Abstract
We evaluated the prognostic value of C-reactive protein (CRP), albumin, CRP clearance (CRPc) and CRP/albumin ratio (CAR) in neurocritically ill patients with acute stroke. This is a retrospective, observational study. We included acute stroke patients who were hospitalized in the neurosurgical ICU from January 2013 to September 2019. The primary outcome was in-hospital mortality. A total of 307 patients were enrolled in the study. Among them, 267 (87.0%) survived until discharge from the hospital. CRP and CAR were significantly higher in non-survivors than in survivors (both p < 0.001). Serum albumin levels were significantly lower in the non-survivors than in the survivors (p < 0.001). In receiver operating characteristic curve analysis for prediction of in-hospital mortality, the area under the curve of CRP (C-statistic: 0.820) and CAR (C-statistic: 0.824) were greater than that of CRPc (C-statistic: 0.650) and albumin (C-statistic: 0.734) (all p < 0.005). However, there was no significant difference in the predictive performance between CRP and CAR (p = 0.287). In this study, CRP and CAR were more important than CRPc and albumin in predicting mortality of neurocritically ill patients with stroke. Early CRP level and CAR determination may help to predict the in-hospital mortality of these patients.
1. Introduction
Serum biomarkers may help predict the prognosis of critically ill patients and make early decisions for their treatment [1]. Among the numerous biomarkers, C-reactive protein (CRP) and serum albumin are commonly used predictors of morbidity and mortality in critically ill patients [2,3]. CRP can be increased in the presence of non-specific acute-phase inflammation [4,5]. Markedly increased CRP levels may be associated with poor outcomes in critically ill patients [6] and changes in CRP concentrations have been associated with the outcomes of critically ill septic patients [7]. In addition, malnutrition is associated with poor outcomes in intensive care unit patients (ICU) [8,9,10]; albumin may reflect the nutritional state of critically ill patients and a low level of serum albumin may be associated with a malnutritional state in these patients [11,12]. Therefore, serum albumin levels may help to predict the clinical outcomes of critically ill patients [13,14]. Consequentially, the CRP/albumin ratio (CAR) can reflect inflammation as well as malnutrition [15,16]. Therefore, CAR may be a useful biochemical marker for predicting clinical outcomes in critically ill patients [17].
High CRP levels may reflect the progression of vascular disease [4,5] and may be associated with poor neurological outcomes in patients with ischemic stroke [5,18]. On the other hand, low serum albumin is associated with poor prognosis and mortality in aneurysmal subarachnoid hemorrhage [19]. Therefore, high CRP levels and low serum albumin may be also associated with poor neurological outcomes in neurocritically ill patients with stroke [19,20,21,22].
However, it is unclear whether absolute CRP levels and their changes are important, and whether CAR is also associated with the clinical prognosis of neurocritically ill patients with stroke. Therefore, in the present study, we evaluated the prognostic value of CRP, albumin, CRP clearance (CRPc), and CAR in neurocritically ill patients with acute stroke.
2. Methods
2.1. Study Population
This is a retrospective, single-center, observational study. Adult patients who were admitted to the neurosurgical ICU in our tertiary hospital (Samsung Medical Center, Seoul, Republic of Korea) from January 2013 to September 2019 were eligible. This study was approved by the Institutional Review Board (IRB) of Samsung Medical Center (IRB approval number: SMC 2020-09-082). The requirement for informed consent was waived by IRB due to its retrospective nature. We included patients (1) who were hospitalized in the neurosurgical ICU, (2) those with acute stroke, and (3) those with initial levels of CRP and serum albumin collected within 12 h after ICU admission and with follow-up levels. Of these patients, we excluded patients (1) who were aged below 18 years, (2) those who underwent neurosurgery and surgical intervention within 7 days after ICU admission, (3) those with acute infectious disease, (4) those with severely neurodegenerative diseases and chronic neurological abnormality, (5) those with insufficient medical records, (6) those with a ‘do not resuscitate’ order, and (7) those who were admitted to departments other than neurosurgery.
2.2. Definitions and Endpoints
In this study, baseline characteristics of comorbidities, ICU management, and laboratory data were collected retrospectively using Clinical Data Warehouse, which was designed for investigators to search and retrieve de-identified medical records from the electronic archives. Acute stroke is defined as the acute onset of focal neurological findings in a vascular territory or altered consciousness as a result of an underlying cerebrovascular disease such as cerebral infarction, intracranial hemorrhage other than trauma, or subarachnoid hemorrhage. The concentration of CRP and serum albumin levels were collected from ICU admission to 7 days. Baseline CRP was defined as the maximal level of CRP within 72 h from ICU admission. Baseline albumin was defined as a minimal level of serum albumin within 72 h after ICU admission. The CAR was defined as the percentage of the ratio of baseline CRP to albumin (100 × baseline CRP/baseline albumin). CRP kinetics was expressed as ΔCRP concentrations, which are the differences between baseline and subsequent measurement (the lowest serum CRP level from the 4th to the 7th day after admission). CRPc was calculated as the percentage of ΔCRP over the baseline CRP level [7]. Serum CRP levels were measured using immunoturbidimetric assays (CRPL3, Roche Diagnostics, Indianapolis, IN, USA) with a lower reference limit of 0.3 m/dL [23]. Acute Physiology and Chronic Health Evaluation (APACHE) II scores were calculated with the worst values recorded during the initial 24 h after the ICU admission [24,25]. This score ranges from a minimum of 0 to a maximum of 71; increasing score is associated with an increasing risk of hospital death. If the patient was intubated, the verbal score of the Glasgow Coma Scale (GCS) was estimated using the eye and motor scores as described previously [26]. The primary outcome was in-hospital mortality.
2.3. Statistical Analyses
Continuous variables were presented as means plus or minus standard deviations, and categorical variables were represented as numbers with subsequent percentages. Data comparison was carried out using Student’s t-test for continuous variables, whereas the Chi-square test or Fisher’s exact test was used for categorical variables. We assessed the predictive performance of CRP, CRP variants, and albumin using the areas under the curve (AUCs) of the receiver operating characteristic (ROC) curves for sensitivity vs. 100-specificity. We compared AUCs using the nonparametric approach published by DeLong et al. [27] for two correlated AUCs. Clinically relevant variables, including CRP, CRPc, CAR, albumin, age, sex, comorbidities, habitual risk factors, classification of stroke subtypes, APACHE II score on ICU admission, and ICU management were subjected to multiple logistic regression analyses to obtain statistically meaningful predictors. Due to the high proportion of malignant patients in this study, it was not possible to exclude all cancer patients from the study. Therefore, multiple logistic regression and subgroup analyzes according to the presence of malignancies were performed, because CRP can also be increased by cancer itself. Adequacy of the prediction model was determined using the Hosmer-Lemeshow test, along with the areas under the curve (AUC). All tests were two-sided and p < 0.05 was considered statistically significant. All data were analyzed using R Statistical Software (version 4.2.1; R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Baseline Characteristics and Clinical Outcome
A total of 307 patients were enrolled in the study (Figure 1). Among them, 267 (87.0%) survived until discharge from the hospital. The patients’ median age was 59.5 ± 16.2 years. One hundred and forty-seven patients (47.9%) were males. Hypertension (47.9%) and malignancy (31.3%) were the most common comorbidities in the study population. Intracranial hemorrhage (55.4%) was the most common cause of ICU admission. APACHE II score on ICU admission was higher in the non-survivors than in the survivors (p < 0.001). GCS on ICU admission was lower in the non-survivors than in the survivors (p < 0.001). In addition, mechanical ventilation, more than one hyperosmolar agent, and vasopressor were more commonly used in the non-survivors than in the survivors. A comparison of baseline characteristics of both survivors and non-survivors is presented in Table 1.
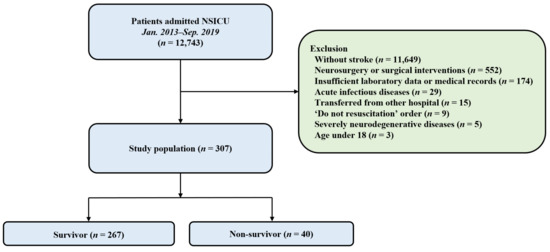
Figure 1.
A flowchart of the study. A total of 12,743 patients admitted to the NSICU were enrolled, and 307 patients were included for the analysis. NSICU, Neurosurgical intensive care unit.

Table 1.
Comparison of baseline characteristics between the survivors and non-survivors.
3.2. Relationship between CRP, CRP Variants, and Albumin and Clinical Outcomes
Levels of serum CRP, albumin, and CAR along with ΔCRP, and CRPC were compared between the survivors and the non-survivors (Table 2). CRP and CAR were significantly higher in non-survivors than in survivors (both p < 0.001). Also, in the subgroup analysis in patients with neurological deficit with a GCS of less than 13, CRP and CAR were significantly higher in non-survivors (p = 0.019 and p = 0.036, respectively). In addition, CRP and CAR were also significantly higher in non-survivors in a subgroup analysis of patients who underwent mechanical ventilation (both p = 0.004). Serum albumin levels were significantly lower in non-survivors than in survivors (p < 0.001). However, there were no significant differences in ΔCRP and CRPC between the two groups (p = 0.261, p = 0.701, respectively). In multivariable analysis, CRP (adjusted odds ratio [OR]: 1.41, 95% confidence interval [CI]: 1.05–1.91), APACHE II score on ICU admission (adjusted OR: 1.09, 95% CI: 1.01–1.17), mechanical ventilation (adjusted OR: 7.74, 95% CI: 2.48–30.03), use of vasopressor (adjusted OR: 4.46, 95% CI: 1.15–18.25), use of mannitol (adjusted OR: 0.24, 95% CI: 0.07–0.67), and use of more than one hyperosmolar agent (adjusted OR: 4.21, 95% CI: 1.67–11.33) were significantly associated with in-hospital mortality (Hosmer–Lemeshow Chi-squared = 5.074, df = 8, p = 0.680) with AUCs of 0.936 (95% CI 0.902–0.970) (Table 3). In ROC curve analysis for prediction of in-hospital mortality, the AUCs of CRP (C-statistic: 0.820, 95% CI: 0.772–0.863) and CAR (C-statistic: 0.824, 95% CI: 0.776–0.866) were greater than that of CRPc (C-statistic: 0.650, 95% CI: 0.592–0.704) and albumin (C-statistic: 0.734, 95% CI: 0.679–0.784) (all p < 0.05). However, there was no significant difference in the predictive performance between CRP and CAR (p = 0.287) (Figure 2).

Table 2.
Comparison of serum CRP and albumin levels between survivors and non-survivors.

Table 3.
Predicting factors for in-hospital mortality in patients assessed using logistic regression model.
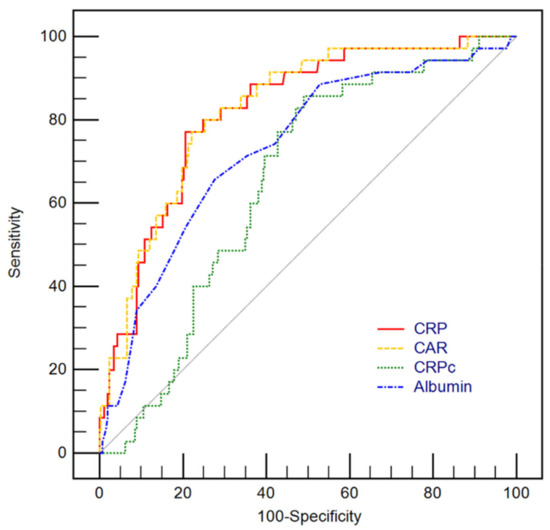
Figure 2.
Receiver operating characteristic curves for predicting in-hospital mortality using levels of C-reactive protein (CRP), CRP to albumin ratio (CAR), CRP clearance (CRPc) along with albumin. The area under the curves (AUCs) of CRP (C-statistic: 0.820, 95% confidence interval [CI]: 0.772–0.863) and CAR (C-statistic: 0.824, 95% CI: 0.776–0.866) were greater than that of CRPc (C-statistic: 0.650, 95% CI: 0.592–0.704) and Albumin (C-statistic: 0.734, 95% CI: 0.679–0.784) with significant statistical differences (all p < 0.05). However, there were no statistically significant differences between AUC of CAR and CRP (p = 0.287).
There were no significant differences in CRP and CAR between stroke patients with malignancy and those without malignancy (Table S1). In addition, in-hospital mortality was similar between these two groups. Although CRP level and CAR were different between stroke subtypes (Table S2), patterns of ROC curves for predicting in-hospital mortality were similar for the patients with intracranial hemorrhage, the patients with subarachnoid hemorrhage, and total patients (Figure S1).
4. Discussion
In the present study, we investigated the prognostic value of CRP, albumin, CRPc, and CAR in neurocritically ill patients with acute stroke. The major findings were as follows: First, there were significant differences in CRP, CAR, and albumin between survivors and non-survivors. However, there were no significant differences in ΔCRP and CRPc between the two groups. Second, in the multivariable analysis, CRP, APACHE II score on ICU admission, mechanical ventilation, use of vasopressor, use of mannitol, and use of more than one hyperosmolar agent were significantly associated with in-hospital mortality. Finally, CRP was demonstrated as a useful biomarker for predicting in-hospital mortality. However, there was no significant difference in the predictive performance between CRP and CAR. Therefore, CRP and CAR may be equally helpful to predict the prognosis of neurocritically ill patients with stroke and to make early decisions for their treatment.
Proinflammatory cytokines may be associated with neurological deterioration in patients with stroke [28]. CRP is an acute-phase reactant that synthesized by hepatocytes and regulated by proinflammatory cytokines, especially interleukin (IL)-6 [29]. CRP levels can be obtained quickly and easily compared to proinflammatory cytokines including IL-6, IL-1β, and nucleotide-binding domain and leucine-rich repeat protein-3 (NLRP3), which makes CRP testing superior to proinflammatory cytokines. In addition, CRP levels positively correlated with infarction volume compared with other laboratory tests and proinflammatory cytokines [30]. As a result, CRP plays an important role in the progression of cerebral tissue injury [30].
High CRP levels are associated with long-term poor functional outcomes in patients with ischemic stroke [23]. Acute local inflammation and changes in inflammatory cytokines levels develop in patients with ischemic brain injury due to arterial occlusion [18,23]. CRP levels can reflect the extent of cerebral infarction [18]. Ischemic stroke patients with early CRP elevation have an increased risk of death and cardiovascular mortality [18]. In addition, high CRP levels are associated with delayed cerebral ischemia and vasospasm after subarachnoid hemorrhage [23,31,32]. Therefore, in the early stage, high levels of CRP may be associated with clinical outcomes in patients with stroke. However, it is unclear whether absolute CRP levels and their change are important. In septic patients, rapidly decreased CRP levels have been associated with favorable clinical outcomes [7]. However, in this study, the change of CRP was not associated with clinical outcomes of neurocritically ill patients with stroke. Therefore, the absolute value of CRP may be more important in predicting mortality than its change and may be associated with vascular inflammation and disease severity in neurocritically ill patients with stroke.
Serum albumin levels may be associated with nutritional state and clinical outcomes in critically ill patients. In addition, as an inflammatory response, albumin levels can be reduced due to a decreased hepatic synthesis and an increased vascular permeability. Similarly, in patients with intracranial hemorrhage, serum albumin may be associated with acute inflammatory response and the severity of intracranial hemorrhage. [33]. In the present study, serum albumin levels were significantly different between the survivors and non-survivors. However, the absolute difference was small between the two groups. Therefore, in this study, the CAR predictive performance may largely be due to CRP other than serum albumin. Notably, there was no significant difference in the predictive performance between CRP and CAR. Considering the clinically relevant factors including APACHE II score on ICU admission, mechanical ventilation, vasopressor, and hyperosmolar therapy, an early CRP level may help to predict in-hospital mortality of neurocritically ill patients with stroke.
Serum CRP level can be elevated in patients with malignancies. CRP level may be associated with disease activity (i.e., quiescence or rapid growth phase) and clinical prognosis [34,35,36]. Several studies have shown that factors such as D-dimer, fibrinogen, and lactate dehydrogenase (LDH) are also highly correlated with malignant tumor activity and prognosis. [37,38,39,40,41]. In this study, there were no statistically significant differences in serum levels of D-dimer, fibrinogen, and LDH between stroke patients with malignancies and those without malignancies (Supplementary Table S2). Therefore, the stroke patients with malignancies would have had relatively low disease activity in this study. Indeed, CRP levels were similar between two groups. In addition, in this study, malignancy itself was not an important prognostic factor in multivariate analysis.
This study has several limitations. First, this was a retrospective and observational study and some data on CRP and albumin values were missing. Second, the non-randomized nature of the registry data might have resulted in selection bias. Third, elevated CRP levels can also be observed in patients with chronic inflammatory and neurodegenerative diseases [42]. The patients with severely neurodegenerative diseases and chronic neurological abnormality were excluded, but the patients with mild or undiagnosed illnesses might not be excluded. In addition, elevated CRP levels in patients with advanced or active cancers have not been evaluated in this study. Finally, a small sample size may limit statistical power in this study. Although the present study provides valuable insights, prospective large-scale studies are needed to further confirm the usefulness of CRP, CAR, and albumin in predicting clinical outcomes of stroke patients with evidence-based conclusions.
5. Conclusions
In this study, CRP and CAR were more important than CRPc and albumin in predicting mortality of neurocritically ill patients with stroke. However, there was no significant difference in the predictive performance between CRP and CAR. Therefore, CRP and CAR may be equally helpful in predicting the prognosis of neurocritically ill patients with stroke and in making early decisions for their treatment. Eventually, early CRP levels and CAR may help to predict the in-hospital mortality of these patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11175067/s1. Table S1. Comparison of serum CRP and Albumin levels between stroke patients with malignancy and those without malignancy. Table S2. Comparison of serum CRP and Albumin levels according to stroke subtypes. Figure S1. Receiver operating characteristic curves for predicting in-hospital mortality using levels of C-reactive protein (CRP), CRP to albumin ratio (CAR), CRP clearance (CRPc) and albumin according to intracranial hemorrhage and subarachnoid hemorrhage.
Author Contributions
Conceptualization, J.H.J. and J.-A.R.; Data curation, S.H.; Formal analysis, J.H.J., S.H. and J.-A.R.; Investigation, J.-A.R.; Methodology, J.H.J. and J.-A.R.; Resources, S.H.; Supervision, J.-A.R.; Validation, J.-A.R.; Visualization, S.H.; Writing—original draft, J.H.J. and J.-A.R.; Writing—review & editing, J.-A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This is a retrospective, single-center, observational study. Adult patients who were admitted to the neurosurgical ICU in our tertiary hospital (Samsung Medical Center, Seoul, Republic of Korea) from January 2013 to September 2019 were eligible. This study was approved by the Institutional Review Board (IRB) of Samsung Medical Center (IRB approval number: SMC 2020-09-082). The requirement for informed consent was waived by IRB due to its retrospective nature.
Informed Consent Statement
Not applicable. This study does not contain individual or personal data in any form (including individual details, images, or videos).
Data Availability Statement
Our data are available on Harvard Dataverse Network (http://dx.doi.org/10.7910/DVN/JG0ILD, accessed on 8 July 2022) as recommended repositories.
Acknowledgments
We would like to thank the nursing director of the neurosurgical intensive care unit, Suk Kyung Choo, for providing excellent advice and fruitful discussions. We would also like to thank all the nurses of the neurosurgical intensive care unit at the Samsung Medical Center for their support in the completion of this study.
Conflicts of Interest
The authors declare that they have no competing interest.
References
- Bender, M.; Haferkorn, K.; Friedrich, M.; Uhl, E.; Stein, M. Impact of Early C-Reactive Protein/Albumin Ratio on Intra-Hospital Mortality Among Patients with Spontaneous Intracerebral Hemorrhage. J. Clin. Med. 2020, 9, 1236. [Google Scholar] [CrossRef] [PubMed]
- Devran, O.; Karakurt, Z.; Adıgüzel, N.; Güngör, G.; Moçin, O.Y.; Balcı, M.K.; Celik, E.; Saltürk, C.; Takır, H.B.; Kargın, F.; et al. C-reactive protein as a predictor of mortality in patients affected with severe sepsis in intensive care unit. Multidiscip. Respir. Med. 2012, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Quispe, E.Á.; Li, X.M.; Yi, H. Comparison and relationship of thyroid hormones, IL-6, IL-10 and albumin as mortality predictors in case-mix critically ill patients. Cytokine 2016, 81, 94–100. [Google Scholar] [CrossRef]
- Juvela, S.; Kuhmonen, J.; Siironen, J. C-reactive protein as predictor for poor outcome after aneurysmal subarachnoid haemorrhage. Acta Neurochir. 2012, 154, 397–404. [Google Scholar] [CrossRef] [PubMed]
- VanGilder, R.L.; Davidov, D.M.; Stinehart, K.R.; Huber, J.D.; Turner, R.C.; Wilson, K.S.; Haney, E.; Davis, S.M.; Chantler, P.D.; Theeke, L.; et al. C-reactive protein and long-term ischemic stroke prognosis. J. Clin. Neurosci. 2014, 21, 547–553. [Google Scholar] [CrossRef]
- Qu, R.; Hu, L.; Ling, Y.; Hou, Y.; Fang, H.; Zhang, H.; Liang, S.; He, Z.; Fang, M.; Li, J.; et al. C-reactive protein concentration as a risk predictor of mortality in intensive care unit: A multicenter, prospective, observational study. BMC Anesthesiol. 2020, 20, 292. [Google Scholar] [CrossRef]
- Ryu, J.A.; Yang, J.H.; Lee, D.; Park, C.M.; Suh, G.Y.; Jeon, K.; Cho, J.; Baek, S.Y.; Carriere, K.C.; Chung, C.R. Clinical Usefulness of Procalcitonin and C-Reactive Protein as Outcome Predictors in Critically Ill Patients with Severe Sepsis and Septic Shock. PLoS ONE 2015, 10, e0138150. [Google Scholar]
- Villet, S.; Chiolero, R.L.; Bollmann, M.D.; Revelly, J.P.; Cayeux, R.N.M.; Delarue, J.; Berger, M.M. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin. Nutr. 2005, 24, 502–509. [Google Scholar] [CrossRef]
- Dvir, D.; Cohen, J.; Singer, P. Computerized energy balance and complications in critically ill patients: An observational study. Clin. Nutr. 2006, 25, 37–44. [Google Scholar] [CrossRef]
- Moisey, L.L.; Mourtzakis, M.; Cotton, B.A.; Premji, T.; Heyland, D.K.; Wade, C.E.; Bulger, E.; Kozar, R.A.; Nutrition and Rehabilitation Investigators Consortium (NUTRIC). Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit. Care 2013, 17, R206. [Google Scholar] [CrossRef]
- Carriere, I.; Dupuy, A.M.; Lacroux, A.; Cristol, J.P.; Delcourt, C. Biomarkers of inflammation and malnutrition associated with early death in healthy elderly people. J. Am. Geriatr. Soc. 2008, 56, 840–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domínguez de Villota, E.; Mosquera, J.M.; Rubio, J.J.; Galdos, P.; Díez Balda, V.; de la Serna, J.L.; Tomás, M.I. Association of a low serum albumin with infection and increased mortality in critically ill patients. Intensive Care Med. 1980, 7, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Kendall, H.; Abreu, E.; Cheng, A.L. Serum Albumin Trend Is a Predictor of Mortality in ICU Patients With Sepsis. Biol. Res. Nurs. 2019, 21, 237–244. [Google Scholar] [CrossRef]
- Yap, F.H.; Joynt, G.M.; Buckley, T.A.; Wong, E.L. Association of serum albumin concentration and mortality risk in critically ill patients. Anaesth. Intensive Care 2002, 30, 202–207. [Google Scholar] [CrossRef]
- Iwata, M.; Kuzuya, M.; Kitagawa, Y.; Iguchi, A. Prognostic value of serum albumin combined with serum C-reactive protein levels in older hospitalized patients: Continuing importance of serum albumin. Aging Clin. Exp. Res. 2006, 18, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Donini, L.M.; Savina, C.; Ricciardi, L.M.; Coletti, C.; Paolini, M.; Scavone, L.; de Felice, M.R.; Laviano, A.; Rossi Fanelli, F.; Cannella, C. Predicting the outcome of artificial nutrition by clinical and functional indices. Nutrition 2009, 25, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.K.; Song, I.A.; Lee, J.H. Clinical usefulness of C-reactive protein to albumin ratio in predicting 30-day mortality in critically ill patients: A retrospective analysis. Sci. Rep. 2018, 8, 14977. [Google Scholar] [CrossRef]
- Di Napoli, M.; Papa, F.; Bocola, V. C-reactive protein in ischemic stroke: An independent prognostic factor. Stroke 2001, 32, 917–924. [Google Scholar] [CrossRef]
- Kapoor, A.; Dhandapani, S.; Gaudihalli, S.; Dhandapani, M.; Singh, H.; Mukherjee, K.K. Serum albumin level in spontaneous subarachnoid haemorrhage: More than a mere nutritional marker! Br. J. Neurosurg. 2018, 32, 47–52. [Google Scholar] [CrossRef]
- Schuss, P.; Hadjiathanasiou, A.; Brandecker, S.; Güresir, Á.; Vatter, H.; Güresir, E. Elevated C-reactive protein and white blood cell count at admission predict functional outcome after non-aneurysmal subarachnoid hemorrhage. J. Neurol. 2018, 265, 2944–2948. [Google Scholar] [CrossRef]
- Jeon, Y.T.; Lee, J.H.; Lee, H.; Lee, H.K.; Hwang, J.W.; Lim, Y.J.; Park, H.P. The postoperative C-reactive protein level can be a useful prognostic factor for poor outcome and symptomatic vasospasm in patients with aneurysmal subarachnoid hemorrhage. J. Neurosurg. Anesth. 2012, 24, 317–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Yan, H.; Wei, Y.; Liu, X.; Zhuang, Z.; Dai, W.; Li, J.; Li, W.; Hang, C. C-Reactive Protein/Albumin Ratio Correlates With Disease Severity and Predicts Outcome in Patients With Aneurysmal Subarachnoid Hemorrhage. Front. Neurol. 2019, 10, 1186. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.O.; Ryu, J.A. Clinical usefulness of early serial measurements of C-reactive protein as outcome predictors in patients with subarachnoid hemorrhage. BMC Neurol. 2020, 20, 112. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Capuzzo, M.; Valpondi, V.; Sgarbi, A.; Bortolazzi, S.; Pavoni, V.; Gilli, G.; Candini, G.; Gritti, G.; Alvisi, R. Validation of severity scoring systems SAPS II and APACHE II in a single-center population. Intensive Care Med. 2000, 26, 1779–1785. [Google Scholar] [CrossRef]
- Meredith, W.; Rutledge, R.; Fakhry, S.M.; Emery, S.; Kromhout-Schiro, S. The conundrum of the Glasgow Coma Scale in intubated patients: A linear regression prediction of the Glasgow verbal score from the Glasgow eye and motor scores. J. Trauma 1998, 44, 839–844. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Vila, N.; Castillo, J.; Dávalos, A.; Chamorro, A. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke 2000, 31, 2325–2329. [Google Scholar] [CrossRef]
- Castell, J.V.; Gomez-Lechon, M.J.; David, M.; Fabra, R.; Trullenque, R.; Heinrich, P.C. Acute-phase response of human hepatocytes: Regulation of acute-phase protein synthesis by interleukin-6. Hepatology 1990, 12, 1179–1186. [Google Scholar] [CrossRef]
- Ormstad, H.; Aass, H.C.; Lund-Sørensen, N.; Amthor, K.F.; Sandvik, L. Serum levels of cytokines and C-reactive protein in acute ischemic stroke patients, and their relationship to stroke lateralization, type, and infarct volume. J. Neurol. 2011, 258, 677–685. [Google Scholar] [CrossRef]
- Turner, C.L.; Budohoski, K.; Smith, C.; Hutchinson, P.J.; Kirkpatrick, P.J.; Murray, G.D. Elevated Baseline C-Reactive Protein as a Predictor of Outcome After Aneurysmal Subarachnoid Hemorrhage: Data From the Simvastatin in Aneurysmal Subarachnoid Hemorrhage (STASH) Trial. Neurosurgery 2015, 77, 786–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, F.R.; Bertolini Ede, F.; Figueiredo, E.G.; Teixeira, M.J. Serum C-reactive protein levels predict neurological outcome after aneurysmal subarachnoid hemorrhage. Arq. Neuropsiquiatr. 2012, 70, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, M.; Behrouz, R.; Topel, C.H.; Misra, V.; Pomero, F.; Giraudo, A.; Pennati, P.; Masotti, L.; Schreuder, F.; Staals, J.; et al. Hypoalbuminemia, systemic inflammatory response syndrome, and functional outcome in intracerebral hemorrhage. J. Crit. Care 2017, 41, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.C.; Rajab, I.M.; Alebraheem, M.; Potempa, L.A. C-Reactive Protein and Cancer-Diagnostic and Therapeutic Insights. Front. Immunol. 2020, 11, 595835. [Google Scholar] [CrossRef]
- Shrotriya, S.; Walsh, D.; Bennani-Baiti, N.; Thomas, S.; Lorton, C. C-Reactive Protein Is an Important Biomarker for Prognosis Tumor Recurrence and Treatment Response in Adult Solid Tumors: A Systematic Review. PLoS ONE 2015, 10, e0143080. [Google Scholar]
- Shrotriya, S.; Walsh, D.; Nowacki, A.S.; Lorton, C.; Aktas, A.; Hullihen, B.; Benanni-Baiti, N.; Hauser, K.; Ayvaz, S.; Estfan, B. Serum C-reactive protein is an important and powerful prognostic biomarker in most adult solid tumors. PLoS ONE 2018, 13, e0202555. [Google Scholar] [CrossRef]
- Ay, C.; Dunkler, D.; Pirker, R.; Thaler, J.; Quehenberger, P.; Wagner, O.; Zielinski, C.; Pabinger, I. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica 2012, 97, 1158–1164. [Google Scholar] [CrossRef]
- Inal, T.; Anar, C.; Polat, G.; Unsal, I.; Halilcolar, H. The prognostic value of D-dimer in lung cancer. Clin. Respir. J. 2015, 9, 305–313. [Google Scholar] [CrossRef]
- Gallo, M.; Sapio, L.; Spina, A.; Naviglio, D.; Calogero, A.; Naviglio, S. Lactic dehydrogenase and cancer: An overview. Front. Biosci. 2015, 20, 1234–1249. [Google Scholar]
- Forkasiewicz, A.; Dorociak, M.; Stach, K.; Szelachowski, P.; Tabola, R.; Augoff, K. The usefulness of lactate dehydrogenase measurements in current oncological practice. Cell Mol. Biol. Lett. 2020, 25, 35. [Google Scholar] [CrossRef]
- Caliskan, S.; Sungur, M. Fibrinogen and D-dimer levels in prostate cancer: Preliminary results. Prostate Int. 2017, 5, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.Y.; Yao, Y.M. The Clinical Significance and Potential Role of C-Reactive Protein in Chronic Inflammatory and Neurodegenerative Diseases. Front. Immunol. 2018, 9, 1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).