, S.; Niméus, E.; Holm, K.; et al. Comprehensive molecular comparison of BRCA1 hypermethylated and BRCA1 mutated triple negative breast cancers. Nat. Commun. 2020, 11, 3747. [Google Scholar] [CrossRef]
- Parvathareddy, S.K.; Siraj, A.K.; Ahmed, S.O.; Ghazwani, L.O.; Aldughaither, S.M.; Al-Dayel, F.; Tulbah, A.; Ajarim, D.; Al-Kuraya, K.S. PD-L1 Protein Expression in Middle Eastern Breast Cancer Predicts Favorable Outcome in Triple-Negative Breast Cancer. Cells 2021, 10, 229. [Google Scholar] [CrossRef]
- Sabatier, R.; Finetti, P.; Mamessier, E.; Adelaide, J.; Chaffanet, M.; Ali, H.R.; Viens, P.; Caldas, C.; Birnbaum, D.; Bertucci, F. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 2015, 6, 5449–5464. [Google Scholar] [CrossRef]
- Barrett, M.T.; Lenkiewicz, E.; Malasi, S.; Basu, A.; Yearley, J.H.; Annamalai, L.; McCullough, A.E.; Kosiorek, H.E.; Narang, P.; Wilson Sayres, M.A.; et al. The Association of Genomic Lesions and PD-1/PD-L1 Expression in Resected Triple-Negative Breast Cancers. Breast Cancer Res. 2018, 20, 71. [Google Scholar] [CrossRef]
- Botti, G.; Collina, F.; Scognamiglio, G.; Rao, F.; Peluso, V.; De Cecio, R.; Piezzo, M.; Landi, G.; De Laurentiis, M.; Cantile, M.; et al. Programmed Death Ligand 1 (PD-L1) Tumor Expression Is Associated With a Better Prognosis and Diabetic Disease in Triple Negative Breast Cancer Patients. Int. J. Mol. Sci. 2017, 18, 459. [Google Scholar] [CrossRef]
- Lotfinejad, P.; Jafarabadi, M.A.; Shadbad, M.A.; Kazemi, T.; Pashazadeh, F.; Shotorbani, S.S.; Niaragh, F.J.; Baghbanzadeh, A.; Vahed, N.; Silvestris, N.; et al. Prognostic Role and Clinical Significance of Tumor-Infiltrating Lymphocyte (TIL) and Programmed Death Ligand 1 (PD-L1) Expression in Triple-Negative Breast Cancer (TNBC): A Systematic Review and Meta-Analysis Study. Diagnostics 2020, 10, 704. [Google Scholar] [CrossRef] [PubMed]
- Uchimiak, K.; Badowska-Kozakiewicz, A.M.; Sobiborowicz-Sadowska, A.; Deptała, A. Current State of Knowledge on the Immune Checkpoint Inhibitors in Triple-Negative Breast Cancer Treatment: Approaches, Efficacy, and Challenges. Clin. Med. Insights Oncol. 2022, 16, 11795549221099869. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Nanda, R.; Liu, M.C.; Yau, C.; Shatsky, R.; Pusztai, L.; Wallace, A.; Chien, A.J.; Forero-Torres, A.; Ellis, E.; Han, H.; et al. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women with Early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial. JAMA 2020, 6, 676–684. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Dieci, M.V.; Radosevic-Robin, N.; Fineberg, S.; van den Eynden, G.; Ternes, N.; Penault-Llorca, F.; Pruneri, G.; D’alfonso, T.M.; Demaria, S.; Castaneda, C.; et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: A report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin. Cancer Biol. 2018, 52, 16–25. [Google Scholar]
- VENTANA PD-L1 (SP142) Assay Interpretation Guide for Triple Negative Breast Carcinoma. Available online: https://diagnostics.roche.com/content/dam/diagnostics/us/en/products/v/ventana-pd-l1-sp142-assay/Ventana-PD-L1-SP142-PI-1019497USa.pdf (accessed on 1 May 2020).
- Badve, S.S.; Penault-Llorca, F.; Reis-Filho, J.S.; Deurloo, R.; Siziopikou, K.P.; D’Arrigo, C.; Viale, G. Determining PD-L1 Status in Patients With Triple-Negative Breast Cancer: Lessons Learned From IMpassion130. J. Natl. Cancer Inst. 2022, 114, 664–675. [Google Scholar] [CrossRef]
- Pinder, S.E.; Provenzano, E.; Earl, H.; Ellis, I.O. Laboratory handling and histology reporting of breast specimens from patients who have received neoadjuvant chemotherapy. Histopathology 2007, 50, 409–417. [Google Scholar] [CrossRef]
- Residual Cancer Burden Calculator. Available online: http://www3.mdanderson.org/app/medcalc/index.cfm?pagename=jsconvert3 (accessed on 4 September 2022).
- Gonzalez-Ericsson, P.I.; Stovgaard, E.S.; Sua, L.F.; Reisenbichler, E.; Kos, Z.; Carter, J.M.; Michiels, S.; le Quesne, J.; Nielsen, T.O.; Laenkholm, A.-V.; et al. The path to a better biomarker: Application of a risk management framework for the implementation of PD-L1 and TILs as immuno-oncology biomarkers in breast cancer clinical trials and daily practice. J. Pathol. 2020, 250, 667–684. [Google Scholar] [CrossRef]
- Huang, R.; Haberberger, J.; Severson, E.; Duncan, D.L.; Hemmerich, A.; Edgerly, C.; Ferguson, N.L.; Williams, E.; Elvin, J.; Vergilio, J.A.; et al. A pan-cancer analysis of PD-L1 immunohistochemistry and gene amplification, tumor mutation burden and microsatellite instability in 48,782 cases. Mod. Pathol. 2021, 34, 252–263. [Google Scholar] [CrossRef]
- List of Cleared or Approved Companion Diagnostic Devices (In Vitro and Imaging Tools). Available online: https://www.fda.gov/medical-devices/in-vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-in-vitro-and-imaging-tools (accessed on 4 September 2022).
- Guo, H.; Ding, Q.; Gong, Y.; Gilcrease, M.Z.; Zhao, M.; Zhao, J.; Sui, D.; Wu, Y.; Chen, H.; Liu, H.; et al. Comparison of three scoring methods using the FDA-approved 22C3 immunohistochemistry assay to evaluate PD-L1 expression in breast cancer and their association with clinicopathologic factors. Breast Cancer Res. 2020, 22, 69. [Google Scholar] [CrossRef] [PubMed]
- Reisenbichler, E.S.; Han, G.; Bellizzi, A.; Bossuyt, V.; Brock, J.; Cole, K.; Fadare, O.; Hameed, O.; Hanley, K.; Harrison, B.T.; et al. Prospective multi-institutional evaluation of pathologist assessment of PD-L1 assays for patient selection in triple negative breast cancer. Mod. Pathol. 2020, 33, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Loi, S.; Adams, S.; Schmid, P.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Dieras, V.C.; Winer, E.P.; Kockx, M.; et al. Performance of PD-L1 immunohistochemistry (IHC) assays in unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC): Post-hoc analysis of IMpassion130. Ann. Oncol. 2019, 30, v858–v859. [Google Scholar] [CrossRef]
- Tsao, M.S.; Kerr, K.M.; Kockx, M.; Beasley, M.-B.; Borczuk, A.C.; Botling, J.; Bubendorf, L.; Chirieac, L.; Chen, G.; Chou, T.-Y.; et al. PD-L1 Immunohistochemistry Comparability Study in Real-Life Clinical Samples: Results of Blueprint Phase 2 Project. J. Thorac. Oncol. 2018, 13, 1302–1311. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Milne, K.; Derocher, H.; Webb, J.R.; Nelson, B.H.; Watson, P.H. PD-L1 and intratumoral immune response in breast cancer. Oncotarget 2017, 8, 51641–51651. [Google Scholar] [CrossRef]
- Freire, R.; Gomez-Fernandez, C.; Salehiazar Sara Jorda, M.; Millan, N.; Akgun, Y.; Nadji, M. The Importance of Histologic Subtypes of Triple Negative Breast Cancers on Immune Cell Density and PD-L1 Expression. Mod. Pathol. 2020, 33, 240–443. [Google Scholar]
- Peters, J.; Bleiweiss, I.; Zhang, P.; Feldman, M.; Dumoff, K.; Nayak, A. Early Results of PD-L1 Staining and Atezolizumab Treatment in Patients with Metastatic Triple Negative Breast Carcinoma. Mod. Pathol. 2020, 33, 240–443. [Google Scholar]
- Li, Y.; Chang, C.-W.; Tran, D.; Denker, M.; Hegde, P.; Molinero, L. Abstract PD6-01: Prevalence of PDL1 and tumor infiltrating lymphocytes (TILs) in primary and metastatic TNBC. Cancer Res. 2018, 78 (Suppl. S4), PD6-01. [Google Scholar] [CrossRef]
- Wimberly, H.; Brown, J.R.; Schalper, K.; Haack, H.; Silver, M.R.; Nixon, C.; Bossuyt, V.; Pusztai, L.; Lannin, D.R.; Rimm, D.L. PD-L1 Expression Correlates with Tumor-Infiltrating Lymphocytes and Response to Neoadjuvant Chemotherapy in Breast Cancer. Cancer Immunol. Res. 2015, 3, 326–332. [Google Scholar] [CrossRef]
- Gianni, L.; Huang, C.S.; Egle, D.; Bermejo, B.; Zamagni, C.; Thill, M.; Anton, A.; Zambelli, S.; Bianchini, G.; Russo, S.; et al. Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple-negative, early high-risk and locally advanced breast cancer: NeoTRIP Michelangelo randomized study. Ann. Oncol. 2022, 33, 534–543. [Google Scholar] [CrossRef]
- Oner, G.; Önder, S.; Karatay, H.; Ak, N.; Tükenmez, M.; Müslümanoğlu, M.; İğci, A.; Dincçağ, A.; Özmen, V.; Aydiner, A.; et al. Clinical impact of PD-L1 expression in triple-negative breast cancer patients with residual tumor burden after neoadjuvant chemotherapy. World J. Surg. Oncol. 2021, 19, 264. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Campeau, P.M.; Foulkes, W.D.; Tischkowitz, M.D. Hereditary breast cancer: New genetic developments, new therapeutic avenues. Hum. Genet. 2008, 124, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Al-Khadairi, G.; Decock, J. Immune Checkpoint Inhibitors in Triple Negative Breast Cancer Treatment: Promising Future Prospects. Front. Oncol. 2021, 10, 600573. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Salgado, R.; Park, Y.H.; Muñoz-Couselo, E.; Kim, S.B.; Sohn, J.; Im, S.-A.; Foukakis, T.; Kuemmel, S.; Dent, R.; et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: Results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann. Oncol. 2020, 31, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Nowecki, I.Z.; Jagiello-Gruszweld, A.; Pogoda, K.; Niwinska, A.; Olszewski, P.W.; Winter, P.; Matkowski, R.; Wysocki, M.W. Neoadjuvant therapy for breast cancer patients and its impact on surgical treatment and radiotherapy (part 1.). Nowotwory. J. Oncol. 2021, 71, 17–25. [Google Scholar]
- Marra, A.; Viale, G.; Curigliano, G. Recent advances in triple negative breast cancer: The immunotherapy era. BMC Med. 2019, 17, 90. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef]
- Dirix, L.Y.; Takacs, I.; Jerusalem, G.; Nikolinakos, P.; Arkenau, H.T.; Forero-Torres, A.; Boccia, R.; Lippman, M.E.; Somer, R.; Smakal, M.; et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase 1b JAVELIN Solid Tumor study. Breast Cancer Res. Treat. 2018, 167, 671–686. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Keenan, T.E.; Pernas, S.; Exman, P.; Jain, E.; Garrido-Castro, A.C.; Hughes, M.; Bychkovsky, B.; Umeton, R.; Files, J.L.; et al. Tumor Mutational Burden and PTEN Alterations as Molecular Correlates of Response to PD-1/L1 Blockade in Metastatic Triple-Negative Breast Cancer. Clin. Cancer Res. 2020, 26, 2565–2572. [Google Scholar] [CrossRef]

| Overall (n = 93) | PD-L1+ (n = 53) | PD-L1− (n = 40) |
|---|---|---|
| Age, year | 54.0 (32.0–81.0) | 61.5 (32.0–80.0) |
| Stage | ||
| IIA (n = 30) | 17 (32.1%) | 13 (32.5%) |
| IIIA (n = 28) | 16 (30.2%) | 12 (30.0%) |
| IIIB (n = 4) | 1 (1.9%) | 3 (7.5%) |
| IIIC (n = 27) | 17 (32.1%) | 10 (25.0%) |
| IV (n = 4) | 2 (3.8%) | 2 (5.0%) |
| MIB-1, (%) | 80.0 (25.0–100.0) | 65.0 (5.0–100.0) |
| MIB-1 ≥ 40 (n = 76), no/yes (%) | 4/49 (7.5/92.5) | 13/27 (32.5/67.5) |
| BRCAness (n = 22), no/yes (%) | 20/15 (57.1/42.9) | 13/7 (65.0/35.0) |
| TILs, % | 30.0 (5.0–90.0] | 15.0 [0.0–60.0] |
| TILs > 30% (n = 42), no/yes (%) | 18/35 (34.0/66.0) | 33/7 (82.5/17.5) |
| Neoadjuvant (n = 75) no/yes (%) | 10/43 (18.9/81.1) | 8/32 (20.0/80.0) |
| Neoadjuvant+Carbo (n = 23), no/yes (%) | 32/14 (69.6/30.4) | 26/9 (74.3/25.7) |
| Neoadjuvant+ddAC (n = 15), no/yes (%) | 32/11 (74.4/25.6) | 28/4 (87.5/12.5) |
| pCR (n = 39), no/yes (%) | 16/27 (37.2/62.8) | 20/12 (62.5/37.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanowska, O.; Kuczkiewicz-Siemion, O.; Dębowska, M.; Olszewski, W.P.; Jagiełło-Gruszfeld, A.; Tysarowski, A.; Prochorec-Sobieszek, M. PD-L1-Positive High-Grade Triple-Negative Breast Cancer Patients Respond Better to Standard Neoadjuvant Treatment—A Retrospective Study of PD-L1 Expression in Relation to Different Clinicopathological Parameters. J. Clin. Med. 2022, 11, 5524. https://doi.org/10.3390/jcm11195524
Stanowska O, Kuczkiewicz-Siemion O, Dębowska M, Olszewski WP, Jagiełło-Gruszfeld A, Tysarowski A, Prochorec-Sobieszek M. PD-L1-Positive High-Grade Triple-Negative Breast Cancer Patients Respond Better to Standard Neoadjuvant Treatment—A Retrospective Study of PD-L1 Expression in Relation to Different Clinicopathological Parameters. Journal of Clinical Medicine. 2022; 11(19):5524. https://doi.org/10.3390/jcm11195524
Chicago/Turabian StyleStanowska, Olga, Olga Kuczkiewicz-Siemion, Małgorzata Dębowska, Wojciech P. Olszewski, Agnieszka Jagiełło-Gruszfeld, Andrzej Tysarowski, and Monika Prochorec-Sobieszek. 2022. "PD-L1-Positive High-Grade Triple-Negative Breast Cancer Patients Respond Better to Standard Neoadjuvant Treatment—A Retrospective Study of PD-L1 Expression in Relation to Different Clinicopathological Parameters" Journal of Clinical Medicine 11, no. 19: 5524. https://doi.org/10.3390/jcm11195524
APA StyleStanowska, O., Kuczkiewicz-Siemion, O., Dębowska, M., Olszewski, W. P., Jagiełło-Gruszfeld, A., Tysarowski, A., & Prochorec-Sobieszek, M. (2022). PD-L1-Positive High-Grade Triple-Negative Breast Cancer Patients Respond Better to Standard Neoadjuvant Treatment—A Retrospective Study of PD-L1 Expression in Relation to Different Clinicopathological Parameters. Journal of Clinical Medicine, 11(19), 5524. https://doi.org/10.3390/jcm11195524






