High Uric Acid Levels in Acute Myocardial Infarction Provide Better Long-Term Prognosis Predictive Power When Combined with Traditional Risk Factors
Abstract
1. Introduction
2. Materials and Methods
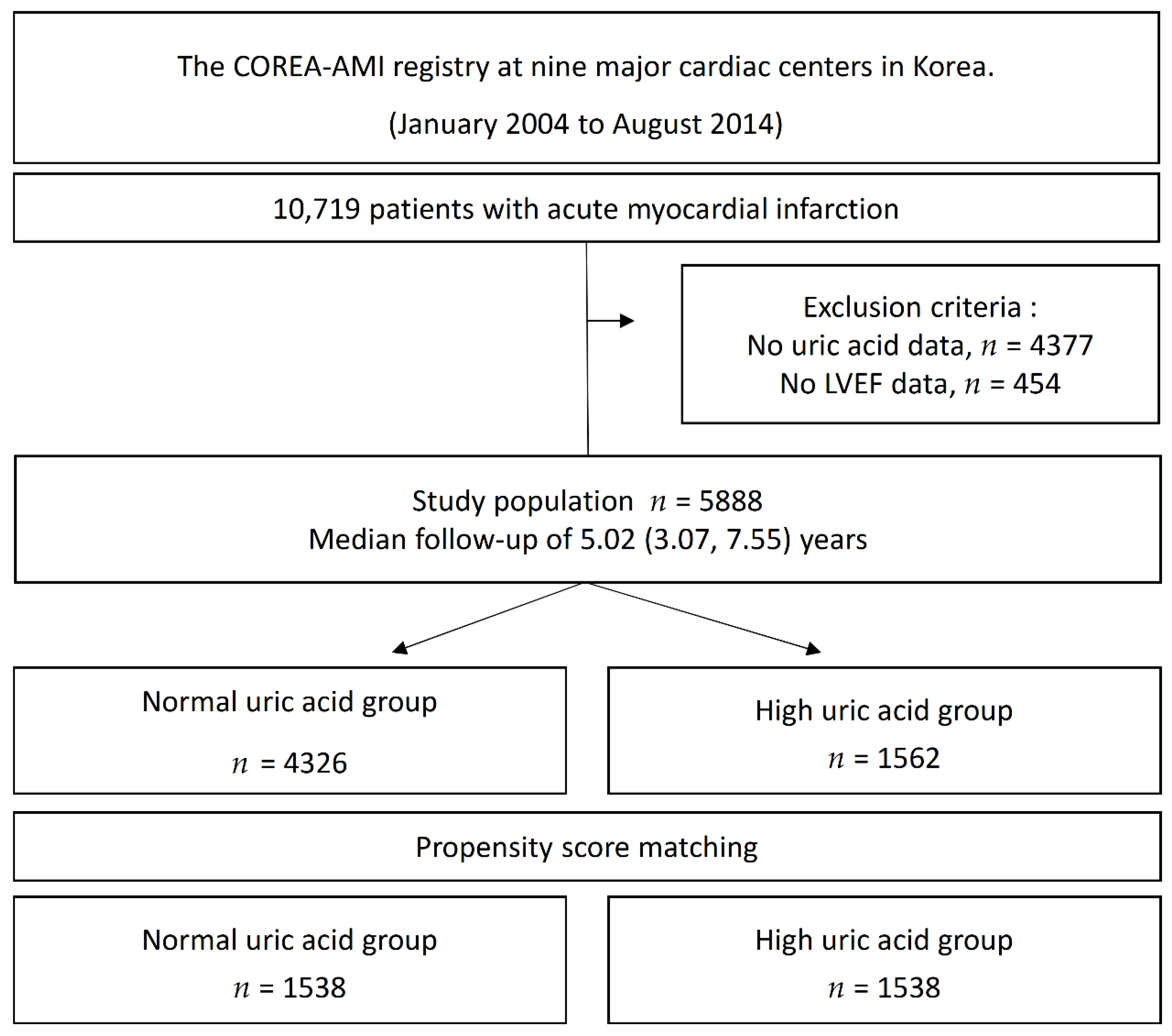
2.1. Study Protocols and Population Selection
2.2. Treatment and Data Collection
2.3. Study Endpoints and Definition
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Primary Outcomes
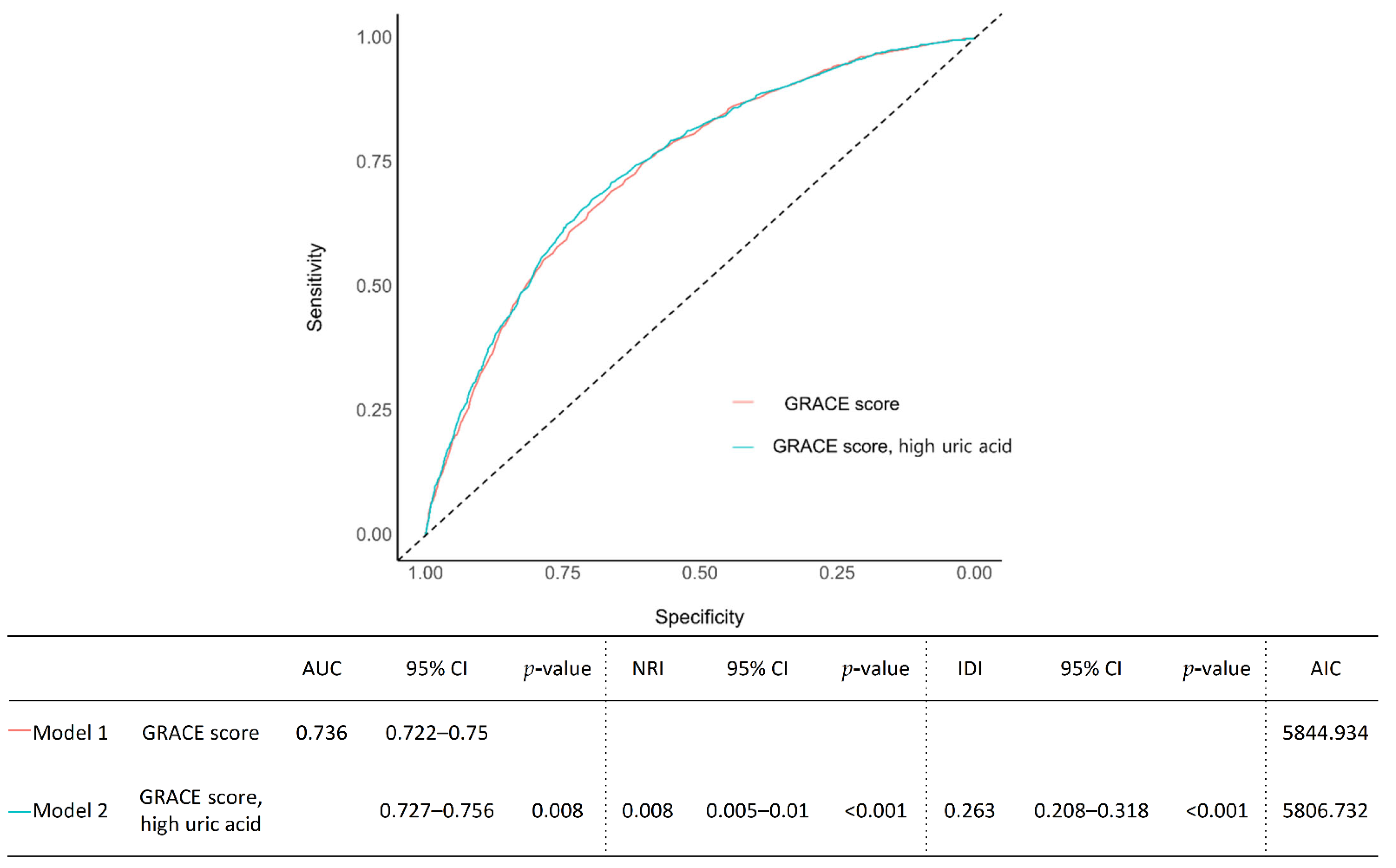
3.3. Risk Prediction, Discrimination, and Reclassification
4. Discussion
4.1. Pathophysiology: Uric Acid and the Development of Cardiovascular Diseases
4.2. Limited Evidence Regarding Hyperuricemia as a Cardiovascular Risk Factor
4.3. The Optimal Cutoff Value of Uric Acid Level in AMI Population
4.4. Predictive Value of the Hyperuricemia in Patients with AMI
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kannel, W.B.; Castelli, W.P.; McNamara, P.M. The coronary profile: 12-year follow-up in the Framingham study. J. Occup. Med. 1967, 9, 611–619. [Google Scholar] [PubMed]
- Cannon, P.J.; Stason, W.B.; Demartini, F.E.; Sommers, S.C.; Laragh, J.H. Hyperuricemia in primary and renal hypertension. N. Engl. J. Med. 1966, 275, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Segal, M.; Afsar, B.; Kang, D.H.; Rodriguez-Iturbe, B.; Johnson, R.J. The role of uric acid in the pathogenesis of human cardiovascular disease. Heart 2013, 99, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Glantzounis, G.K.; Tsimoyiannis, E.C.; Kappas, A.M.; Galaris, D.A. Uric acid and oxidative stress. Curr. Pharm. Des. 2005, 11, 4145–4151. [Google Scholar] [CrossRef] [PubMed]
- Timóteo, A.T.; Lousinha, A.; Labandeiro, J.; Miranda, F.; Papoila, A.L.; Oliveira, J.A.; Ferreira, M.L.; Ferreira, R.C. Serum uric acid: A forgotten prognostic marker in acute coronary syndromes? Eur. Heart J. Acute Cardiovasc. Care 2013, 2, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Nakahashi, T.; Sakata, K.; Masuda, J.; Kumagai, N.; Higuma, T.; Ogimoto, A.; Tanigawa, T.; Hanada, H.; Nakamura, M.; Takamura, M.; et al. Impact of hyperuricemia on coronary blood flow and in-hospital mortality in patients with acute myocardial infarction undergoing percutaneous coronary intervention. J. Cardiol. 2022, 80, 268–274. [Google Scholar] [CrossRef]
- Shen, S.; He, F.; Cheng, C.; Xu, B.; Sheng, J. Uric acid aggravates myocardial ischemia-reperfusion injury via ROS/NLRP3 pyroptosis pathway. Biomed. Pharmacother. 2021, 133, 110990. [Google Scholar] [CrossRef] [PubMed]
- Braga, F.; Pasqualetti, S.; Ferraro, S.; Panteghini, M. Hyperuricemia as risk factor for coronary heart disease incidence and mortality in the general population: A systematic review and meta-analysis. Clin. Chem. Lab. Med. 2016, 54, 7–15. [Google Scholar] [CrossRef]
- Li, M.; Hu, X.; Fan, Y.; Li, K.; Zhang, X.; Hou, W.; Tang, Z. Hyperuricemia and the risk for coronary heart disease morbidity and mortality a systematic review and dose-response meta-analysis. Sci. Rep. 2016, 6, 19520. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Guevara, J.P.; Kim, K.M.; Choi, H.K.; Heitjan, D.F.; Albert, D.A. Hyperuricemia and coronary heart disease: A systematic review and meta-analysis. Arthritis Care Res. 2010, 62, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Gazi, E.; Temiz, A.; Altun, B.; Barutçu, A.; Bekler, A.; Güngör, O.; Yener, A.U.; Kurt, T.; Ozcan, S.; Gazi, S. The association between serum uric acid level and heart failure and mortality in the early period of ST-elevation acute myocardial infarction. Turk. Soc. Cardiol. 2014, 42, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Levantesi, G.; Marfisi, R.M.; Franzosi, M.G.; Maggioni, A.P.; Nicolosi, G.L.; Schweiger, C.; Silletta, M.G.; Tavazzi, L.; Tognoni, G.; Marchioli, R. Uric acid: A cardiovascular risk factor in patients with recent myocardial infarction. Int. J. Cardiol. 2013, 167, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Feig, D.I.; Kang, D.H.; Johnson, R.J. Uric acid and cardiovascular risk. N. Engl. J. Med. 2008, 359, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Culleton, B.F.; Larson, M.G.; Kannel, W.B.; Levy, D. Serum uric acid and risk for cardiovascular disease and death: The Framingham Heart Study. Ann. Intern. Med. 1999, 131, 7–13. [Google Scholar] [CrossRef]
- Panero, F.; Gruden, G.; Perotto, M.; Fornengo, P.; Barutta, F.; Greco, E.; Runzo, C.; Ghezzo, G.; Cavallo-Perin, P.; Bruno, G. Uric acid is not an independent predictor of cardiovascular mortality in type 2 diabetes: A population-based study. Atherosclerosis 2012, 221, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Antman, E.M.; Armstrong, P.W.; Green, L.A.; Halasyamani, L.K.; Hochman, J.S.; Krumholz, H.M.; Lamas, G.A.; Smith, S.C.; Hand, M.; Bates, E.R.; et al. 2007 Focused Update of the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2008, 51, 210–247. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.C.; Wang, C.C.; Kao, T.W.; Chan, J.Y.; Yang, Y.H.; Chang, Y.W.; Chen, W.L. Relationship between hyperuricemia and lipid profiles in US adults. Biomed. Res. Int. 2015, 2015, 127596. [Google Scholar] [CrossRef]
- Lehto, S.; Niskanen, L.; Rönnemaa, T.; Laakso, M. Serum uric acid is a strong predictor of stroke in patients with non-insulin-dependent diabetes mellitus. Stroke 1998, 29, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Li, C.; Cook, S.; Choi, H.K. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation 2007, 115, 2526–2532. [Google Scholar] [CrossRef]
- Siu, Y.P.; Leung, K.T.; Tong, M.K.; Kwan, T.H. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am. J. Kidney Dis. 2006, 47, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Talaat, K.M.; el-Sheikh, A.R. The effect of mild hyperuricemia on urinary transforming growth factor beta and the progression of chronic kidney disease. Am. J. Nephrol. 2007, 27, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G. Serum uric acid is not an independent risk factor for coronary heart disease. Curr. Hypertens. Rep. 2001, 3, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Liao, P.C.; Wu, Y.W.; Ke, S.R. Hyperuricemia as an Outcome Predictor in Patients with ST-Segment Elevation Myocardial Infarction: Too Good to be True? Acta Cardiol. Sin. 2015, 31, 87–88. [Google Scholar] [CrossRef]
- Lazzeri, C.; Valente, S.; Chiostri, M.; Spini, V.; Angelotti, P.; Gensini, G.F. Uric acid and mild renal impairment in patients with ST-elevation myocardial infarction. Scand. Cardiovasc. J. 2015, 49, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Borghi, C.; Bentivenga, C.; Cosentino, E.R. Uric acid and risk of myocardial infarction. A dynamic duo. Int. J. Cardiol. 2020, 320, 23–24. [Google Scholar] [CrossRef]
- Lopez-Pineda, A.; Cordero, A.; Carratala-Munuera, C.; Orozco-Beltran, D.; Quesada, J.A.; Bertomeu-Gonzalez, V.; Gil-Guillen, V.F.; Bertomeu-Martinez, V. Hyperuricemia as a prognostic factor after acute coronary syndrome. Atherosclerosis 2018, 269, 229–235. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Epidemiology 2007, 18, 805–835. [Google Scholar] [CrossRef]
- Granger, C.B.; Goldberg, R.J.; Dabbous, O.; Pieper, K.S.; Eagle, K.A.; Cannon, C.P.; Van De Werf, F.; Avezum, A.; Goodman, S.G.; Flather, M.D.; et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch. Intern. Med. 2003, 163, 2345–2353. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Short, R.A.; Johnson, R.J. Sex differences in uric acid and risk factors for coronary artery disease. Am. J. Cardiol. 2001, 87, 1411–1414. [Google Scholar] [CrossRef]
- Schretlen, D.J.; Inscore, A.B.; Vannorsdall, T.D.; Kraut, M.; Pearlson, G.D.; Gordon, B.; Jinnah, H.A. Serum uric acid and brain ischemia in normal elderly adults. Neurology 2007, 69, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Bodnar, L.M.; Lain, K.Y.; Hubel, C.A.; Markovic, N.; Ness, R.B.; Powers, R.W. Uric acid is as important as proteinuria in identifying fetal risk in women with gestational hypertension. Hypertension 2005, 46, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Bos, M.J.; Koudstaal, P.J.; Hofman, A.; Witteman, J.C.; Breteler, M.M. Uric acid is a risk factor for myocardial infarction and stroke: The Rotterdam study. Stroke 2006, 37, 1503–1507. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Sakamoto, T.; Ishihara, M.; Kimura, K.; Miyazaki, S.; Yamagishi, M.; Tei, C.; Hiraoka, H.; Sonoda, M.; Tsuchihashi, K.; et al. Prognostic usefulness of serum uric acid after acute myocardial infarction (the Japanese Acute Coronary Syndrome Study). Am. J. Cardiol. 2005, 96, 489–495. [Google Scholar] [CrossRef]
- Guo, W.; Yang, D.; Wu, D.; Liu, H.; Chen, S.; Liu, J.; Lei, L.; Liu, Y.; Rao, L.; Zhang, L. Hyperuricemia and long-term mortality in patients with acute myocardial infarction undergoing percutaneous coronary intervention. Ann. Transl. Med. 2019, 7, 636. [Google Scholar] [CrossRef]
- Mohammed, A.Q.; Abdu, F.A.; Liu, L.; Zhang, W.; Yin, G.; Xu, Y.; Che, W. Hyperuricemia Predicts Adverse Outcomes After Myocardial Infarction with Non-obstructive Coronary Arteries. Front. Med. 2021, 8, 716840. [Google Scholar] [CrossRef]
- Kaya, M.G.; Uyarel, H.; Akpek, M.; Kalay, N.; Ergelen, M.; Ayhan, E.; Isik, T.; Cicek, G.; Elcik, D.; Sahin, Ö.; et al. Prognostic Value of Uric Acid in Patients With ST-Elevated Myocardial Infarction Undergoing Primary Coronary Intervention. Am. J. Cardiol. 2012, 109, 486–491. [Google Scholar] [CrossRef]
- Zoppini, G.; Targher, G.; Negri, C.; Stoico, V.; Perrone, F.; Muggeo, M.; Bonora, E. Elevated Serum Uric Acid Concentrations Independently Predict Cardiovascular Mortality in Type 2 Diabetic Patients. Diabetes Care 2009, 32, 1716–1720. [Google Scholar] [CrossRef]
- Bardin, T.; Richette, P. Definition of hyperuricemia and gouty conditions. Curr. Opin. Rheumatol. 2014, 26, 186–191. [Google Scholar] [CrossRef]
- Virdis, A.; Masi, S.; Casiglia, E.; Tikhonoff, V.; Cicero, A.F.G.; Ungar, A.; Rivasi, G.; Salvetti, M.; Barbagallo, C.M.; Bombelli, M.; et al. Identification of the Uric Acid Thresholds Predicting an Increased Total and Cardiovascular Mortality Over 20 Years. Hypertension 2020, 75, 302–308. [Google Scholar] [CrossRef]
- Zuo, T.; Liu, X.; Jiang, L.; Mao, S.; Yin, X.; Guo, L. Hyperuricemia and coronary heart disease mortality: A meta-analysis of prospective cohort studies. BMC Cardiovasc. Disord. 2016, 16, 207. [Google Scholar] [CrossRef] [PubMed]
- Pencina, M.J.; D’Agostino, R.B., Sr.; D’Agostino, R.B., Jr.; Vasan, R.S. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat. Med. 2008, 27, 157–172. [Google Scholar] [CrossRef] [PubMed]
| Before PS-Matching | After PS-Matching | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n = 5888) | Normal Uric Acid (n = 4326) | High Uric Acid (n = 1562) | p-Value | Absolute SMD | Normal Uric Acid (n = 1538) | High Uric Acid (n = 1538) | p-Value | Absolute SMD | |
| Clinical characteristics | |||||||||
| Age, years | 64.0 ± 12.7 | 63.6 ± 12.4 | 65.4 ± 13.3 | <0.001 | 0.141 | 65.0 ± 12.4 | 65.3 ± 13.3 | 0.487 | 0.025 |
| Age ≥65 year | 3010 (51.1) | 2119 (49.0) | 891 (57.0) | <0.001 | 0.162 | 844 (54.9) | 876 (57.0) | 0.26 | 0.042 |
| Male | 4141 (70.3) | 3085 (71.3) | 1056 (67.6) | 0.007 | 0.081 | 1049 (68.2) | 1039 (67.6) | 0.728 | 0.014 |
| BMI, kg/m2 | 24.1 ± 3.2 | 24.0 ± 3.2 | 24.3 ± 3.4 | 0.022 | 0.069 | 24.3 ± 3.3 | 24.3 ± 3.4 | 0.713 | 0.013 |
| BMI >25 kg/m2 | 2069 (35.1) | 1485 (34.3) | 584 (37.4) | 0.032 | 0.064 | 588 (38.2) | 576 (37.5) | 0.683 | 0.016 |
| DM | 2232 (37.9) | 1584 (36.6) | 648 (41.5) | 0.001 | 0.1 | 630 (41.0) | 634 (41.2) | 0.912 | 0.005 |
| Hypertension | 4182 (71.0) | 3008 (69.5) | 1174 (75.2) | <0.001 | 0.126 | 1151 (74.8) | 1151 (74.8) | 1 | <0.001 |
| History of dyslipidemia | 1371 (23.3) | 1013 (23.4) | 358 (22.9) | 0.716 | 0.012 | 362 (23.5) | 351 (22.8) | 0.669 | 0.017 |
| History of Stroke | 472 (8.0) | 326 (7.5) | 146 (9.3) | 0.027 | 0.065 | 145 (9.4) | 143 (9.3) | 0.951 | 0.004 |
| Current smoker | 2280 (38.7) | 1724 (39.9) | 556 (35.6) | 0.003 | 0.088 | 569 (37.0) | 551 (35.8) | 0.524 | 0.024 |
| Previous MI | 259 (4.4) | 187 (4.3) | 72 (4.6) | 0.688 | 0.014 | 68 (4.4) | 69 (4.5) | 1 | 0.003 |
| Previous PCI | 456 (7.7) | 327 (7.6) | 129 (8.3) | 0.406 | 0.026 | 129 (8.4) | 127 (8.3) | 0.948 | 0.005 |
| Previous CABG | 32 (0.5) | 22 (0.5) | 10 (0.6) | 0.685 | 0.017 | 10 (0.7) | 10 (0.7) | 1 | <0.001 |
| Atrial fibrillation on baseline ECG | 224 (3.8) | 144 (3.3) | 80 (5.1) | 0.002 | 0.089 | 85 (5.5) | 78 (5.1) | 0.629 | 0.02 |
| eGFR <30 | 412 (7.0) | 183 (4.2) | 229 (14.7) | <0.001 | 0.362 | 181 (11.8) | 214 (13.9) | 0.085 | 0.064 |
| Chronic liver disease | 59 (1.0) | 43 (1.0) | 16 (1.0) | 1 | 0.003 | 18 (1.2) | 16 (1.0) | 0.863 | 0.012 |
| Chronic lung disease | 153 (2.6) | 101 (2.3) | 52 (3.3) | 0.043 | 0.06 | 62 (4.0) | 52 (3.4) | 0.39 | 0.034 |
| Cancer | 198 (3.4) | 142 (3.3) | 56 (3.6) | 0.626 | 0.017 | 53 (3.4) | 56 (3.6) | 0.845 | 0.011 |
| LVEF | 52.7 ± 11.6 | 53.4 ± 11.1 | 50.5 ± 12.6 | <0.001 | 0.248 | 50.6 ± 12.6 | 50.7 ± 12.5 | 0.89 | 0.005 |
| LVEF ≤35% | 505 (8.6) | 288 (6.7) | 217 (13.9) | <0.001 | 0.24 | 227 (14.8) | 207 (13.5) | 0.325 | 0.037 |
| ST-segment elevation MI | 3095 (52.6) | 2313 (53.5) | 782 (50.1) | 0.023 | 0.068 | 801 (52.1) | 775 (50.4) | 0.367 | 0.034 |
| Medication at discharge | |||||||||
| Aspirin | 5793 (98.4) | 4258 (98.4) | 1535 (98.3) | 0.761 | 0.012 | 1504 (97.8) | 1511 (98.2) | 0.438 | 0.033 |
| Clopidogrel | 5151 (87.5) | 3761 (86.9) | 1390 (89.0) | 0.04 | 0.063 | 1361 (88.5) | 1369 (89.0) | 0.69 | 0.016 |
| Ticagrelor | 331 (5.6) | 240 (5.5) | 91 (5.8) | 0.73 | 0.012 | 94 (6.1) | 90 (5.9) | 0.82 | 0.011 |
| Prasugrel | 371 (6.3) | 301 (7.0) | 70 (4.5) | 0.001 | 0.107 | 66 (4.3) | 69 (4.5) | 0.86 | 0.01 |
| Potent P2Y12 inhibitor | 702 (11.9) | 541 (12.5) | 161 (10.3) | 0.024 | 0.069 | 160 (10.4) | 159 (10.3) | 1 | 0.002 |
| Beta-blocker | 5131 (87.1) | 3824 (88.4) | 1307 (83.7) | <0.001 | 0.137 | 1283 (83.4) | 1291 (83.9) | 0.733 | 0.014 |
| ACEi or ARB | 4443 (75.5) | 3333 (77.0) | 1110 (71.1) | <0.001 | 0.137 | 1096 (71.3) | 1093 (71.1) | 0.937 | 0.004 |
| Oral anticoagulant | 154 (2.6) | 97 (2.2) | 57 (3.6) | 0.004 | 0.083 | 57 (3.7) | 55 (3.6) | 0.923 | 0.007 |
| Statin | 5638 (95.8) | 4154 (96.0) | 1484 (95.0) | 0.102 | 0.049 | 1457 (94.7) | 1463 (95.1) | 0.681 | 0.018 |
| Before PS-Matching | After PS-Matching | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n = 5888) | Normal Uric Acid (n = 4326) | High Uric Acid (n = 1562) | p-Value | Absolute SMD | Normal Uric Acid (n = 1538) | High Uric Acid (n = 1538) | p-Value | Absolute SMD | |
| Laboratory findings | |||||||||
| Uric acid, mg/dL | 5.6 ± 4.0 | 4.3 ± 1.4 | 9.1 ± 6.1 | <0.001 | 1.071 | 4.3 ± 1.4 | 9.1 ± 6.2 | <0.001 | 1.065 |
| Uric acid, µmol/L | (333.1 ± 237.9) | (255.8 ± 83.3) | (541.3 ± 362.8) | (255.8 ± 83.3) | (541.3 ± 368.8) | ||||
| CK-MB, peak, ng/mL | 130.0 ± 753.8 | 124.4 ± 267.3 | 145.2 ± 1394.5 | 0.558 | 0.021 | 130.5 ± 378.1 | 146.6 ± 1405.2 | 0.665 | 0.016 |
| Hemoglobin, mg/dL | 13.4 ± 2.2 | 13.5 ± 2.1 | 13.1 ± 2.5 | <0.001 | 0.154 | 13.2 ± 2.2 | 13.1 ± 2.5 | 0.284 | 0.039 |
| Creatinine, mg/dL | 1.2 ± 1.2 | 1.1 ± 1.0 | 1.5 ± 1.5 | <0.001 | 0.344 | 1.4 ± 1.6 | 1.5 ± 1.4 | 0.081 | 0.063 |
| high-sensitivity CRP, mg/dL | 5.3 ± 18.5 | 5.1 ± 18.1 | 5.7 ± 19.6 | 0.286 | 0.032 | 6.6 ± 22.8 | 5.7 ± 19.7 | 0.236 | 0.043 |
| Total cholesterol, mg/dL | 176.8 ± 43.2 | 176.7 ± 42.6 | 177.1 ± 45.0 | 0.772 | 0.009 | 174.5 ± 44.6 | 177.5 ± 45.1 | 0.065 | 0.067 |
| Triglyceride, mg/dL | 122.2 ± 89.4 | 119.2 ± 84.4 | 130.3 ± 101.7 | <0.001 | 0.119 | 132.4 ± 107.7 | 130.7 ± 102.2 | 0.644 | 0.017 |
| High-density lipoprotein, mg/dL | 40.6 ± 10.8 | 41.0 ± 10.9 | 39.4 ± 10.7 | <0.001 | 0.149 | 39.1 ± 10.4 | 39.5 ± 10.7 | 0.359 | 0.033 |
| Low-density lipoprotein, mg/dL | 112.3 ± 37.1 | 112.5 ± 37.0 | 111.8 ± 37.6 | 0.52 | 0.019 | 110.1 ± 37.6 | 112.0 ± 37.7 | 0.167 | 0.05 |
| Angiographic characteristics | |||||||||
| Multivessel disease | 3198 (54.3) | 2259 (52.2) | 939 (60.1) | <0.001 | 0.16 | 881 (57.3) | 921 (59.9) | 0.153 | 0.053 |
| Left main PCI | 230 (3.9) | 156 (3.6) | 74 (4.7) | 0.057 | 0.057 | 70 (4.6) | 68 (4.4) | 0.931 | 0.006 |
| Left anterior descending PCI | 3515 (59.7) | 2595 (60.0) | 920 (58.9) | 0.471 | 0.022 | 934 (60.7) | 902 (58.6) | 0.255 | 0.042 |
| Left circumflex PCI | 1589 (27.0) | 1188 (27.5) | 401 (25.7) | 0.183 | 0.041 | 438 (28.5) | 396 (25.7) | 0.096 | 0.061 |
| Right coronary artery PCI | 2364 (40.1) | 1718 (39.7) | 646 (41.4) | 0.269 | 0.033 | 630 (41.0) | 636 (41.4) | 0.855 | 0.008 |
| Total stent number | 1.6 ± 0.9 | 1.6 ± 0.9 | 1.6 ± 0.9 | 0.103 | 0.048 | 1.6 ± 1.0 | 1.6 ± 0.9 | 0.72 | 0.013 |
| Total stent length | 37.6 ± 25.0 | 37.2 ± 24.8 | 38.6 ± 25.5 | 0.05 | 0.058 | 38.5 ± 25.8 | 38.5 ± 25.3 | 0.949 | 0.002 |
| Bifurcation PCI with two stents | 91 (1.5) | 57 (1.3) | 34 (2.2) | 0.025 | 0.066 | 36 (2.3) | 31 (2.0) | 0.621 | 0.022 |
| Long stenting >60 mm | 283 (4.8) | 209 (4.8) | 74 (4.7) | 0.937 | 0.004 | 85 (5.5) | 73 (4.7) | 0.369 | 0.035 |
| Restenosis lesion | 91 (1.5) | 67 (1.5) | 24 (1.5) | 1 | 0.001 | 24 (1.6) | 22 (1.4) | 0.882 | 0.011 |
| Ostial lesion | 232 (3.9) | 158 (3.7) | 74 (4.7) | 0.07 | 0.054 | 65 (4.2) | 73 (4.7) | 0.542 | 0.025 |
| Second-generation DES | 3499 (59.4) | 2616 (60.5) | 883 (56.5) | 0.007 | 0.08 | 869 (56.5) | 872 (56.7) | 0.942 | 0.004 |
| Normal Uric Acid (n = 4326) | High Uric Acid (n = 1562) | Unadjusted | Multivariable-Adjusted | Propensity-Score Matched | IPW-Adjusted | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value † | HR (95% CI) | p-Value | HR (95% CI) | p-Value † | HR (95% CI) | p-Value † | |||
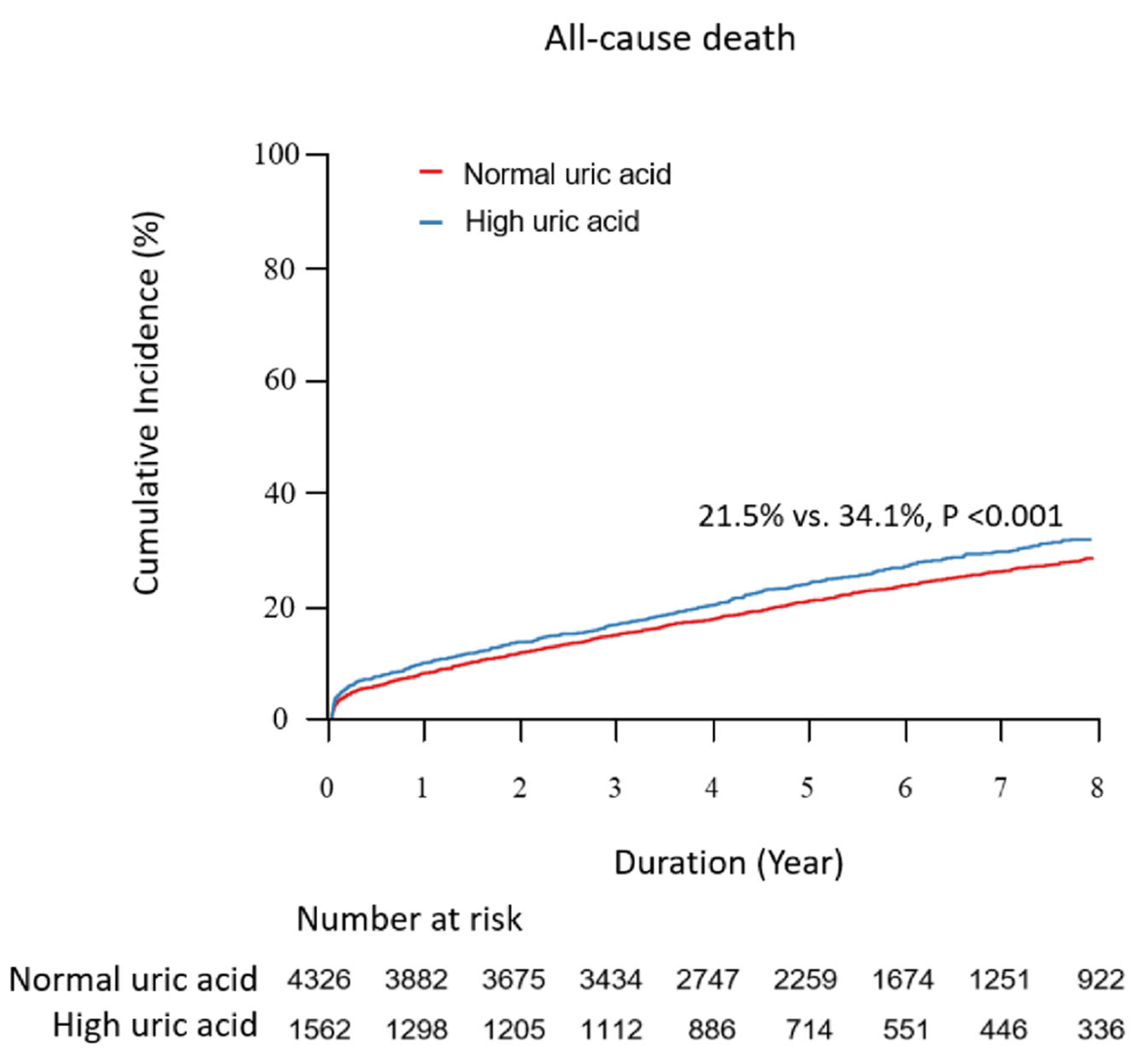
| All cause of death | 929 (21.5) | 532 (34.1) | 1.69 (1.52–1.88) | <0.001 | 1.18 (1.05–1.32) | 0.005 | 1.19 (1.05,1.35) | 0.008 | 1.18 (1.05,1.33) | 0.005 |
| Cardiac death | 688 (15.9) | 417 (26.7) | 1.79 (1.58–2.02) | <0.001 | 1.23 (1.08–1.4) | 0.002 | 1.23 (1.06,1.42) | 0.005 | 1.2 (1.05,1.37) | 0.009 |
| Readmission for HF | 158 (3.7) | 98 (6.3) | 1.86 (1.45–2.39) | <0.001 | 1.78 (1.31–2.41) | <0.001 | 1.14 (0.85,1.54) | 0.368 | 1.28 (0.97,1.67) | 0.076 |
| Readmission for UA | 470 (10.9) | 132 (8.5) | 0.83 (0.69–1.01) | 0.063 | 0.79 (0.64–0.96) | 0.018 | 0.8 (0.63,1) | 0.051 | 0.82 (0.66,1) | 0.051 |
| Recurrent MI | 231 (5.3) | 96 (6.1) | 1.25 (0.98–1.58) | 0.068 | 1.02 (0.79–1.31) | 0.892 | 1.01 (0.76,1.33) | 0.968 | 1.06 (0.83,1.37) | 0.629 |
| Definite or probable ST | 66 (1.5) | 32 (2.0) | 1.43 (0.94–2.19) | 0.094 | 1.46 (0.94–2.26) | 0.092 | 1.64 (0.94,2.88) | 0.081 | 1.42 (0.91,2.2) | 0.121 |
| Revascularization | 659 (15.2) | 254 (16.3) | 1.17 (1.01–1.35) | 0.036 | 1.06 (0.91–1.23) | 0.464 | 1.02 (0.85,1.21) | 0.857 | 1.05 (0.9,1.22) | 0.572 |
| Ischemic stroke | 124 (2.9) | 54 (3.5) | 1.29 (0.94–1.77) | 0.121 | 1.13 (0.81–1.58) | 0.47 | 1.15 (0.78,1.7) | 0.484 | 1.08 (0.77,1.51) | 0.655 |
| Model | C-Index | 95% CI | p-Value | NRI | 95% CI | p-Value for NRI | IDI | 95% CI | p-Value for IDI | AIC | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| For predicting mortality | |||||||||||
| Model A | Old age, gender, obesity, HBP, DM, dyslipidemia, stroke, smoker, AF, CLD | 0.75 | 0.736–0.763 | 5703.343 | |||||||
| Model B | Old age, gender, obesity, HBP, DM, dyslipidemia, stroke, smoker, AF, CLD, high UA | 0.759 | 0.745–0.772 | <0.001 | 0.012 | 0.008–0.015 | <0.001 | 0.263 | 0.208–0.318 | <0.001 | 5644.191 |
| Model A′ | Old age, gender, obesity, HBP, DM, dyslipidemia, stroke, smoker, AF, CLD, CKD | 0.773 | 0.76–0.786 | 5495.279 | |||||||
| Model B′ | Old age, gender, obesity, HBP, DM, dyslipidemia, stroke, smoker, AF, CLD, CKD, high UA | 0.779 | 0.766–0.792 | <0.001 | 0.005 | 0.003–0.008 | <0.001 | 0.263 | 0.208–0.318 | <0.001 | 5468.476 |
| Model A″ | Old age, gender, obesity, HBP, DM, dyslipidemia, stroke, smoker, AF, CLD, CKD, low LVEF | 0.785 | 0.772–0.797 | 5413.460 | |||||||
| Model B″ | Old age, gender, obesity, HBP, DM, dyslipidemia, stroke, smoker, AF, CLD, CKD, low LVEF, high UA | 0.788 | 0.775–0.801 | 0.005 | 0.004 | 0.002–0.006 | <0.001 | 0.263 | 0.208–0.318 | <0.001 | 5395.117 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Hwang, B.-H.; Lee, K.Y.; Kim, C.J.; Choo, E.-H.; Lim, S.; Kim, J.-J.; Choi, I.J.; Park, M.-W.; Oh, G.C.; et al. High Uric Acid Levels in Acute Myocardial Infarction Provide Better Long-Term Prognosis Predictive Power When Combined with Traditional Risk Factors. J. Clin. Med. 2022, 11, 5531. https://doi.org/10.3390/jcm11195531
Kim S, Hwang B-H, Lee KY, Kim CJ, Choo E-H, Lim S, Kim J-J, Choi IJ, Park M-W, Oh GC, et al. High Uric Acid Levels in Acute Myocardial Infarction Provide Better Long-Term Prognosis Predictive Power When Combined with Traditional Risk Factors. Journal of Clinical Medicine. 2022; 11(19):5531. https://doi.org/10.3390/jcm11195531
Chicago/Turabian StyleKim, Soohyun, Byung-Hee Hwang, Kwan Yong Lee, Chan Jun Kim, Eun-Ho Choo, Sungmin Lim, Jin-Jin Kim, Ik Jun Choi, Mahn-Won Park, Gyu Chul Oh, and et al. 2022. "High Uric Acid Levels in Acute Myocardial Infarction Provide Better Long-Term Prognosis Predictive Power When Combined with Traditional Risk Factors" Journal of Clinical Medicine 11, no. 19: 5531. https://doi.org/10.3390/jcm11195531
APA StyleKim, S., Hwang, B.-H., Lee, K. Y., Kim, C. J., Choo, E.-H., Lim, S., Kim, J.-J., Choi, I. J., Park, M.-W., Oh, G. C., Yoo, K. D., Chung, W. S., Ahn, Y., Jeong, M. H., & Chang, K. (2022). High Uric Acid Levels in Acute Myocardial Infarction Provide Better Long-Term Prognosis Predictive Power When Combined with Traditional Risk Factors. Journal of Clinical Medicine, 11(19), 5531. https://doi.org/10.3390/jcm11195531





