Antimicrobial Peptides in Early-Life Host Defense, Perinatal Infections, and Necrotizing Enterocolitis—An Update
Abstract
1. Introduction
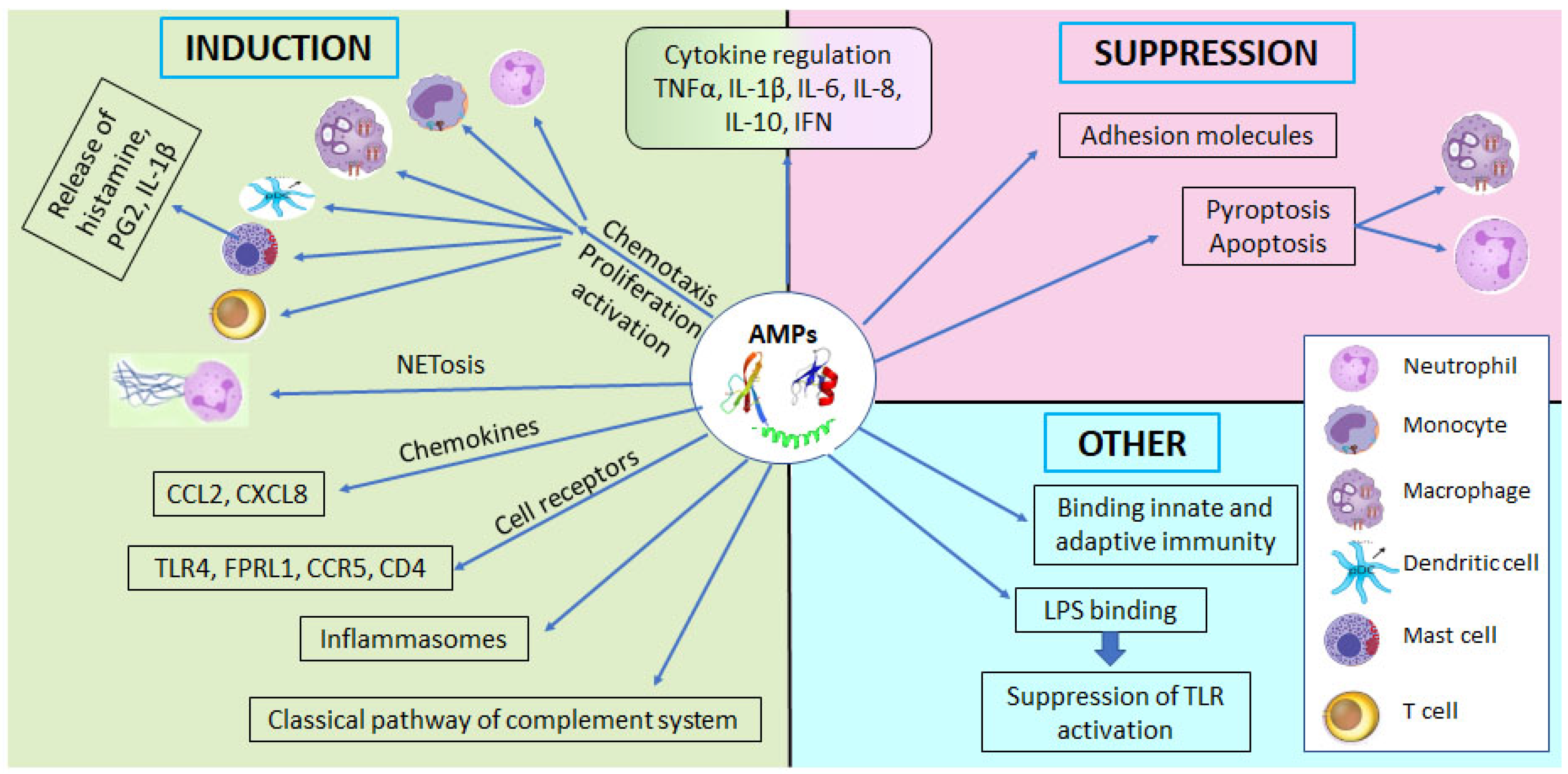
2. Families and Functions of Human AMPs
2.1. Defensins and Cathelicidin LL-37
2.1.1. Structure, Regulation, and Sources of Human Defensins
2.1.2. Structure, Regulation, and Sources of Human Cathelicidin LL-37
2.1.3. Antimicrobial Actions of Defensins and LL-37
2.1.4. Immunomodulating Properties of Defensins and LL-37
2.2. Antileukoproteases Elafin and Secretory Leukocyte Protease Inhibitor
Functions of Antileukoproteases
2.3. Hepcidin
2.4. Human Milk-Derived AMPs
3. AMP Sources in the Intrauterine Environment, the Fetus, and the Neonate
3.1. AMPs Originating from the Maternal Reproductive Tract during Pregnancy
3.2. AMP Production by the Fetus and Neonate
3.2.1. The Fetal and Neonatal Skin as a Source of AMPs
3.2.2. The Developing Lung as a Source of AMPs
3.2.3. The Fetal and Neonatal Gastrointestinal System as a Source of AMPs
3.3. Effect of Labor on AMP Expression
3.4. Effect of Prematurity on Fetal and Neonatal AMP Levels
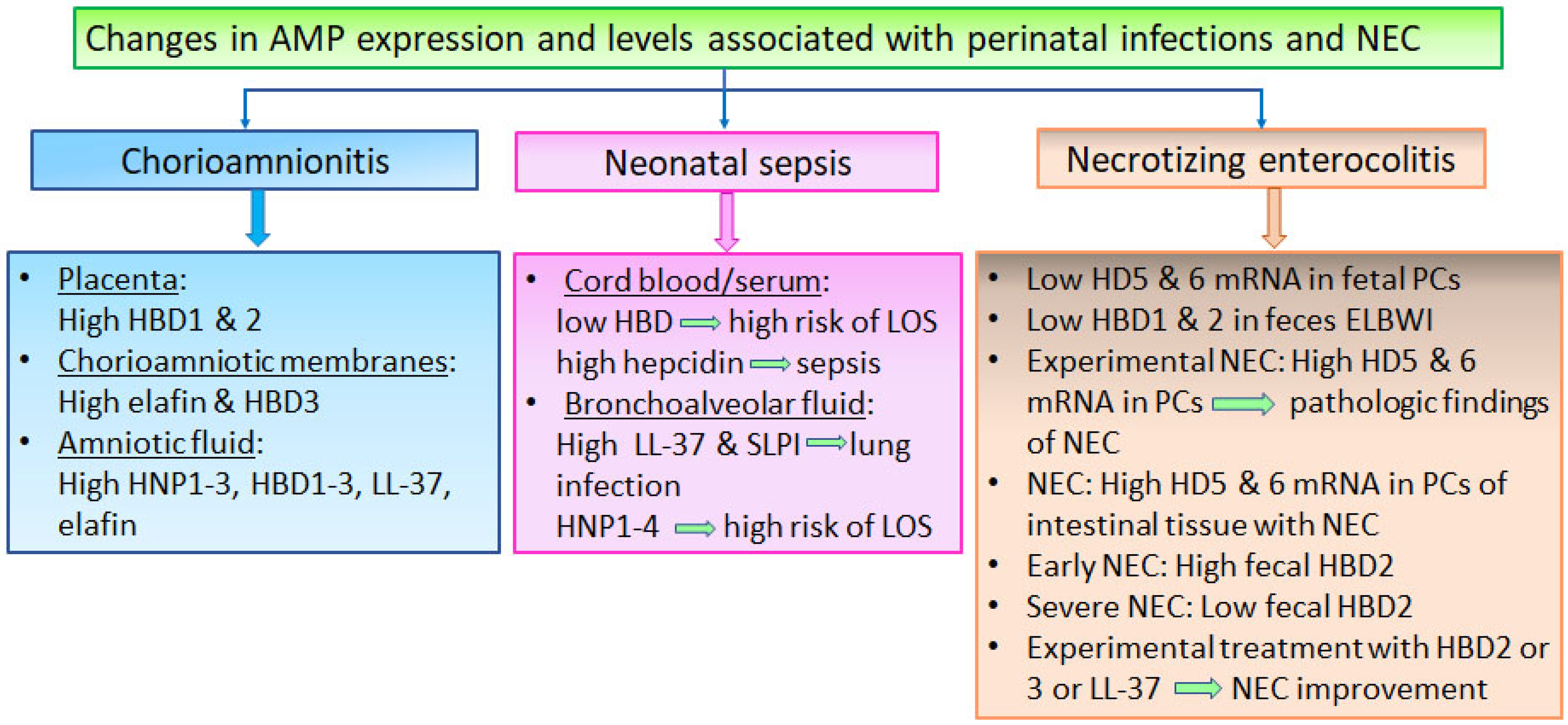
4. Changes in AMP Expression and Levels in Perinatal Infections and NEC
4.1. Changes in AMP Expression and Levels Associated with Chorioamnionitis and Neonatal Infections
4.2. Changes in Gastrointestinal AMPs Associated with NEC Development and Severity
5. Clinical Applications of AMPs in Neonates and Future Perspectives
5.1. The Potential Role of AMPs as Surrogate Biomarkers of Intrauterine and Neonatal Infection and NEC
5.2. The Role of AMPs as Therapeutic Agents in Neonatal Sepsis and NEC
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stoll, B.J.; Hansen, N.; Fanaroff, A.A.; Wright, L.L.; Carlo, W.A.; Ehrenkranz, R.A.; Lemons, J.A.; Donovan, E.F.; Stark, A.R.; Tyson, J.E.; et al. Late-Onset Sepsis in Very Low Birth Weight Neonates: The Experience of the NICHD Neonatal Research Network. Pediatrics 2002, 110, 285–291. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Higgins, R.D.; Fanaroff, A.A.; Duara, S.; Goldberg, R.; Laptook, A.; Walsh, M.; Oh, W.; Hale, E.; et al. Very Low Birth Weight Preterm Infants with Early Onset Neonatal Sepsis: The Predominance of Gram-Negative Infections Continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002–2003. Pediatr. Infect. Dis. J. 2005, 24, 635–639. [Google Scholar] [CrossRef]
- Cuenca, A.G.; Wynn, J.L.; Moldawer, L.L.; Levy, O. Role of Innate Immunity in Neonatal Infection. Am. J. Perinatol. 2013, 30, 105–112. [Google Scholar] [CrossRef]
- Ageitos, J.M.; Sánchez-Pérez, A.; Calo-Mata, P.; Villa, T.G. Antimicrobial Peptides (AMPs): Ancient Compounds That Represent Novel Weapons in the Fight against Bacteria. Biochem. Pharmacol. 2017, 133, 117–138. [Google Scholar] [CrossRef]
- Jenssen, H.; Hamill, P.; Hancock, R.E.W. Peptide Antimicrobial Agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Lazzaro, B.P.; Zasloff, M.; Rolff, J. Antimicrobial Peptides: Application Informed by Evolution. Science 2020, 368, eaau5480. [Google Scholar] [CrossRef]
- Bin Hafeez, A.; Jiang, X.; Bergen, P.J.; Zhu, Y. Antimicrobial Peptides: An Update on Classifications and Databases. Int. J. Mol. Sci. 2021, 22, 11691. [Google Scholar] [CrossRef]
- Nagarajan, K.; Marimuthu, S.K.; Palanisamy, S.; Subbiah, L. Peptide Therapeutics Versus Superbugs: Highlight on Current Research and Advancements. Int. J. Pept. Res. Ther. 2018, 24, 19–33. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Yan, Z.-B.; Meng, Y.-M.; Hong, X.-Y.; Shao, G.; Ma, J.-J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial Peptides: Mechanism of Action, Activity and Clinical Potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Matsuzaki, K. Why and How Are Peptide-Lipid Interactions Utilized for Self-Defense? Magainins and Tachyplesins as Archetypes. Biochim. Biophys. Acta 1999, 1462, 1–10. [Google Scholar] [CrossRef]
- Kumari, S.; Booth, V. Antimicrobial Peptide Mechanisms Studied by Whole-Cell Deuterium NMR. Int. J. Mol. Sci. 2022, 23, 2740. [Google Scholar] [CrossRef]
- Nagaoka, I.; Tamura, H.; Hirata, M. An Antimicrobial Cathelicidin Peptide, Human CAP18/LL-37, Suppresses Neutrophil Apoptosis via the Activation of Formyl-Peptide Receptor-like 1 and P2X7. J. Immunol. 2006, 176, 3044–3052. [Google Scholar] [CrossRef]
- Prasad, S.V.; Fiedoruk, K.; Daniluk, T.; Piktel, E.; Bucki, R. Expression and Function of Host Defense Peptides at Inflammation Sites. Int. J. Mol. Sci. 2019, 21, 104. [Google Scholar] [CrossRef]
- Šket, T.; Ramuta, T.Ž.; Starčič Erjavec, M.; Kreft, M.E. The Role of Innate Immune System in the Human Amniotic Membrane and Human Amniotic Fluid in Protection Against Intra-Amniotic Infections and Inflammation. Front. Immunol. 2021, 12, 735324. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The Antimicrobial Peptide Database as a Tool for Research and Education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef]
- Haney, E.F.; Nazmi, K.; Lau, F.; Bolscher, J.G.M.; Vogel, H.J. Novel Lactoferrampin Antimicrobial Peptides Derived from Human Lactoferrin. Biochimie 2009, 91, 141–154. [Google Scholar] [CrossRef]
- Lee, P.; Peng, H.; Gelbart, T.; Wang, L.; Beutler, E. Regulation of Hepcidin Transcription by Interleukin-1 and Interleukin-6. Proc. Natl. Acad. Sci. USA 2005, 102, 1906–1910. [Google Scholar] [CrossRef]
- Doss, M.; White, M.R.; Tecle, T.; Hartshorn, K.L. Human Defensins and LL-37 in Mucosal Immunity. J. Leukoc. Biol. 2010, 87, 79–92. [Google Scholar] [CrossRef]
- Auvynet, C.; Rosenstein, Y. Multifunctional Host Defense Peptides: Antimicrobial Peptides, the Small yet Big Players in Innate and Adaptive Immunity. FEBS J. 2009, 276, 6497–6508. [Google Scholar] [CrossRef]
- Aquino-Domínguez, A.S.; de los Romero-Tlalolini, M.A.; Torres-Aguilar, H.; Aguilar-Ruiz, S.R. Recent Advances in the Discovery and Function of Antimicrobial Molecules in Platelets. Int. J. Mol. Sci. 2021, 22, 10230. [Google Scholar] [CrossRef]
- Magrone, T.; Russo, M.A.; Jirillo, E. Antimicrobial Peptides: Phylogenic Sources and Biological Activities. First of Two Parts. Curr. Pharm. Des. 2018, 24, 1043–1053. [Google Scholar] [CrossRef]
- Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Antimicrobial Peptides: Key Components of the Innate Immune System. Crit. Rev. Biotechnol. 2012, 32, 143–171. [Google Scholar] [CrossRef]
- Lehrer, R.I.; Lu, W. α-Defensins in Human Innate Immunity. Immunol. Rev. 2012, 245, 84–112. [Google Scholar] [CrossRef]
- Ayabe, T.; Satchell, D.P.; Wilson, C.L.; Parks, W.C.; Selsted, M.E.; Ouellette, A.J. Secretion of Microbicidal Alpha-Defensins by Intestinal Paneth Cells in Response to Bacteria. Nat. Immunol. 2000, 1, 113–118. [Google Scholar] [CrossRef]
- Quayle, A.J.; Porter, E.M.; Nussbaum, A.A.; Wang, Y.M.; Brabec, C.; Yip, K.P.; Mok, S.C. Gene Expression, Immunolocalization, and Secretion of Human Defensin-5 in Human Female Reproductive Tract. Am. J. Pathol. 1998, 152, 1247–1258. [Google Scholar]
- Schneider, J.J.; Unholzer, A.; Schaller, M.; Schäfer-Korting, M.; Korting, H.C. Human Defensins. J. Mol. Med. 2005, 83, 587–595. [Google Scholar] [CrossRef]
- Svinarich, D.M.; Gomez, R.; Romero, R. Detection of Human Defensins in the Placenta. Am. J. Reprod. Immunol. 1997, 38, 252–255. [Google Scholar] [CrossRef]
- Mathews, M.; Jia, H.P.; Guthmiller, J.M.; Losh, G.; Graham, S.; Johnson, G.K.; Tack, B.F.; McCray, P.B. Production of Beta-Defensin Antimicrobial Peptides by the Oral Mucosa and Salivary Glands. Infect. Immun. 1999, 67, 2740–2745. [Google Scholar] [CrossRef]
- Singh, G.; Archana, G. Unraveling the Mystery of Vernix Caseosa. Indian J. Dermatol. 2008, 53, 54–60. [Google Scholar] [CrossRef]
- Cieślik, M.; Bagińska, N.; Górski, A.; Jończyk-Matysiak, E. Human β-Defensin 2 and Its Postulated Role in Modulation of the Immune Response. Cells 2021, 10, 2991. [Google Scholar] [CrossRef]
- Bals, R.; Wang, X.; Wu, Z.; Freeman, T.; Bafna, V.; Zasloff, M.; Wilson, J.M. Human Beta-Defensin 2 Is a Salt-Sensitive Peptide Antibiotic Expressed in Human Lung. J. Clin. Investig. 1998, 102, 874–880. [Google Scholar] [CrossRef]
- Chadebech, P.; Goidin, D.; Jacquet, C.; Viac, J.; Schmitt, D.; Staquet, M.J. Use of Human Reconstructed Epidermis to Analyze the Regulation of Beta-Defensin HBD-1, HBD-2, and HBD-3 Expression in Response to LPS. Cell Biol. Toxicol. 2003, 19, 313–324. [Google Scholar] [CrossRef]
- Harder, J.; Bartels, J.; Christophers, E.; Schroder, J.M. Isolation and Characterization of Human Beta-Defensin-3, a Novel Human Inducible Peptide Antibiotic. J. Biol. Chem. 2001, 276, 5707–5713. [Google Scholar] [CrossRef]
- Alford, M.A.; Baquir, B.; Santana, F.L.; Haney, E.F.; Hancock, R.E.W. Cathelicidin Host Defense Peptides and Inflammatory Signaling: Striking a Balance. Front. Microbiol. 2020, 11, 1902. [Google Scholar] [CrossRef]
- Murakami, M.; Dorschner, R.A.; Stern, L.J.; Lin, K.H.; Gallo, R.L. Expression and Secretion of Cathelicidin Antimicrobial Peptides in Murine Mammary Glands and Human Milk. Pediatr. Res. 2005, 57, 10–15. [Google Scholar] [CrossRef]
- Bals, R.; Wang, X.; Zasloff, M.; Wilson, J.M. The Peptide Antibiotic LL-37/HCAP-18 Is Expressed in Epithelia of the Human Lung Where It Has Broad Antimicrobial Activity at the Airway Surface. Proc. Natl. Acad. Sci. USA 1998, 95, 9541–9546. [Google Scholar] [CrossRef]
- García, J.R.; Krause, A.; Schulz, S.; Rodríguez-Jiménez, F.J.; Klüver, E.; Adermann, K.; Forssmann, U.; Frimpong-Boateng, A.; Bals, R.; Forssmann, W.G. Human Beta-Defensin 4: A Novel Inducible Peptide with a Specific Salt-Sensitive Spectrum of Antimicrobial Activity. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2001, 15, 1819–1821. [Google Scholar]
- De Smet, K.; Contreras, R. Human Antimicrobial Peptides: Defensins, Cathelicidins and Histatins. Biotechnol. Lett. 2005, 27, 1337–1347. [Google Scholar] [CrossRef]
- García, J.R.; Jaumann, F.; Schulz, S.; Krause, A.; Rodríguez-Jiménez, J.; Forssmann, U.; Adermann, K.; Klüver, E.; Vogelmeier, C.; Becker, D.; et al. Identification of a Novel, Multifunctional Beta-Defensin (Human Beta-Defensin 3) with Specific Antimicrobial Activity. Its Interaction with Plasma Membranes of Xenopus Oocytes and the Induction of Macrophage Chemoattraction. Cell Tissue Res. 2001, 306, 257–264. [Google Scholar] [CrossRef]
- Dürr, U.H.N.; Sudheendra, U.S.; Ramamoorthy, A. LL-37, the Only Human Member of the Cathelicidin Family of Antimicrobial Peptides. Biochim. Biophys. Acta 2006, 1758, 1408–1425. [Google Scholar] [CrossRef]
- Bastian, A.; Schäfer, H. Human Alpha-Defensin 1 (HNP-1) Inhibits Adenoviral Infection In Vitro. Regul. Pept. 2001, 101, 157–161. [Google Scholar] [CrossRef]
- Duplantier, A.J.; van Hoek, M.L. The Human Cathelicidin Antimicrobial Peptide LL-37 as a Potential Treatment for Polymicrobial Infected Wounds. Front. Immunol. 2013, 4, 143. [Google Scholar] [CrossRef]
- Bedran, T.B.L.; Mayer, M.P.A.; Spolidorio, D.P.; Grenier, D. Synergistic Anti-Inflammatory Activity of the Antimicrobial Peptides Human Beta-Defensin-3 (HBD-3) and Cathelicidin (LL-37) in a Three-Dimensional Co-Culture Model of Gingival Epithelial Cells and Fibroblasts. PLoS ONE 2014, 9, e106766. [Google Scholar] [CrossRef]
- Nagaoka, I.; Hirota, S.; Yomogida, S.; Ohwada, A.; Hirata, M. Synergistic Actions of Antibacterial Neutrophil Defensins and Cathelicidins. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. Al 2000, 49, 73–79. [Google Scholar] [CrossRef]
- Ou, J.; Liang, S.; Guo, X.-K.; Hu, X. α-Defensins Promote Bacteroides Colonization on Mucosal Reservoir to Prevent Antibiotic-Induced Dysbiosis. Front. Immunol. 2020, 11, 2065. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Alford, M.A.; Haney, E.F. Antibiofilm Activity of Host Defence Peptides: Complexity Provides Opportunities. Nat. Rev. Microbiol. 2021, 19, 786–797. [Google Scholar] [CrossRef]
- Pletzer, D.; Coleman, S.R.; Hancock, R.E. Anti-Biofilm Peptides as a New Weapon in Antimicrobial Warfare. Curr. Opin. Microbiol. 2016, 33, 35–40. [Google Scholar] [CrossRef]
- Chaly, Y.V.; Paleolog, E.M.; Kolesnikova, T.S.; Tikhonov, I.I.; Petratchenko, E.V.; Voitenok, N.N. Neutrophil Alpha-Defensin Human Neutrophil Peptide Modulates Cytokine Production in Human Monocytes and Adhesion Molecule Expression in Endothelial Cells. Eur. Cytokine Netw. 2000, 11, 257–266. [Google Scholar]
- Bian, T.; Li, H.; Zhou, Q.; Ni, C.; Zhang, Y.; Yan, F. Human β-Defensin 3 Reduces TNF-α-Induced Inflammation and Monocyte Adhesion in Human Umbilical Vein Endothelial Cells. Mediat. Inflamm. 2017, 2017, 8529542. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Q.; Schmidt, A.P.; Anderson, G.M.; Wang, J.M.; Wooters, J.; Oppenheim, J.J.; Chertov, O. LL-37, the Neutrophil Granule- and Epithelial Cell-Derived Cathelicidin, Utilizes Formyl Peptide Receptor-like 1 (FPRL1) as a Receptor to Chemoattract Human Peripheral Blood Neutrophils, Monocytes, and T Cells. J. Exp. Med. 2000, 192, 1069–1074. [Google Scholar] [CrossRef]
- Zhang, Z.; Cherryholmes, G.; Chang, F.; Rose, D.M.; Schraufstatter, I.; Shively, J.E. Evidence That Cathelicidin Peptide LL-37 May Act as a Functional Ligand for CXCR2 on Human Neutrophils. Eur. J. Immunol. 2009, 39, 3181–3194. [Google Scholar] [CrossRef]
- Hu, Z.; Murakami, T.; Suzuki, K.; Tamura, H.; Reich, J.; Kuwahara-Arai, K.; Iba, T.; Nagaoka, I. Antimicrobial Cathelicidin Peptide LL-37 Inhibits the Pyroptosis of Macrophages and Improves the Survival of Polybacterial Septic Mice. Int. Immunol. 2016, 28, 245–253. [Google Scholar] [CrossRef]
- Nagaoka, I.; Hirota, S.; Niyonsaba, F.; Hirata, M.; Adachi, Y.; Tamura, H.; Heumann, D. Cathelicidin Family of Antibacterial Peptides CAP18 and CAP11 Inhibit the Expression of TNF-Alpha by Blocking the Binding of LPS to CD14(+) Cells. J. Immunol. 2001, 167, 3329–3338. [Google Scholar] [CrossRef]
- Nagaoka, I.; Tamura, H.; Reich, J. Therapeutic Potential of Cathelicidin Peptide LL-37, an Antimicrobial Agent, in a Murine Sepsis Model. Int. J. Mol. Sci. 2020, 21, E5973. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Faiyaz, S.; Choi, K.-Y.G.; Krokhin, O.V.; Mookherjee, N. Immunomodulatory Functions of the Human Cathelicidin LL-37 (Aa 13-31)-Derived Peptides Are Associated with Predicted α-Helical Propensity and Hydrophobic Index. Biomolecules 2019, 9, 501. [Google Scholar] [CrossRef]
- Wu, Z.; Hoover, D.M.; Yang, D.; Boulègue, C.; Santamaria, F.; Oppenheim, J.J.; Lubkowski, J.; Lu, W. Engineering Disulfide Bridges to Dissect Antimicrobial and Chemotactic Activities of Human Beta-Defensin 3. Proc. Natl. Acad. Sci. USA 2003, 100, 8880–8885. [Google Scholar] [CrossRef]
- Chertov, O.; Michiel, D.F.; Xu, L.; Wang, J.M.; Tani, K.; Murphy, W.J.; Longo, D.L.; Taub, D.D.; Oppenheim, J.J. Identification of Defensin-1, Defensin-2, and CAP37/Azurocidin as T-Cell Chemoattractant Proteins Released from Interleukin-8-Stimulated Neutrophils. J. Biol. Chem. 1996, 271, 2935–2940. [Google Scholar] [CrossRef]
- Conejo-Garcia, J.R.; Benencia, F.; Courreges, M.-C.; Kang, E.; Mohamed-Hadley, A.; Buckanovich, R.J.; Holtz, D.O.; Jenkins, A.; Na, H.; Zhang, L.; et al. Tumor-Infiltrating Dendritic Cell Precursors Recruited by a Beta-Defensin Contribute to Vasculogenesis under the Influence of Vegf-A. Nat. Med. 2004, 10, 950–958. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Q.; Chertov, O.; Oppenheim, J.J. Human Neutrophil Defensins Selectively Chemoattract Naive T and Immature Dendritic Cells. J. Leukoc. Biol. 2000, 68, 9–14. [Google Scholar]
- Wu, J.; Gong, R.-L.; Hu, Q.-F.; Chen, X.-T.; Zhao, W.; Chen, T.-X. Immunoregulatory Effect of Human β-Defensin 1 on Neonatal Cord Blood Monocyte-Derived Dendritic Cells and T Cells. Mol. Immunol. 2019, 109, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Shelley, J.R.; Davidson, D.J.; Dorin, J.R. The Dichotomous Responses Driven by β-Defensins. Front. Immunol. 2020, 11, 1176. [Google Scholar] [CrossRef] [PubMed]
- Niyonsaba, F.; Someya, A.; Hirata, M.; Ogawa, H.; Nagaoka, I. Evaluation of the Effects of Peptide Antibiotics Human Beta-Defensins-1/-2 and LL-37 on Histamine Release and Prostaglandin D(2) Production from Mast Cells. Eur. J. Immunol. 2001, 31, 1066–1075. [Google Scholar] [CrossRef]
- Van den Berg, R.H.; Faber-Krol, M.C.; van Wetering, S.; Hiemstra, P.S.; Daha, M.R. Inhibition of Activation of the Classical Pathway of Complement by Human Neutrophil Defensins. Blood 1998, 92, 3898–3903. [Google Scholar] [CrossRef]
- Takahashi, M.; Umehara, Y.; Yue, H.; Trujillo-Paez, J.V.; Peng, G.; Nguyen, H.L.T.; Ikutama, R.; Okumura, K.; Ogawa, H.; Ikeda, S.; et al. The Antimicrobial Peptide Human β-Defensin-3 Accelerates Wound Healing by Promoting Angiogenesis, Cell Migration, and Proliferation through the FGFR/JAK2/STAT3 Signaling Pathway. Front. Immunol. 2021, 12, 712781. [Google Scholar] [CrossRef]
- Heilborn, J.D.; Nilsson, M.F.; Kratz, G.; Weber, G.; Sørensen, O.; Borregaard, N.; Ståhle-Bäckdahl, M. The Cathelicidin Anti-Microbial Peptide LL-37 Is Involved in Re-Epithelialization of Human Skin Wounds and Is Lacking in Chronic Ulcer Epithelium. J. Investig. Dermatol. 2003, 120, 379–389. [Google Scholar] [CrossRef]
- Brogden, K.A.; Heidari, M.; Sacco, R.E.; Palmquist, D.; Guthmiller, J.M.; Johnson, G.K.; Jia, H.P.; Tack, B.F.; McCray, P.B. Defensin-Induced Adaptive Immunity in Mice and Its Potential in Preventing Periodontal Disease. Oral Microbiol. Immunol. 2003, 18, 95–99. [Google Scholar] [CrossRef]
- Sallenave, J.M. Antimicrobial Activity of Antiproteinases. Biochem. Soc. Trans. 2002, 30, 111–115. [Google Scholar] [CrossRef]
- Williams, S.E.; Brown, T.I.; Roghanian, A.; Sallenave, J.-M. SLPI and Elafin: One Glove, Many Fingers. Clin. Sci. 2006, 110, 21–35. [Google Scholar] [CrossRef]
- Sallenave, J.M.; Shulmann, J.; Crossley, J.; Jordana, M.; Gauldie, J. Regulation of Secretory Leukocyte Proteinase Inhibitor (SLPI) and Elastase-Specific Inhibitor (ESI/Elafin) in Human Airway Epithelial Cells by Cytokines and Neutrophilic Enzymes. Am. J. Respir. Cell Mol. Biol. 1994, 11, 733–741. [Google Scholar] [CrossRef]
- De Rose, V.; Molloy, K.; Gohy, S.; Pilette, C.; Greene, C.M. Airway Epithelium Dysfunction in Cystic Fibrosis and COPD. Mediators Inflamm. 2018, 2018, 1309746. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Furukawa, M.; Abe, J.; Kashiwagi, M.; Hirose, S. Localization of Porcine Trappin-2 (SKALP/Elafin) in Trachea and Large Intestine by in Situ Hybridization and Immunohistochemistry. Histochem. Cell Biol. 2000, 114, 15–20. [Google Scholar] [CrossRef] [PubMed]
- King, A.E.; Critchley, H.O.D.; Sallenave, J.-M.; Kelly, R.W. Elafin in Human Endometrium: An Antiprotease and Antimicrobial Molecule Expressed during Menstruation. J. Clin. Endocrinol. Metab. 2003, 88, 4426–4431. [Google Scholar] [CrossRef]
- Mihaila, A.; Tremblay, G.M. Human Alveolar Macrophages Express Elafin and Secretory Leukocyte Protease Inhibitor. Z. Naturforsch. C 2001, 56, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, H.; Masuda, T.; Shimura, S.; Fushimi, T.; Shirato, K. Secretion and Gene Expression of Secretory Leukocyte Protease Inhibitor by Human Airway Submucosal Glands. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2001, 280, L79–L87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shimoya, K.; Moriyama, A.; Yamanaka, K.; Nakajima, A.; Nobunaga, T.; Koyama, M.; Azuma, C.; Murata, Y. Production of Secretory Leukocyte Protease Inhibitor by Human Amniotic Membranes and Regulation of Its Concentration in Amniotic Fluid. Mol. Hum. Reprod. 2001, 7, 573–579. [Google Scholar] [CrossRef][Green Version]
- McKelvey, M.C.; Brown, R.; Ryan, S.; Mall, M.A.; Weldon, S.; Taggart, C.C. Proteases, Mucus, and Mucosal Immunity in Chronic Lung Disease. Int. J. Mol. Sci. 2021, 22, 5018. [Google Scholar] [CrossRef]
- Roghanian, A.; Williams, S.E.; Sheldrake, T.A.; Brown, T.I.; Oberheim, K.; Xing, Z.; Howie, S.E.M.; Sallenave, J.-M. The Antimicrobial/Elastase Inhibitor Elafin Regulates Lung Dendritic Cells and Adaptive Immunity. Am. J. Respir. Cell Mol. Biol. 2006, 34, 634–642. [Google Scholar] [CrossRef]
- Baranger, K.; Zani, M.-L.; Chandenier, J.; Dallet-Choisy, S.; Moreau, T. The Antibacterial and Antifungal Properties of Trappin-2 (Pre-Elafin) Do Not Depend on Its Protease Inhibitory Function. FEBS J. 2008, 275, 2008–2020. [Google Scholar] [CrossRef]
- Doumas, S.; Kolokotronis, A.; Stefanopoulos, P. Anti-Inflammatory and Antimicrobial Roles of Secretory Leukocyte Protease Inhibitor. Infect. Immun. 2005, 73, 1271–1274. [Google Scholar] [CrossRef]
- Yapakçi, E.; Tarcan, A.; Celik, B.; Ozbek, N.; Gürakan, B. Serum Pro-Hepcidin Levels in Term and Preterm Newborns with Sepsis. Pediatr. Int. Off. J. Jpn. Pediatr. Soc. 2009, 51, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Hepcidin—A Peptide Hormone at the Interface of Innate Immunity and Iron Metabolism. Curr. Top. Microbiol. Immunol. 2006, 306, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Çelik, H.T.; Yurdakök, M.; Korkmaz, A.; Yiğit, Ş. Serum Prohepcidin Levels in Premature Newborns with Oxygen Radical Diseases. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2015, 28, 2228–2233. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, K.; Lv, C.; Wang, H.; Cheng, B.; Jin, Y.; Chen, Q.; Lian, Q.; Fang, X. Nuclear Factor-ΚB Mediated Lipopolysaccharide-Induced MRNA Expression of Hepcidin in Human Peripheral Blood Leukocytes. Innate Immun. 2012, 18, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Chen, Q.; Zhang, K.; Chen, Q.; Song, S.; Fang, X. Hepatic Hepcidin Protects against Polymicrobial Sepsis in Mice by Regulating Host Iron Status. Anesthesiology 2015, 122, 374–386. [Google Scholar] [CrossRef]
- Mohanty, D.P.; Mohapatra, S.; Misra, S.; Sahu, P.S. Milk Derived Bioactive Peptides and Their Impact on Human Health—A Review. Saudi J. Biol. Sci. 2016, 23, 577–583. [Google Scholar] [CrossRef]
- Khan, M.U.; Pirzadeh, M.; Förster, C.Y.; Shityakov, S.; Shariati, M.A. Role of Milk-Derived Antibacterial Peptides in Modern Food Biotechnology: Their Synthesis, Applications and Future Perspectives. Biomolecules 2018, 8, 110. [Google Scholar] [CrossRef]
- Pellegrini, A.; Thomas, U.; Bramaz, N.; Hunziker, P.; von Fellenberg, R. Isolation and Identification of Three Bactericidal Domains in the Bovine Alpha-Lactalbumin Molecule. Biochim. Biophys. Acta 1999, 1426, 439–448. [Google Scholar] [CrossRef]
- Gruden, Š.; Poklar Ulrih, N. Diverse Mechanisms of Antimicrobial Activities of Lactoferrins, Lactoferricins, and Other Lactoferrin-Derived Peptides. Int. J. Mol. Sci. 2021, 22, 11264. [Google Scholar] [CrossRef]
- Hayes, M.; Ross, R.P.; Fitzgerald, G.F.; Hill, C.; Stanton, C. Casein-Derived Antimicrobial Peptides Generated by Lactobacillus acidophilus DPC6026. Appl. Environ. Microbiol. 2006, 72, 2260–2264. [Google Scholar] [CrossRef]
- López-Expósito, I.; Quirós, A.; Amigo, L.; Recio, I. Casein Hydrolysates as a Source of Antimicrobial, Antioxidant and Antihypertensive Peptides. Lait 2007, 87, 241–249. [Google Scholar] [CrossRef]
- Trend, S.; Strunk, T.; Lloyd, M.L.; Kok, C.H.; Metcalfe, J.; Geddes, D.T.; Lai, C.T.; Richmond, P.; Doherty, D.A.; Simmer, K.; et al. Levels of Innate Immune Factors in Preterm and Term Mothers’ Breast Milk during the 1st Month Postpartum. Br. J. Nutr. 2016, 115, 1178–1193. [Google Scholar] [CrossRef] [PubMed]
- Stinson, L.F.; Boyce, M.C.; Payne, M.S.; Keelan, J.A. The Not-so-Sterile Womb: Evidence That the Human Fetus Is Exposed to Bacteria Prior to Birth. Front. Microbiol. 2019, 10, 1124. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The Placenta Harbors a Unique Microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef] [PubMed]
- Mei, C.; Yang, W.; Wei, X.; Wu, K.; Huang, D. The Unique Microbiome and Innate Immunity During Pregnancy. Front. Immunol. 2019, 10, 2886. [Google Scholar] [CrossRef]
- Blaser, M.J.; Devkota, S.; McCoy, K.D.; Relman, D.A.; Yassour, M.; Young, V.B. Lessons Learned from the Prenatal Microbiome Controversy. Microbiome 2021, 9, 8. [Google Scholar] [CrossRef]
- Lauder, A.P.; Roche, A.M.; Sherrill-Mix, S.; Bailey, A.; Laughlin, A.L.; Bittinger, K.; Leite, R.; Elovitz, M.A.; Parry, S.; Bushman, F.D. Comparison of Placenta Samples with Contamination Controls Does Not Provide Evidence for a Distinct Placenta Microbiota. Microbiome 2016, 4, 29. [Google Scholar] [CrossRef]
- Tomlinson, M.S.; Lu, K.; Stewart, J.R.; Marsit, C.J.; O’Shea, T.M.; Fry, R.C. Microorganisms in the Placenta: Links to Early-Life Inflammation and Neurodevelopment in Children. Clin. Microbiol. Rev. 2019, 32, e00103-18. [Google Scholar] [CrossRef]
- Yarbrough, V.L.; Winkle, S.; Herbst-Kralovetz, M.M. Antimicrobial Peptides in the Female Reproductive Tract: A Critical Component of the Mucosal Immune Barrier with Physiological and Clinical Implications. Hum. Reprod. Update 2015, 21, 353–377. [Google Scholar] [CrossRef]
- Hein, M.; Valore, E.V.; Helmig, R.B.; Uldbjerg, N.; Ganz, T. Antimicrobial Factors in the Cervical Mucus Plug. Am. J. Obstet. Gynecol. 2002, 187, 137–144. [Google Scholar] [CrossRef]
- King, A.E.; Kelly, R.W.; Sallenave, J.-M.; Bocking, A.D.; Challis, J.R.G. Innate Immune Defences in the Human Uterus during Pregnancy. Placenta 2007, 28, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Erez, O.; Romero, R.; Tarca, A.L.; Chaiworapongsa, T.; Kim, Y.M.; Than, N.G.; Vaisbuch, E.; Draghici, S.; Tromp, G. Differential Expression Pattern of Genes Encoding for Anti-Microbial Peptides in the Fetal Membranes of Patients with Spontaneous Preterm Labor and Intact Membranes and Those with Preterm Prelabor Rupture of the Membranes. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2009, 22, 1103–1115. [Google Scholar] [CrossRef]
- Espinoza, J.; Chaiworapongsa, T.; Romero, R.; Edwin, S.; Rathnasabapathy, C.; Gomez, R.; Bujold, E.; Camacho, N.; Kim, Y.M.; Hassan, S.; et al. Antimicrobial Peptides in Amniotic Fluid: Defensins, Calprotectin and Bacterial/Permeability-Increasing Protein in Patients with Microbial Invasion of the Amniotic Cavity, Intra-Amniotic Inflammation, Preterm Labor and Premature Rupture of Membranes. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2003, 13, 2–21. [Google Scholar] [CrossRef]
- Heine, R.P.; Wiesenfeld, H.; Mortimer, L.; Greig, P.C. Amniotic Fluid Defensins: Potential Markers of Subclinical Intrauterine Infection. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1998, 27, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Yoshio, H.; Tollin, M.; Gudmundsson, G.H.; Lagercrantz, H.; Jornvall, H.; Marchini, G.; Agerberth, B. Antimicrobial Polypeptides of Human Vernix Caseosa and Amniotic Fluid: Implications for Newborn Innate Defense. Pediatr. Res. 2003, 53, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Akinbi, H.T.; Narendran, V.; Pass, A.K.; Markart, P.; Hoath, S.B. Host Defense Proteins in Vernix Caseosa and Amniotic Fluid. Am. J. Obstet. Gynecol. 2004, 191, 2090–2096. [Google Scholar] [CrossRef]
- Kai-Larsen, Y.; Gudmundsson, G.H.; Agerberth, B. A Review of the Innate Immune Defence of the Human Foetus and Newborn, with the Emphasis on Antimicrobial Peptides. Acta Paediatr. 2014, 103, 1000–1008. [Google Scholar] [CrossRef]
- Son, G.-H.; Lee, J.-J.; Kim, Y.; Lee, K.-Y. The Role of Antimicrobial Peptides in Preterm Birth. Int. J. Mol. Sci. 2021, 22, 8905. [Google Scholar] [CrossRef]
- Starner, T.D.; Agerberth, B.; Gudmundsson, G.H.; McCray, P.B. Expression and Activity of Beta-Defensins and LL-37 in the Developing Human Lung. J. Immunol. 2005, 174, 1608–1615. [Google Scholar] [CrossRef]
- Strunk, T.; Hibbert, J.; Doherty, D.; Granland, C.; Trend, S.; Simmer, K.; Burgner, D.; Patole, S.; Currie, A. Probiotics and Antimicrobial Protein and Peptide Levels in Preterm Infants. Acta Paediatr. 2017, 106, 1747–1753. [Google Scholar] [CrossRef]
- Tirone, C.; Boccacci, S.; Inzitari, R.; Tana, M.; Aurilia, C.; Fanali, C.; Cabras, T.; Messana, I.; Castagnola, M.; Romagnoli, C.; et al. Correlation of Levels of Alpha-Defensins Determined by HPLC-ESI-MS in Bronchoalveolar Lavage Fluid with the Diagnosis of Pneumonia in Premature Neonates. Pediatr. Res. 2010, 68, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Varrey, A.; Romero, R.; Panaitescu, B.; Miller, D.; Chaiworapongsa, T.; Patwardhan, M.; Faro, J.; Pacora, P.; Hassan, S.S.; Hsu, C.-D.; et al. Human β-Defensin-1: A Natural Antimicrobial Peptide Present in Amniotic Fluid That Is Increased in Spontaneous Preterm Labor with Intra-Amniotic Infection. Am. J. Reprod. Immunol. 2018, 80, e13031. [Google Scholar] [CrossRef] [PubMed]
- Vornhagen, J.; Quach, P.; Santana-Ufret, V.; Alishetti, V.; Brokaw, A.; Armistead, B.; Qing Tang, H.; MacDonald, J.W.; Bammler, T.K.; Adams Waldorf, K.M.; et al. Human Cervical Mucus Plugs Exhibit Insufficiencies in Antimicrobial Activity towards Group B Streptococcus. J. Infect. Dis. 2018, 217, 1626–1636. [Google Scholar] [CrossRef] [PubMed]
- Scheid, A.; Li, N.; Jeffers, C.; Borriello, F.; Joshi, S.; Ozonoff, A.; Pettengill, M.; Levy, O. Antimicrobial Peptide LL-37 and Recombinant Human Mannose-Binding Lectin Express Distinct Age- and Pathogen-Specific Antimicrobial Activity in Human Newborn Cord Blood In Vitro. F1000Research 2018, 7, 616. [Google Scholar] [CrossRef] [PubMed]
- Ramuta, T.Ž.; Tratnjek, L.; Janev, A.; Seme, K.; Starčič Erjavec, M.; Kreft, M.E. The Antibacterial Activity of Human Amniotic Membrane against Multidrug-Resistant Bacteria Associated with Urinary Tract Infections: New Insights from Normal and Cancerous Urothelial Models. Biomedicines 2021, 9, 218. [Google Scholar] [CrossRef]
- Klaffenbach, D.; Friedrich, D.; Strick, R.; Strissel, P.L.; Beckmann, M.W.; Rascher, W.; Gessner, A.; Dötsch, J.; Meissner, U.; Schnare, M. Contribution of Different Placental Cells to the Expression and Stimulation of Antimicrobial Proteins (AMPs). Placenta 2011, 32, 830–837. [Google Scholar] [CrossRef]
- Zare-Bidaki, M.; Sadrinia, S.; Erfani, S.; Afkar, E.; Ghanbarzade, N. Antimicrobial Properties of Amniotic and Chorionic Membranes: A Comparative Study of Two Human Fetal Sacs. J. Reprod. Infertil. 2017, 18, 218–224. [Google Scholar]
- King, A.E.; Paltoo, A.; Kelly, R.W.; Sallenave, J.-M.; Bocking, A.D.; Challis, J.R.G. Expression of Natural Antimicrobials by Human Placenta and Fetal Membranes. Placenta 2007, 28, 161–169. [Google Scholar] [CrossRef]
- Szukiewicz, D.; Alkhalayla, H.; Pyzlak, M.; Watroba, M.; Szewczyk, G.; Wejman, J. Human Beta-Defensin 1, 2 and 3 Production by Amniotic Epithelial Cells with Respect to Human Papillomavirus (HPV) Infection, HPV Oncogenic Potential and the Mode of Delivery. Microb. Pathog. 2016, 97, 154–165. [Google Scholar] [CrossRef]
- Stock, S.J.; Kelly, R.W.; Riley, S.C.; Calder, A.A. Natural Antimicrobial Production by the Amnion. Am. J. Obstet. Gynecol. 2007, 196, 255.e1–255.e6. [Google Scholar] [CrossRef]
- Soto, E.; Espinoza, J.; Nien, J.K.; Kusanovic, J.P.; Erez, O.; Richani, K.; Santolaya-Forgas, J.; Romero, R. Human Beta-Defensin-2: A Natural Antimicrobial Peptide Present in Amniotic Fluid Participates in the Host Response to Microbial Invasion of the Amniotic Cavity. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2007, 20, 15–22. [Google Scholar] [CrossRef]
- Buhimschi, I.A.; Jabr, M.; Buhimschi, C.S.; Petkova, A.P.; Weiner, C.P.; Saed, G.M. The Novel Antimicrobial Peptide Beta3-Defensin Is Produced by the Amnion: A Possible Role of the Fetal Membranes in Innate Immunity of the Amniotic Cavity. Am. J. Obstet. Gynecol. 2004, 191, 1678–1687. [Google Scholar] [CrossRef] [PubMed]
- Olmos-Ortiz, A.; Hernández-Pérez, M.; Flores-Espinosa, P.; Sedano, G.; Helguera-Repetto, A.C.; Villavicencio-Carrisoza, Ó.; Valdespino-Vazquez, M.Y.; Flores-Pliego, A.; Irles, C.; Rivas-Santiago, B.; et al. Compartmentalized Innate Immune Response of Human Fetal Membranes against Escherichia coli Choriodecidual Infection. Int. J. Mol. Sci. 2022, 23, 2994. [Google Scholar] [CrossRef]
- Yoshio, H.; Lagercrantz, H.; Gudmundsson, G.H.; Agerberth, B. First Line of Defense in Early Human Life. Semin. Perinatol. 2004, 28, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Tambor, V.; Kacerovsky, M.; Andrys, C.; Musilova, I.; Hornychova, H.; Pliskova, L.; Link, M.; Stulik, J.; Lenco, J. Amniotic Fluid Cathelicidin in PPROM Pregnancies: From Proteomic Discovery to Assessing Its Potential in Inflammatory Complications Diagnosis. PLoS ONE 2012, 7, e41164. [Google Scholar] [CrossRef]
- Mao, Y.; Hoffman, T.; Singh-Varma, A.; Duan-Arnold, Y.; Moorman, M.; Danilkovitch, A.; Kohn, J. Antimicrobial Peptides Secreted from Human Cryopreserved Viable Amniotic Membrane Contribute to Its Antibacterial Activity. Sci. Rep. 2017, 7, 13722. [Google Scholar] [CrossRef]
- Stock, S.J.; Duthie, L.; Tremaine, T.; Calder, A.A.; Kelly, R.W.; Riley, S.C. Elafin (SKALP/Trappin-2/Proteinase Inhibitor-3) Is Produced by the Cervix in Pregnancy and Cervicovaginal Levels Are Diminished in Bacterial Vaginosis. Reprod. Sci. 2009, 16, 1125–1134. [Google Scholar] [CrossRef]
- Helmig, R.; Uldbjerg, N.; Ohlsson, K. Secretory Leukocyte Protease Inhibitor in the Cervical Mucus and in the Fetal Membranes. Eur. J. Obstet. Gynecol. Reprod. Biol. 1995, 59, 95–101. [Google Scholar] [CrossRef]
- Pfundt, R.; van Ruissen, F.; van Vlijmen-Willems, I.M.; Alkemade, H.A.; Zeeuwen, P.L.; Jap, P.H.; Dijkman, H.; Fransen, J.; Croes, H.; van Erp, P.E.; et al. Constitutive and Inducible Expression of SKALP/Elafin Provides Anti-Elastase Defense in Human Epithelia. J. Clin. Investig. 1996, 98, 1389–1399. [Google Scholar] [CrossRef]
- Battersby, A.J.; Khara, J.; Wright, V.J.; Levy, O.; Kampmann, B. Antimicrobial Proteins and Peptides in Early Life: Ontogeny and Translational Opportunities. Front. Immunol. 2016, 7, 309. [Google Scholar] [CrossRef]
- Faust, K.; Göpel, W.; Moser, K.; Temole, G.; Bartels, M.; Wieg, C.; Tröger, B.; Herting, E.; Härtel, C. Differential Expression of Antimicrobial Polypeptides in Cord Blood Samples of Preterm and Term Infants. Acta Paediatr. 2014, 103, e143–e147. [Google Scholar] [CrossRef] [PubMed]
- Mallow, E.B.; Harris, A.; Salzman, N.; Russell, J.P.; DeBerardinis, R.J.; Ruchelli, E.; Bevins, C.L. Human Enteric Defensins. Gene Structure and Developmental Expression. J. Biol. Chem. 1996, 271, 4038–4045. [Google Scholar] [CrossRef] [PubMed]
- Salzman, N.H.; Polin, R.A.; Harris, M.C.; Ruchelli, E.; Hebra, A.; Zirin-Butler, S.; Jawad, A.; Martin Porter, E.; Bevins, C.L. Enteric Defensin Expression in Necrotizing Enterocolitis. Pediatr. Res. 1998, 44, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Puiman, P.J.; Burger-Van Paassen, N.; Schaart, M.W.; De Bruijn, A.C.J.M.; De Krijger, R.R.; Tibboel, D.; Van Goudoever, J.B.; Renes, I.B. Paneth Cell Hyperplasia and Metaplasia in Necrotizing Enterocolitis. Pediatr. Res. 2011, 69, 217–223. [Google Scholar] [CrossRef]
- Heida, F.H.; Beyduz, G.; Bulthuis, M.L.C.; Kooi, E.M.W.; Bos, A.F.; Timmer, A.; Hulscher, J.B.F. Paneth Cells in the Developing Gut: When Do They Arise and When Are They Immune Competent? Pediatr. Res. 2016, 80, 306–310. [Google Scholar] [CrossRef]
- Jenke, A.C.W.; Zilbauer, M.; Postberg, J.; Wirth, S. Human β-Defensin 2 Expression in ELBW Infants with Severe Necrotizing Enterocolitis. Pediatr. Res. 2012, 72, 513–520. [Google Scholar] [CrossRef]
- Richter, M.; Topf, H.-G.; Gröschl, M.; Fröhlich, T.; Tzschoppe, A.; Wenzl, T.G.; Köhler, H. Influence of Gestational Age, Cesarean Section, and Type of Feeding on Fecal Human Beta-Defensin 2 and Tumor Necrosis Factor-Alpha. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 103–105. [Google Scholar] [CrossRef]
- Campeotto, F.; Baldassarre, M.; Laforgia, N.; Viallon, V.; Kalach, N.; Amati, L.; Butel, M.J.; Dupont, C.; Kapel, N. Fecal Expression of Human β-Defensin-2 Following Birth. Neonatology 2010, 98, 365–369. [Google Scholar] [CrossRef]
- Olbrich, P.; Pavón, A.; Rosso, M.L.; Molinos, A.; de Felipe, B.; Sanchez, B.; Praena-Fernández, J.M.; Jimenez, F.; Obando, I.; Neth, O. Association of Human Beta-Defensin-2 Serum Levels and Sepsis in Preterm Neonates. Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc. 2013, 14, 796–800. [Google Scholar] [CrossRef]
- Corebima, B.I.R.V.; Rohsiswatmo, R.; Gayatri, P.; Patole, S. Fecal Human β-Defensin-2 (HBD-2) Levels and Gut Microbiota Patterns in Preterm Neonates with Different Feeding Patterns. Iran. J. Microbiol. 2019, 11, 151–159. [Google Scholar]
- Braff, M.H.; Hawkins, M.A.; Di Nardo, A.; Lopez-Garcia, B.; Howell, M.D.; Wong, C.; Lin, K.; Streib, J.E.; Dorschner, R.; Leung, D.Y.M.; et al. Structure-Function Relationships among Human Cathelicidin Peptides: Dissociation of Antimicrobial Properties from Host Immunostimulatory Activities. J. Immunol. 2005, 174, 4271–4278. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.; Hultenby, K.; Hell, E.; Riedel, H.M.; Brismar, H.; Flock, J.-I.; Lundahl, J.; Giske, C.G.; Marchini, G. Staphylococcus Epidermidis Isolated from Newborn Infants Express Pilus-like Structures and Are Inhibited by the Cathelicidin-Derived Antimicrobial Peptide LL37. Pediatr. Res. 2009, 66, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Misawa, Y.; Baba, A.; Ito, S.; Tanaka, M.; Shiohara, M. Vitamin D(3) Induces Expression of Human Cathelicidin Antimicrobial Peptide 18 in Newborns. Int. J. Hematol. 2009, 90, 561–570. [Google Scholar] [CrossRef]
- Mandic Havelka, A.; Yektaei-Karin, E.; Hultenby, K.; Sørensen, O.E.; Lundahl, J.; Berggren, V.; Marchini, G. Maternal Plasma Level of Antimicrobial Peptide LL37 Is a Major Determinant Factor of Neonatal Plasma LL37 Level. Acta Paediatr. 2010, 99, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Gad, G.I.; Abushady, N.M.; Fathi, M.S.; Elsaadany, W. Diagnostic Value of Anti-Microbial Peptide, Cathelicidin in Congenital Pneumonia. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2015, 28, 2197–2200. [Google Scholar] [CrossRef]
- Kai-Larsen, Y.; Bergsson, G.; Gudmundsson, G.H.; Printz, G.; Jörnvall, H.; Marchini, G.; Agerberth, B. Antimicrobial Components of the Neonatal Gut Affected upon Colonization. Pediatr. Res. 2007, 61, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Strunk, T.; Doherty, D.; Richmond, P.; Simmer, K.; Charles, A.; Levy, O.; Liyanage, K.; Smith, T.; Currie, A.; Burgner, D. Reduced Levels of Antimicrobial Proteins and Peptides in Human Cord Blood Plasma. Arch. Dis. Child. Fetal Neonatal Ed. 2009, 94, F230–F231. [Google Scholar] [CrossRef]
- Marchini, G.; Lindow, S.; Brismar, H.; Ståbi, B.; Berggren, V.; Ulfgren, A.-K.; Lonne-Rahm, S.; Agerberth, B.; Gudmundsson, G.H. The Newborn Infant Is Protected by an Innate Antimicrobial Barrier: Peptide Antibiotics Are Present in the Skin and Vernix Caseosa. Br. J. Dermatol. 2002, 147, 1127–1134. [Google Scholar] [CrossRef]
- Schaller-Bals, S.; Schulze, A.; Bals, R. Increased Levels of Antimicrobial Peptides in Tracheal Aspirates of Newborn Infants during Infection. Am. J. Respir. Crit. Care Med. 2002, 165, 992–995. [Google Scholar] [CrossRef]
- Dorschner, R.A.; Lin, K.H.; Murakami, M.; Gallo, R.L. Neonatal Skin in Mice and Humans Expresses Increased Levels of Antimicrobial Peptides: Innate Immunity during Development of the Adaptive Response. Pediatr. Res. 2003, 53, 566–572. [Google Scholar] [CrossRef]
- Gschwandtner, M.; Zhong, S.; Tschachler, A.; Mlitz, V.; Karner, S.; Elbe-Bürger, A.; Mildner, M. Fetal Human Keratinocytes Produce Large Amounts of Antimicrobial Peptides: Involvement of Histone-Methylation Processes. J. Investig. Dermatol. 2014, 134, 2192–2201. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, K.; Sveger, T.; Svenningsen, N. Protease Inhibitors in Bronchoalveolar Lavage Fluid from Neonates with Special Reference to Secretory Leukocyte Protease Inhibitor. Acta Paediatr. 1992, 81, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Watterberg, K.L.; Carmichael, D.F.; Gerdes, J.S.; Werner, S.; Backstrom, C.; Murphy, S. Secretory Leukocyte Protease Inhibitor and Lung Inflammation in Developing Bronchopulmonary Dysplasia. J. Pediatr. 1994, 125, 264–269. [Google Scholar] [CrossRef]
- Sveger, T.; Ohlsson, K.; Polberger, S.; Noack, G.; Mörse, H.; Laurin, S. Tracheobronchial Aspirate Fluid Neutrophil Lipocalin, Elastase- and Neutrophil Protease-4-Alpha1-Antitrypsin Complexes, Protease Inhibitors and Free Proteolytic Activity in Respiratory Distress Syndrome. Acta Paediatr. 2002, 91, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Kothiyal, P.; Schulkers, K.; Liu, X.; Hazrati, S.; Vilboux, T.; Gomez, L.M.; Huddleston, K.; Wong, W.S.W.; Niederhuber, J.E.; Conrads, T.P.; et al. Differences in Maternal Gene Expression in Cesarean Section Delivery Compared with Vaginal Delivery. Sci. Rep. 2020, 10, 17797. [Google Scholar] [CrossRef] [PubMed]
- Cizmeci, M.N.; Kara, S.; Kanburoglu, M.K.; Simavli, S.; Duvan, C.I.; Tatli, M.M. Detection of Cord Blood Hepcidin Levels as a Biomarker for Early-Onset Neonatal Sepsis. Med. Hypotheses 2014, 82, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Georgountzou, A.; Papadopoulos, N.G. Postnatal Innate Immune Development: From Birth to Adulthood. Front. Immunol. 2017, 8, 957. [Google Scholar] [CrossRef]
- Schauber, J.; Gallo, R.L. Antimicrobial Peptides and the Skin Immune Defense System. J. Allergy Clin. Immunol. 2008, 122, 261–266. [Google Scholar] [CrossRef]
- Nishijima, K.; Yoneda, M.; Hirai, T.; Takakuwa, K.; Enomoto, T. Biology of the Vernix Caseosa: A Review. J. Obstet. Gynaecol. Res. 2019, 45, 2145–2149. [Google Scholar] [CrossRef]
- Rogan, M.P.; Geraghty, P.; Greene, C.M.; O’Neill, S.J.; Taggart, C.C.; McElvaney, N.G. Antimicrobial Proteins and Polypeptides in Pulmonary Innate Defence. Respir. Res. 2006, 7, 29. [Google Scholar] [CrossRef]
- Magrone, T.; Russo, M.A.; Jirillo, E. Antimicrobial Peptides in Human Disease: Therapeutic Approaches. Second of Two Parts. Curr. Pharm. Des. 2018, 24, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Agerberth, B.; Grunewald, J.; Castaños-Velez, E.; Olsson, B.; Jörnvall, H.; Wigzell, H.; Eklund, A.; Gudmundsson, G.H. Antibacterial Components in Bronchoalveolar Lavage Fluid from Healthy Individuals and Sarcoidosis Patients. Am. J. Respir. Crit. Care Med. 1999, 160, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Bonadies, L.; Zaramella, P.; Porzionato, A.; Perilongo, G.; Muraca, M.; Baraldi, E. Present and Future of Bronchopulmonary Dysplasia. J. Clin. Med. 2020, 9, 1539. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.L.; Spiller, O.B.; Beeton, M.L.; Maxwell, N.C.; Remold-O’Donnell, E.; Kotecha, S. Relationship of Proteinases and Proteinase Inhibitors with Microbial Presence in Chronic Lung Disease of Prematurity. Thorax 2010, 65, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Lueschow, S.R.; McElroy, S.J. The Paneth Cell: The Curator and Defender of the Immature Small Intestine. Front. Immunol. 2020, 11, 587. [Google Scholar] [CrossRef]
- Kandasamy, J.; Huda, S.; Ambalavanan, N.; Jilling, T. Inflammatory Signals That Regulate Intestinal Epithelial Renewal, Differentiation, Migration and Cell Death: Implications for Necrotizing Enterocolitis. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 2014, 21, 67–80. [Google Scholar] [CrossRef]
- McElroy, S.J.; Underwood, M.A.; Sherman, M.P. Paneth Cells and Necrotizing Enterocolitis: A Novel Hypothesis for Disease Pathogenesis. Neonatology 2013, 103, 10–20. [Google Scholar] [CrossRef]
- Stanford, A.H.; Gong, H.; Noonan, M.; Lewis, A.N.; Gong, Q.; Lanik, W.E.; Hsieh, J.J.; Lueschow, S.R.; Frey, M.R.; Good, M.; et al. A Direct Comparison of Mouse and Human Intestinal Development Using Epithelial Gene Expression Patterns. Pediatr. Res. 2020, 88, 66–76. [Google Scholar] [CrossRef]
- Salzman, N.H.; Hung, K.; Haribhai, D.; Chu, H.; Karlsson-Sjöberg, J.; Amir, E.; Teggatz, P.; Barman, M.; Hayward, M.; Eastwood, D.; et al. Enteric Defensins Are Essential Regulators of Intestinal Microbial Ecology. Nat. Immunol. 2010, 11, 76–83. [Google Scholar] [CrossRef]
- Ménard, S.; Förster, V.; Lotz, M.; Gütle, D.; Duerr, C.U.; Gallo, R.L.; Henriques-Normark, B.; Pütsep, K.; Andersson, M.; Glocker, E.O.; et al. Developmental Switch of Intestinal Antimicrobial Peptide Expression. J. Exp. Med. 2008, 205, 183–193. [Google Scholar] [CrossRef]
- Bry, L.; Falk, P.; Huttner, K.; Ouellette, A.; Midtvedt, T.; Gordon, J.I. Paneth Cell Differentiation in the Developing Intestine of Normal and Transgenic Mice. Proc. Natl. Acad. Sci. USA 1994, 91, 10335–10339. [Google Scholar] [CrossRef] [PubMed]
- Para, R.; Romero, R.; Miller, D.; Panaitescu, B.; Varrey, A.; Chaiworapongsa, T.; Hassan, S.S.; Hsu, C.-D.; Gomez-Lopez, N. Human β-Defensin-3 Participates in Intra-Amniotic Host Defense in Women with Labor at Term, Spontaneous Preterm Labor and Intact Membranes, and Preterm Prelabor Rupture of Membranes. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2020, 33, 4117–4132. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-F.; Cao, R.-M.; Li, J.; Wu, J.; Wu, S.-M.; Chen, T.-X. Identification of Sociodemographic and Clinical Factors Associated with the Levels of Human β-Defensin-1 and Human β-Defensin-2 in the Human Milk of Han Chinese. Br. J. Nutr. 2014, 111, 867–874. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sevelsted, A.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H. Cesarean Section and Chronic Immune Disorders. Pediatrics 2015, 135, e92–e98. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Korzeniewski, S.J. Are Infants Born by Elective Cesarean Delivery without Labor at Risk for Developing Immune Disorders Later in Life? Am. J. Obstet. Gynecol. 2013, 208, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Strunk, T.; Currie, A.; Richmond, P.; Simmer, K.; Burgner, D. Innate Immunity in Human Newborn Infants: Prematurity Means More than Immaturity. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2011, 24, 25–31. [Google Scholar] [CrossRef]
- Book, M.; Chen, Q.; Lehmann, L.E.; Klaschik, S.; Weber, S.; Schewe, J.-C.; Luepertz, M.; Hoeft, A.; Stuber, F. Inducibility of the Endogenous Antibiotic Peptide Beta-Defensin 2 Is Impaired in Patients with Severe Sepsis. Crit. Care 2007, 11, R19. [Google Scholar] [CrossRef]
- Al Mansour, N.; Al-Kafaji, G.; Al Mahmeed, A.; Bindayna, K.M. Dysregulation of Human Beta-Defensin-3 Expression in the Peripheral Blood of Patients with Sepsis. SAGE Open Med. 2021, 9, 20503121211041516. [Google Scholar] [CrossRef]
- Berkestedt, I.; Herwald, H.; Ljunggren, L.; Nelson, A.; Bodelsson, M. Elevated Plasma Levels of Antimicrobial Polypeptides in Patients with Severe Sepsis. J. Innate Immun. 2010, 2, 478–482. [Google Scholar] [CrossRef]
- Thomas, N.J.; Carcillo, J.A.; Doughty, L.A.; Sasser, H.; Heine, R.P. Plasma Concentrations of Defensins and Lactoferrin in Children with Severe Sepsis. Pediatr. Infect. Dis. J. 2002, 21, 34–38. [Google Scholar] [CrossRef]
- Hiratsuka, T.; Mukae, H.; Iiboshi, H.; Ashitani, J.; Nabeshima, K.; Minematsu, T.; Chino, N.; Ihi, T.; Kohno, S.; Nakazato, M. Increased Concentrations of Human Beta-Defensins in Plasma and Bronchoalveolar Lavage Fluid of Patients with Diffuse Panbronchiolitis. Thorax 2003, 58, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Cakir, E.; Torun, E.; Gedik, A.H.; Umutoglu, T.; Aktas, E.C.; Topuz, U.; Deniz, G. Cathelicidin and Human β-Defensin 2 in Bronchoalveolar Lavage Fluid of Children with Pulmonary Tuberculosis. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Tuberc. Lung Dis. 2014, 18, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Majewski, K.; Agier, J.; Kozłowska, E.; Brzezińska-Błaszczyk, E. Status of Cathelicidin IL-37, Cytokine TNF, and Vitamin D in Patients with Pulmonary Tuberculosis. J. Biol. Regul. Homeost. Agents 2018, 32, 321–325. [Google Scholar] [PubMed]
- Romero, R.; Pacora, P.; Kusanovic, J.P.; Jung, E.; Panaitescu, B.; Maymon, E.; Erez, O.; Berman, S.; Bryant, D.R.; Gomez-Lopez, N.; et al. Clinical Chorioamnionitis at Term X: Microbiology, Clinical Signs, Placental Pathology, and Neonatal Bacteremia—Implications for Clinical Care. J. Perinat. Med. 2021, 49, 275–298. [Google Scholar] [CrossRef]
- Farzin, A.; Boyer, P.; Ank, B.; Nielsen-Saines, K.; Bryson, Y. Amniotic Fluid Exhibits an Innate Inhibitory Activity against HIV Type 1 Replication In Vitro. AIDS Res. Hum. Retroviruses 2013, 29, 77–83. [Google Scholar] [CrossRef]
- Tambor, V.; Kacerovsky, M.; Lenco, J.; Bhat, G.; Menon, R. Proteomics and Bioinformatics Analysis Reveal Underlying Pathways of Infection Associated Histologic Chorioamnionitis in PPROM. Placenta 2013, 34, 155–161. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Du, C.; Zhang, R.; Feng, Z.; Zhang, J. Diagnostic Value of Amniotic Fluid Inflammatory Biomarkers for Subclinical Chorioamnionitis. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2016, 134, 160–164. [Google Scholar] [CrossRef]
- Buhimschi, C.S.; Dulay, A.T.; Abdel-Razeq, S.; Zhao, G.; Lee, S.; Hodgson, E.J.; Bhandari, V.; Buhimschi, I.A. Fetal Inflammatory Response in Women with Proteomic Biomarkers Characteristic of Intra-Amniotic Inflammation and Preterm Birth. BJOG Int. J. Obstet. Gynaecol. 2009, 116, 257–267. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Romero, R.; Xu, Y.; Leng, Y.; Garcia-Flores, V.; Miller, D.; Jacques, S.M.; Hassan, S.S.; Faro, J.; Alsamsam, A.; et al. Are Amniotic Fluid Neutrophils in Women with Intraamniotic Infection and/or Inflammation of Fetal or Maternal Origin? Am. J. Obstet. Gynecol. 2017, 217, 693.e1–693.e16. [Google Scholar] [CrossRef]
- Vento, G.; Lio, A.; Tirone, C.; Aurilia, C.; Tana, M.; Piras, A.; Ricci, C.; Perelli, S.; Romagnoli, C.; Posteraro, B.; et al. Association of High Levels of α-Defensins and S100A Proteins with Candida Mannan Detection in Bronchoalveolar Lavage Fluid of Preterm Neonates. Pediatr. Res. 2013, 74, 19–25. [Google Scholar] [CrossRef]
- Yoshio, H.; Yamada, M.; Yoshida, M.; Takeuchi, A.; Fujii, S.; Kunii, Y.; Kageyama, M.; Yokoi, Y.; Yamauchi, Y.; Agerberth, B. 426 Expression of a Human Cathelicidin Antimicrobial Peptide, LL-37, in Amniotic Fluid with Neonatal or Maternal Infection. Pediatr. Res. 2005, 58, 427. [Google Scholar] [CrossRef][Green Version]
- Blackburn, R.M.; Verlander, N.Q.; Heath, P.T.; Muller-Pebody, B. The Changing Antibiotic Susceptibility of Bloodstream Infections in the First Month of Life: Informing Antibiotic Policies for Early- and Late-Onset Neonatal Sepsis. Epidemiol. Infect. 2014, 142, 803–811. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Myntti, T.; Rahkonen, L.; Nupponen, I.; Pätäri-Sampo, A.; Tikkanen, M.; Sorsa, T.; Juhila, J.; Andersson, S.; Paavonen, J.; Stefanovic, V. Amniotic Fluid Infection in Preterm Pregnancies with Intact Membranes. Dis. Markers 2017, 2017, 8167276. [Google Scholar] [CrossRef] [PubMed]
- Pillay, K.; Coutsoudis, A.; Agadzi-Naqvi, A.K.; Kuhn, L.; Coovadia, H.M.; Janoff, E.N. Secretory Leukocyte Protease Inhibitor in Vaginal Fluids and Perinatal Human Immunodeficiency Virus Type 1 Transmission. J. Infect. Dis. 2001, 183, 653–656. [Google Scholar] [CrossRef]
- Farquhar, C.; VanCott, T.C.; Mbori-Ngacha, D.A.; Horani, L.; Bosire, R.K.; Kreiss, J.K.; Richardson, B.A.; John-Stewart, G.C. Salivary Secretory Leukocyte Protease Inhibitor Is Associated with Reduced Transmission of Human Immunodeficiency Virus Type 1 through Breast Milk. J. Infect. Dis. 2002, 186, 1173–1176. [Google Scholar] [CrossRef]
- Wu, T.-W.; Tabangin, M.; Kusano, R.; Ma, Y.; Ridsdale, R.; Akinbi, H. The Utility of Serum Hepcidin as a Biomarker for Late-Onset Neonatal Sepsis. J. Pediatr. 2013, 162, 67–71. [Google Scholar] [CrossRef]
- Hackam, D.; Caplan, M. Necrotizing Enterocolitis: Pathophysiology from a Historical Context. Semin. Pediatr. Surg. 2018, 27, 11–18. [Google Scholar] [CrossRef]
- Han, F.; Lu, Z.; Liu, Y.; Xia, X.; Zhang, H.; Wang, X.; Wang, Y. Cathelicidin-BF Ameliorates Lipopolysaccharide-Induced Intestinal Epithelial Barrier Disruption in Rat. Life Sci. 2016, 152, 199–209. [Google Scholar] [CrossRef]
- Otte, J.-M.; Zdebik, A.-E.; Brand, S.; Chromik, A.M.; Strauss, S.; Schmitz, F.; Steinstraesser, L.; Schmidt, W.E. Effects of the Cathelicidin LL-37 on Intestinal Epithelial Barrier Integrity. Regul. Pept. 2009, 156, 104–117. [Google Scholar] [CrossRef]
- Neu, J. Neonatal Necrotizing Enterocolitis: An Update. Acta Paediatr. 2005, 94, 100–105. [Google Scholar] [CrossRef]
- McElroy, S.J.; Weitkamp, J.-H. Innate Immunity in the Small Intestine of the Preterm Infant. NeoReviews 2011, 12, e517–e526. [Google Scholar] [CrossRef] [PubMed]
- Yee, W.H.; Soraisham, A.S.; Shah, V.S.; Aziz, K.; Yoon, W.; Lee, S.K. Canadian Neonatal Network Incidence and Timing of Presentation of Necrotizing Enterocolitis in Preterm Infants. Pediatrics 2012, 129, e298–e304. [Google Scholar] [CrossRef] [PubMed]
- Salzman, N.H.; Underwood, M.A.; Bevins, C.L. Paneth Cells, Defensins, and the Commensal Microbiota: A Hypothesis on Intimate Interplay at the Intestinal Mucosa. Semin. Immunol. 2007, 19, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kai, L.; Zhu, L.; Xu, B.; Chen, N.; Valencak, T.G.; Wang, Y.; Shan, T. Cathelicidin-WA Protects against LPS-Induced Gut Damage through Enhancing Survival and Function of Intestinal Stem Cells. Front. Cell Dev. Biol. 2021, 9, 685363. [Google Scholar] [CrossRef]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.J.; Pulendran, B.; Palucka, K. Immunobiology of Dendritic Cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef]
- Velilla, P.A.; Rugeles, M.T.; Chougnet, C.A. Defective Antigen-Presenting Cell Function in Human Neonates. Clin. Immunol. 2006, 121, 251–259. [Google Scholar] [CrossRef]
- Koeninger, L.; Armbruster, N.S.; Brinch, K.S.; Kjaerulf, S.; Andersen, B.; Langnau, C.; Autenrieth, S.E.; Schneidawind, D.; Stange, E.F.; Malek, N.P.; et al. Human β-Defensin 2 Mediated Immune Modulation as Treatment for Experimental Colitis. Front. Immunol. 2020, 11, 93. [Google Scholar] [CrossRef]
- Sheng, Q.; Lv, Z.; Cai, W.; Song, H.; Qian, L.; Mu, H.; Shi, J.; Wang, X. Human β-Defensin-3 Promotes Intestinal Epithelial Cell Migration and Reduces the Development of Necrotizing Enterocolitis in a Neonatal Rat Model. Pediatr. Res. 2014, 76, 269–279. [Google Scholar] [CrossRef]
- Chen, L.; Lv, Z.; Gao, Z.; Ge, G.; Wang, X.; Zhou, J.; Sheng, Q. Human β-Defensin-3 Reduces Excessive Autophagy in Intestinal Epithelial Cells and in Experimental Necrotizing Enterocolitis. Sci. Rep. 2019, 9, 19890. [Google Scholar] [CrossRef]
- Altobelli, E.; Angeletti, P.M.; Verrotti, A.; Petrocelli, R. The Impact of Human Milk on Necrotizing Enterocolitis: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1322. [Google Scholar] [CrossRef]
- Al-Alaiyan, S.; Abdulaziz, N.; Alkohlani, A.; Almairi, S.O.; Al Hazzani, F.; Binmanee, A.; Alfattani, A. Effects of Probiotics and Lactoferrin on Necrotizing Enterocolitis in Preterm Infants. Cureus 2021, 13, e18256. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Farahbakhsh, N.; Shastri, S.; Sharma, P. Biomarkers for Diagnosis of Neonatal Sepsis: A Literature Review. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2018, 31, 1646–1659. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.C.; Lam, H.S. Biomarkers for Late-Onset Neonatal Sepsis: Cytokines and Beyond. Clin. Perinatol. 2010, 37, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Mwesigye, P.; Rizwan, F.; Alassaf, N.; Khan, R. The Role and Validity of Diagnostic Biomarkers in Late-Onset Neonatal Sepsis. Cureus 2021, 13, e17065. [Google Scholar] [CrossRef]
- Buhimschi, C.S.; Bhandari, V.; Hamar, B.D.; Bahtiyar, M.-O.; Zhao, G.; Sfakianaki, A.K.; Pettker, C.M.; Magloire, L.; Funai, E.; Norwitz, E.R.; et al. Proteomic Profiling of the Amniotic Fluid to Detect Inflammation, Infection, and Neonatal Sepsis. PLoS Med. 2007, 4, e18. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Andrews, W.W.; Goepfert, A.R.; Faye-Petersen, O.; Cliver, S.P.; Carlo, W.A.; Hauth, J.C. The Alabama Preterm Birth Study: Umbilical Cord Blood Ureaplasma Urealyticum and Mycoplasma Hominis Cultures in Very Preterm Newborn Infants. Am. J. Obstet. Gynecol. 2008, 198, 43.e1–43.e5. [Google Scholar] [CrossRef]
- Buttery, J.P. Blood Cultures in Newborns and Children: Optimising an Everyday Test. Arch. Dis. Child. Fetal Neonatal Ed. 2002, 87, F25–F28. [Google Scholar] [CrossRef]
- Buhimschi, I.A.; Christner, R.; Buhimschi, C.S. Proteomic Biomarker Analysis of Amniotic Fluid for Identification of Intra-Amniotic Inflammation. BJOG Int. J. Obstet. Gynaecol. 2005, 112, 173–181. [Google Scholar] [CrossRef]
- Tambor, V.; Fučíková, A.; Lenco, J.; Kacerovský, M.; Řeháček, V.; Stulík, J.; Pudil, R. Application of Proteomics in Biomarker Discovery: A Primer for the Clinician. Physiol. Res. 2010, 59, 471–497. [Google Scholar] [CrossRef]
- Vanzolini, T.; Bruschi, M.; Rinaldi, A.C.; Magnani, M.; Fraternale, A. Multitalented Synthetic Antimicrobial Peptides and Their Antibacterial, Antifungal and Antiviral Mechanisms. Int. J. Mol. Sci. 2022, 23, 545. [Google Scholar] [CrossRef]
- Saravolatz, L.D.; Pawlak, J.; Johnson, L.; Bonilla, H.; Saravolatz, L.D.; Fakih, M.G.; Fugelli, A.; Olsen, W.M. In Vitro Activities of LTX-109, a Synthetic Antimicrobial Peptide, against Methicillin-Resistant, Vancomycin-Intermediate, Vancomycin-Resistant, Daptomycin-Nonsusceptible, and Linezolid-Nonsusceptible Staphylococcus Aureus. Antimicrob. Agents Chemother. 2012, 56, 4478–4482. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, H.; Nakamura, K.; Hu, Z.; Tamura, H.; Reich, J.; Kuwahara-Arai, K.; Iba, T.; Tabe, Y.; Nagaoaka, I. Antimicrobial Cathelicidin Peptide LL-37 Induces NET Formation and Suppresses the Inflammatory Response in a Mouse Septic Model. Mol. Med. Rep. 2017, 16, 5618–5626. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, R.Y.K.; Britton, W.J.; Chan, H.-K. Advances in the Development of Antimicrobial Peptides and Proteins for Inhaled Therapy. Adv. Drug Deliv. Rev. 2022, 180, 114066. [Google Scholar] [CrossRef]
- Berrington, J.E.; McGuire, W.; Embleton, N.D. ELFIN, the United Kingdom Preterm Lactoferrin Trial: Interpretation and Future Questions 1. Biochem. Cell Biol. 2021, 99, 1–6. [Google Scholar] [CrossRef]
- Pammi, M.; Suresh, G. Enteral Lactoferrin Supplementation for Prevention of Sepsis and Necrotizing Enterocolitis in Preterm Infants. Cochrane Database Syst. Rev. 2020, 3, CD007137. [Google Scholar] [CrossRef] [PubMed]
- Vogelmeier, C.; Gillissen, A.; Buhl, R. Use of Secretory Leukoprotease Inhibitor to Augment Lung Antineutrophil Elastase Activity. Chest 1996, 110, 261S–266S. [Google Scholar] [CrossRef]
- Alejandre Alcazar, M.A.; Kaschwich, M.; Ertsey, R.; Preuss, S.; Milla, C.; Mujahid, S.; Masumi, J.; Khan, S.; Mokres, L.M.; Tian, L.; et al. Elafin Treatment Rescues EGFR-Klf4 Signaling and Lung Cell Survival in Ventilated Newborn Mice. Am. J. Respir. Cell Mol. Biol. 2018, 59, 623–634. [Google Scholar] [CrossRef]
- Han, W.; Li, X.; Zhang, H.; Yu, B.; Guo, C.; Deng, C. Recombinant Human Elafin Promotes Alveologenesis in Newborn Mice Exposed to Chronic Hyperoxia. Int. J. Biochem. Cell Biol. 2017, 92, 173–182. [Google Scholar] [CrossRef]
- Boparai, J.K.; Sharma, P.K. Mini Review on Antimicrobial Peptides, Sources, Mechanism and Recent Applications. Protein Pept. Lett. 2020, 27, 4–16. [Google Scholar] [CrossRef]
- Hutchings, C.J.; Koglin, M.; Olson, W.C.; Marshall, F.H. Opportunities for Therapeutic Antibodies Directed at G-Protein-Coupled Receptors. Nat. Rev. Drug Discov. 2017, 16, 787–810. [Google Scholar] [CrossRef]
- Rodríguez, A.A.; Otero-González, A.; Ghattas, M.; Ständker, L. Discovery, Optimization, and Clinical Application of Natural Antimicrobial Peptides. Biomedicines 2021, 9, 1381. [Google Scholar] [CrossRef] [PubMed]
- Malekkhaiat Häffner, S.; Malmsten, M. Influence of Self-Assembly on the Performance of Antimicrobial Peptides. Curr. Opin. Colloid Interface Sci. 2018, 38, 56–79. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Björn, C.; Ekblom, J. Antimicrobial Peptides as Therapeutic Agents: Opportunities and Challenges. Crit. Rev. Biotechnol. 2020, 40, 978–992. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-Y.G.; Mookherjee, N. Multiple Immune-Modulatory Functions of Cathelicidin Host Defense Peptides. Front. Immunol. 2012, 3, 149. [Google Scholar] [CrossRef]
- Ashby, M.; Petkova, A.; Hilpert, K. Cationic Antimicrobial Peptides as Potential New Therapeutic Agents in Neonates and Children: A Review. Curr. Opin. Infect. Dis. 2014, 27, 258–267. [Google Scholar] [CrossRef]
- Adlakha, S.; Sharma, A.; Vaghasiya, K.; Ray, E.; Verma, R.K. Inhalation Delivery of Host Defense Peptides (HDP) Using Nano-Formulation Strategies: A Pragmatic Approach for Therapy of Pulmonary Ailments. Curr. Protein Pept. Sci. 2020, 21, 369–378. [Google Scholar] [CrossRef]
- Nordström, R.; Malmsten, M. Delivery Systems for Antimicrobial Peptides. Adv. Colloid Interface Sci. 2017, 242, 17–34. [Google Scholar] [CrossRef]
- Tripathi, J.K.; Pal, S.; Awasthi, B.; Kumar, A.; Tandon, A.; Mitra, K.; Chattopadhyay, N.; Ghosh, J.K. Variants of Self-Assembling Peptide, KLD-12 That Show Both Rapid Fracture Healing and Antimicrobial Properties. Biomaterials 2015, 56, 92–103. [Google Scholar] [CrossRef]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell. Infect. Microbiol. 2021, 11, 668632. [Google Scholar] [CrossRef]
- Yazici, A.; Ortucu, S.; Taskin, M.; Marinelli, L. Natural-Based Antibiofilm and Antimicrobial Peptides from Microorganisms. Curr. Top. Med. Chem. 2018, 18, 2102–2107. [Google Scholar] [CrossRef]


| AMPs | Sources | Antimicrobial Spectrum | Immunomodulating Actions | Other Properties |
|---|---|---|---|---|
| Human neutrophil peptides (HNPs) 1 to 4 | Neutrophils | Gram+ and gram- bacteria (S. aureus, bacillus subtilis, S. epidermis, E. coli, P. aeruginosa), fungi (C. albicans), and viruses (influenza v., HSV, CMV) | Induction: TNF-α and IL-1β and chemotaxis (neutrophils, immature Dcs and other immune cells). Inhibition: IL-10. | |
| Human defensins (HDs) 5 & 6 | PCs (HD-5 and -6), epithelia of the female reproductive tract (HD-5) | Gram+ and gram- bacteria (E. coli, Listeria monocytogenes, Salmonella typhimurium, S. aureus), and C. albicans). | Induction: IL-8 and chemotaxis of macrophages, T lymphocytes, and mast cells. | Modulation of the commensal bacteria in the small intestine. |
| Human β-defensins (HBDs) 1 to 4 | Neutrophils and other immune cells, keratinocytes, and epithelia of respiratory, GI, and genitourinary tracts. They can be found in blood, urine, heart, and skeletal muscles (HBD-3), and testis. | HBD-1 to -4: Gram- bacteria (P. aeruginosa, E. coli, vancomycin resistant Enterococcus); HBD-1: anaerobic gram+ bacteria. HBD-2 to -4: Gram+ bacteria (S. aureus, S. Mutans, Str. Pneumoniae, Str. pyogenes) | Induction: pro-inflammatory cytokines, chemotaxis of inflammatory cells, differentiation of monocytes, proliferation and activation of CD4+ T cells, activation of mast cells (release of histamine & PGD2). Inhibition: IL-6 and IL-8 (HBD-3), apoptosis of DCs. Linkage of innate with acquired immunity, activation of the classical complement system pathway. | Preservation of epithelial barrier integrity, amelioration and repair of inflammation-induced tissue injury, antioxidant action. |
| Cathelicidin LL-37 | Immune cells (neutrophils, macrophages, monocytes, B-cells, T-cells), and in most types of epithelial cells (GI, skin, lung, etc.). | Bacteria (E. coli, Listeria monocytogenes, Enterococcus faecium), fungi, and viruses. Synergistic effect with HNP-1, HBD-2, and HBD-3. Inhibition of biofilm formation. | Induction: Production of IL-1β, IL-6, IL-8, and TNF-α, IL-10, and chemokines, chemotaxis of neutrophils, monocytes, and mast cells, monocyte differentiation, macrophage pyroptosis and activation, vascular endothelium proliferation. Suppression: neutrophil apoptosis stimulates bactericidal activity. Inhibits sepsis-induced production of pro-inflammatory cytokines. Binds LPS (antiendotoxin action). | Promotion of angiogenesis, arteriogenesis, and re-epithelialization of wounded epithelia and epidermis. |
| Antileukoprotease elafin | Epithelia (skin, respiratory tract, intestine, endometrium), neutrophils, and macrophages. | S. Aureus, P. aeruginosa, Aspergillus fumigatus, and C. albicans. | Promotion of neutrophil and lymphocyte chemotaxis, LPS response, humoral and cellular aspects of adaptive immunity. Inhibition of inflammatory cell recruitment and NF-κB activation. | Inhibition of proteases, promotion of tissue remodeling and cellular differentiation. |
| Antileukoprotease SLPI | Inflammatory cells (neutrophils and macrophages, mast cells), keratinocytes, and epithelial cells of respiratory and GI systems, and amniotic membranes. | Gram+ bacteria (S. aureus and S. epidermidis, group A Streptococcus), Gram- bacteria (E. coli, P. aeruginosa), fungi (Aspergillus fumigatus, C. albicans), and viruses. | Inhibition of inflammatory infiltrate, NF-κB activation, mast cell histamine release, and C5a production. Modulation of adaptive immune responses. | Neutralization of proteases, involvement in cutaneous and oral mucosal wound healing. |
| HM Protein | Production of HM Protein-Derived AMPs | Antimicrobial Spectrum | Ref. |
|---|---|---|---|
| a-lactalbumin (La) | Digestion of La with trypsin releases 2 AMPs: α-La f(1–5)/LTD1, and α-La f(17–31) S-S(109–114)/LTD2. Digestion with chymotrypsin releases AMP α-La f(61–68)S-S(75–80)/LCD. | Mainly gram+ bacteria (Bacillus subtilis, S. epidermidis, S. lentus); α-La f(17–31) S-S(109–114)/LTD2 and α-La f(61–68)S-S(75–80) were also effective against S. aureus, K. pneumoniae, and P. aeruginosa. | [86,87,88] |
| Casein | Digestion of casein with chymocin, proteolysis, or acidification releases the AMPs casecidin, lactenin, isracidin, caseicin A and B, kapacin, and κ-casecidin. Fermentation by L. acidophilus was found to produce casein A, B, and C. | Gram+ and gram- pathogens (S. species, Str. pneumoniae, Listeria, Str. pyogenes, E. coli, Enterobacter faecalis, and P. aeruginosa). | [87,90,91] |
| Lactoferrin | Digestion of lactoferrin with pepsin produces lactoferricin (amino acids 1–40 of lactoferrin) and lactoferricin-derived shorter AMPs. | Effective against gram+ and gram- bacteria (S. aureus, Str. Mutans, E. coli), and viruses (HSV-1 & -2, CMV, HPV). | [87,89] |
| Human lactoferrampin: Synthetic peptide with an amino-acid sequence corresponding to 269–285 amino acids of human lactoferrin. | Broad spectrum of antibacterial activity, although some bacteria are resistant to this peptide (E. coli and Str. sanguis). | [17,89] |
| AMPs | First Author & Year [Ref.] | Aim | Study Design and Population/Material | Main Results | Reference |
|---|---|---|---|---|---|
| HNPs 1–4 in BAF | Tirone C, et al., 2010 | HNP -1 to -4 in BAF and ventilator-associated pneumonia. | Cohort study of 24 PTI (GA <30 wks), nine with pneumonia. Proteomics. | HNP-1 and -2 were detectable in all samples, and were increased in the pneumonia group. | [111] |
| HNP-1 to -3 | Faust K, et al., 2014 | Expression of HNP-1 to -3 in CB, and influencing factors. | Cohort study of 139 preterm (GA 24–36 wks) and 36 term infants (n = 36). HNP-1 to -3 in supernatants of whole-CB cultures. | Increased CB HNP-1 to -3 in clinical chorioamnionitis. | [131] |
| HD-5 & HD-6 | Mallow EB, et al. 1996 | HD-5 & -6 mRNA expression in PCs of the fetus. | Intestinal tissue from fetuses with GA 19–24 wks. | HD-5 and -6 mRNA detected in fetal PCs cells from 13.5 wks of GA, 40- to 250-fold less than in adults. | [132] |
| HD-5 and HD-6 | Salzman NH, et al. 1998 | HD-5 and HD-6 expression in PCs of NEC cases and controls. | Case-control study. Six NEC-cases (GA 25–31 wks) and five controls (GA 35–40 wks). | HD-5 expressed at 24 wks of GA at levels lower than term infants and adults, and increased three-fold in NEC cases. | [133] |
| HC-5 and PCs | Puiman PJ, et al., 2011 | PC developmental changes in PTI with NEC | Intestinal tissue from 55 PTI with NEC, 22 preterm controls, and nine term controls. | Acute NEC, no effect. After NEC recovery, PC hyperplasia and elevated HD-5 expression. | [134] |
| HC-5 and PCs | Heida FH, et al., 2016 | Developmental changes in PC and HD-5 expression. | Studied 57 samples of ileum tissue from fetuses/infants (GA 9–40 wks). | PCs expressing HD-5 observed at GA >29 wks. | [135] |
| HBD-1 | Wu J, et al., 2019 | Immunoregulatory function of HBD-1 in NCBM-dDC&TC. | In vitro; NCBM-dDC&TC from human CB. | HBD-1 promotes the differentiation and maturation of DCs, inhibits apoptosis of CBM-dDC, promotes proliferation and activation of CB CD4 + T cells. | [78] |
| HBD-1 and HBD-2 | Jenke ACW, et al., 2012 | Expression of HBD-1 and -2, IL-8, and TLR4 in NEC. | Cohort study of 68 ELBW infants (GA <27 wks); 12 with NEC, 56 without. Serial stool samples, and intestinal biopsies. | Fecal HBD-1 levels were low in all neonates, HBD-2 levels were increased in chorioamnionitis and moderate NEC (before clinical symptoms) but low in severe NEC. | [136] |
| HBD-2 | Richter M, et al., 2010 | Developmental changes in HBD-2 levels in stool from neonates. | Case-control study of 59 preterm and term infants. Stool samples collected between days three and 28. | HBD-2 levels increased significantly between 24 and 42 wks of GA and were not affected by sex or mode of delivery. | [137] |
| HBD-2 | Campeotto F, et al., 2010 | Levels of HBD-2 in feces of term and preterm infants and effect of intestinal distress. | Case–control study of 30 healthy term and 20 PTI. Fecal samples (up to day 30 or 60). Case-control study of 10 PTI with intestinal distress and 20 controls. | Fecal HBD-2 did not differ either between healthy term and preterm infants or between infants with clinical intestinal distress and controls, although it was increased in two out of three infants with NEC, and on out of seven with rectal bleeding. | [138] |
| HBD-2 | Olbrich P, et al., 2013 | HBD-2 levels in CB and its impact on sepsis. | Cohort study; 42 term and 31 preterm neonates. | HBD-2 was lower in preterm than in term infants. Low HBD-2 was associated with neonatal sepsis. | [139] |
| HBD-2 and gut microbiota | Corebima BIRV, et al., 2019 | Fecal HBD-2 and gut microbiota in PTI in relation to feeding patterns. | Cross-sectional study of 44 PTI, four groups related to type of milk feeding. | The formula milk group had the highest HBD-2, not correlated with microbiota. | [140] |
| HBD-3 | Bian T, et al., 2017 | Effects of HBD-3 on HUVECs triggered by TNF-α and inflammatory response. | In vitro. HUVECs culture. | HBD3 reduced production of inflammatory mediators and ROS by HUVECs, and inhibited NF-кB activation. | [50] |
| LL-37 | Braff et al., 2005 | Effects of LL-37 on neonatal human keratinocytes. | In vitro study. Gene expression in keratinocytes after exposure to LL-37. | LL-37 affected the expression and release by keratinocytes of several chemokines and cytokines. | [141] |
| LL37 | Nelson A, et al., 2009 | Effects of LL-37 on growth of S. epidermidis. | Skin swabs for cultures from 21 term neonates (12 with erythema toxicum). | LL37 was constitutively expressed in the skin, and significantly inhibited growth of S. epidermidis. | [142] |
| LL-37 | Misawa Y, et al., 2009 | LL-37 expression in neutrophils and plasma levels, and effect of 1a(OH)D3. | Included 25 neonates, 25 adults, and CB, as well as human myeloid leukemia cell line. | Expression of LL-37 was impaired in neonates, and was induced by addition of 1a(OH)D3. | [143] |
| LL-37 | Mandic- Havelka A, et al., 2010 | LL-37 levels in CB neutrophils and maternal and neonatal plasma; relation with delivery mode and biochemical markers. | Cohort study of 115 term infants (47 with elective CS) including 50 mother–infant pairs. | In vaginal delivery, cord plasma LL-37 was higher than in CS and was similar to maternal levels. In CS, cord LL-37 was lower than maternal levels. Cord LL-37 was correlated to plasma levels. | [144] |
| LL-37 & 24(OH)D | Gad GI, et al., 2015 | Diagnostic value of LL-37 in congenital pneumonia and in relation to (25 OH)D. | Case-control study; 30 neonates with pneumonia and 30 controls. Serum LL-37 and 25(OH)D assessed. | In congenital pneumonia, LL-37 increased and 25(OH)D decreased. Diagnostic value of LL-37 (cut-off level 17 pg/mmol): 93% sensitivity, 86% specificity. | [145] |
| LL-37 | Scheid A. et al., 2018 | Effects of LL-37 on antimicrobial activity in human newborn CB. | Cross-sectional study. 30 neonates (22 term, eight preterm). Antimicrobial activity tested before and after addition of LL-37. | Preterm CB had impaired antibacterial capacity against S. aureus, S. epidermidis, and Candida Albicans, which was enhanced by LL-37. | [114] |
| LL-37 and HNP-1–3 | Kai-Larsen Y, et al., 2007 | LL-37 and HNP-1 to -3 levels and antimicrobial activity of meconium vs neonatal feces. | Cross-sectional of 20 healthy breast-fed term neonates. | Meconium exhibited higher antimicrobial activity against E. coli and GBS than did neonatal feces. LL-37, HNP-1–2, and HD-5 were present in both meconium and feces; LL-37 higher in feces than in meconium. | [146] |
| LL-37 and HNP-1 to -3 | Strunk T, et al., 2009 | LL-37 and HNP-1 to -3 in CB and maternal blood, and their relation with GA and sepsis. | Cohort study of 105 neonates and 100 mothers. | LL-37 in PTI was lower than in term and maternal plasma. HNP-1 to -3 in neonates were lower than maternal levels. AMP levels were not correlated with chorioamnionitis or delivery mode. | [147] |
| LL-37 and HBD-1 | Marchini G, et al., 2002 | LL-37 and HBD-1 in skin and vernix caseosa of neonates with erythema toxicum. | Cross-sectional study. Skin biopsies of four term neonates with erythema toxicum and four controls, and vernix caseosa of six healthy infants. | LL-37 (inducible) and HBD-1 (constitutive) were expressed in dermal layer cells in erythema toxicum biopsies. LL-37 was detected in the vernix caseosa, also exhibiting antibacterial activity. | [148] |
| HBD-1, HBD-2, and LL-37 | Schaller-Bals S, et al., 2002 | HBD-1, HBD-2, and LL-37 in tracheal aspirates of term and preterm newborns with and without respiratory infections. | Cohort study of 45 ventilated newborns (GA 22–40 wks). Serial BAF samples were obtained daily during mechanical ventilation. | LL-37, HBD-1, and HBD-2 were detected in BAF. Their levels were comparable between term and preterm newborns, correlated with each other and with IL-8 & INF-α, and were increased in pulmonary or systemic infections. | [149] |
| Cathelicidin (CRAMP) and β-defensins | Dorschner RA, et al., 2003 | Expression of cathelicidin and β-defensins in skin of mice and human neonates. | In vitro study in skin of embryonic and newborn mice, and human newborn foreskin. | Cathelicidin expression was increased in the perinatal period. HBD-2 was present in newborn skin. LL-37 and HBD-2 had synergistic activity against GBS. | [150] |
| HBDs and LL-37 | Starner TD, et al., 2005 | Development and antimicrobial spectrum of HBD-1, -2, and -3, and LL-37, in the neonatal lung. | Midgestational fetal lung explants (GA 18–22 wks), and tracheal aspirates at birth, seven months, and 13 years of age. | HBD-2 and HBD-1 expression was detected in term and postnatal tissues, but not in prenatal tissues. HBD-3 was not detected. LL-37 was expressed in tissues from all developmental ages. | [109] |
| HBD-1, HBD-3, and LL-37. | Gschwandtner M, et al., 2014 | Expression and regulation of HBDs and LL-37 in fetal, neonatal, and adult keratinocytes. | In vitro study in cultured keratinocytes from fetal skin (GA 20–23 wks), neonatal foreskin, and adult skin. | The expression of HBD-2, HBD-3, and LL-37 was significantly higher in keratinocytes from fetal skin than in postnatal skin, and further increased after stimulation. | [151] |
| HBD-1, HBD-2 and LL-37 | Strunk T, et al., 2017 | HBD-1, HBD-2, and LL-37 plasma levels in PTI, and effects of Bifidobacterium breve supplementation. | Cohort study of PTI (GA <30 wks). Plasma on days 1, 14, 28, and stool prior to and 21 days after probiotic supplementation. | Stool, plasma, and stimulated blood AMP levels changed significantly during the first month of life. Probiotic supplementation did not affect AMP levels. | [110] |
| SLPI in BAF | Ohlsson K, et al., 1992 | The importance of SLPI in protecting against ventilator-induced lung damage in neonates. | Cohort study. 38 ventilated neonates (25 RDS without BPD, 10 RDS and BPD, three pneumonia). Serial SLPI measurements in BAF. | Infants with pneumonia had higher levels of SLPI and elastase in BAF than those with RDS. Infants who also developed BPD had intermediate values. | [152] |
| SLPI and BPD | Watterberg KL, et al., 1994 | Relation of SLPI with neutrophil counts, elastase activity, and BPD in neonates. | Prospective cohort study of 41 neonates; 24/41 developed BPD. Serial BAF samples up to day 28. | During the first week of life, SLPI levels were similar between BPD and no-BPD groups. Neutrophil counts and elastase activity were higher in the BPD group. | [153] |
| SLPI | Sveger T. et al., 2002 | Effect of protease/protease inhibitor balance and neutrophil activity in BAF on RDS and BPD. | Cohort study of ventilated PTI with RDS (n = 43). BAF obtained in the first wk of life. Elastase and SLPI were determined. | BPD correlated with low SLPI (p = 0.03) (and other anti-protease levels) at seven or eight days of age. CS was correlated with low levels of SLPI on days three and four. | [154] |
| Elafin, HNP-1 to -4 | Kothiyal P, et al., 2020 | Delivery mode relation to postpartum expression of elafin and HNP-1 to -4 genes. | Cross-sectional study of 324 mothers delivering at term; 181 had vaginal delivery and 143 CS. | AMPs were upregulated in vaginal delivery compared to CS either with or without labor. | [155] |
| Pro-hepcidin | Yapakci E, et al., 2009 | Relation of serum pro-hepcidin in septic neonates with serum iron parameters. | Case–control study of 15 septic PTI, 17 healthy PTI, six septic term and 16 healthy term neonates. | Pro-hepcidin was significantly increased in preterm and term neonates with sepsis. Pro-hepcidin was not correlated with iron parameters. | [81] |
| Hepcidin | Cizmeci MN, et al., 2014 | The value of CB hepcidin levels as a biomarker for early-onset neonatal sepsis. | Pilot data of a prospective cohort study. 38 septic infants (GA 24–41 wks), 20 term and 18 preterm. | Newborns with early onset sepsis had an increased range of CB hepcidin, that was not correlated with GA or markers of anemia. | [156] |
| Pro-hepcidin | Celik HT, et al., 2015 | Association of serum pro-hepcidin in PTI with oxygen radical diseases (i.e., BPD, ROP, NEC). | Case–control study. 80 PTI (GA 25–34 wks); 38/80 with oxygen radical diseases and 42/80 controls. | Pro-hepcidin levels were increased in neonates with BPD or ROP, but not NEC. They were lower in preterm than in term newborns, and not correlated with iron parameters. | [83] |
| HNP1–3, HBD-2, l LL37, SLPI, lactoferrin | Akinbi HT, 2004 | HNP-1 to -3, HBD-2, LL37, SLPI, lactoferrin, and lysozyme in vernix caseosa and amniotic fluid in the absence of chorioamnionitis. | Cohort study of term infants delivered via elective CS without labor or PROM. 25 samples of vernix and 10 of amniotic fluid were collected. | HNP-1 to -3, SLPI, lactoferrin, and lysozyme were identified in vernix suspensions and amniotic fluid; HBD-2 and LL-37 were not detected. | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agakidou, E.; Agakidis, C.; Kontou, A.; Chotas, W.; Sarafidis, K. Antimicrobial Peptides in Early-Life Host Defense, Perinatal Infections, and Necrotizing Enterocolitis—An Update. J. Clin. Med. 2022, 11, 5074. https://doi.org/10.3390/jcm11175074
Agakidou E, Agakidis C, Kontou A, Chotas W, Sarafidis K. Antimicrobial Peptides in Early-Life Host Defense, Perinatal Infections, and Necrotizing Enterocolitis—An Update. Journal of Clinical Medicine. 2022; 11(17):5074. https://doi.org/10.3390/jcm11175074
Chicago/Turabian StyleAgakidou, Eleni, Charalampos Agakidis, Angeliki Kontou, William Chotas, and Kosmas Sarafidis. 2022. "Antimicrobial Peptides in Early-Life Host Defense, Perinatal Infections, and Necrotizing Enterocolitis—An Update" Journal of Clinical Medicine 11, no. 17: 5074. https://doi.org/10.3390/jcm11175074
APA StyleAgakidou, E., Agakidis, C., Kontou, A., Chotas, W., & Sarafidis, K. (2022). Antimicrobial Peptides in Early-Life Host Defense, Perinatal Infections, and Necrotizing Enterocolitis—An Update. Journal of Clinical Medicine, 11(17), 5074. https://doi.org/10.3390/jcm11175074







