Protective Role of an Initial Low-Dose Septic Challenge against Lethal Sepsis in Neonatal Mice: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. CS Preparation
2.3. Sepsis Induction
2.4. Initial Low-Dose Septic Challenge
2.5. Bacteriologic Examination
2.6. Polymerase Chain Reaction (PCR) Arrays
2.7. Measurement of Lipid Mediators (LM)
2.8. Statistical Analyses
3. Results
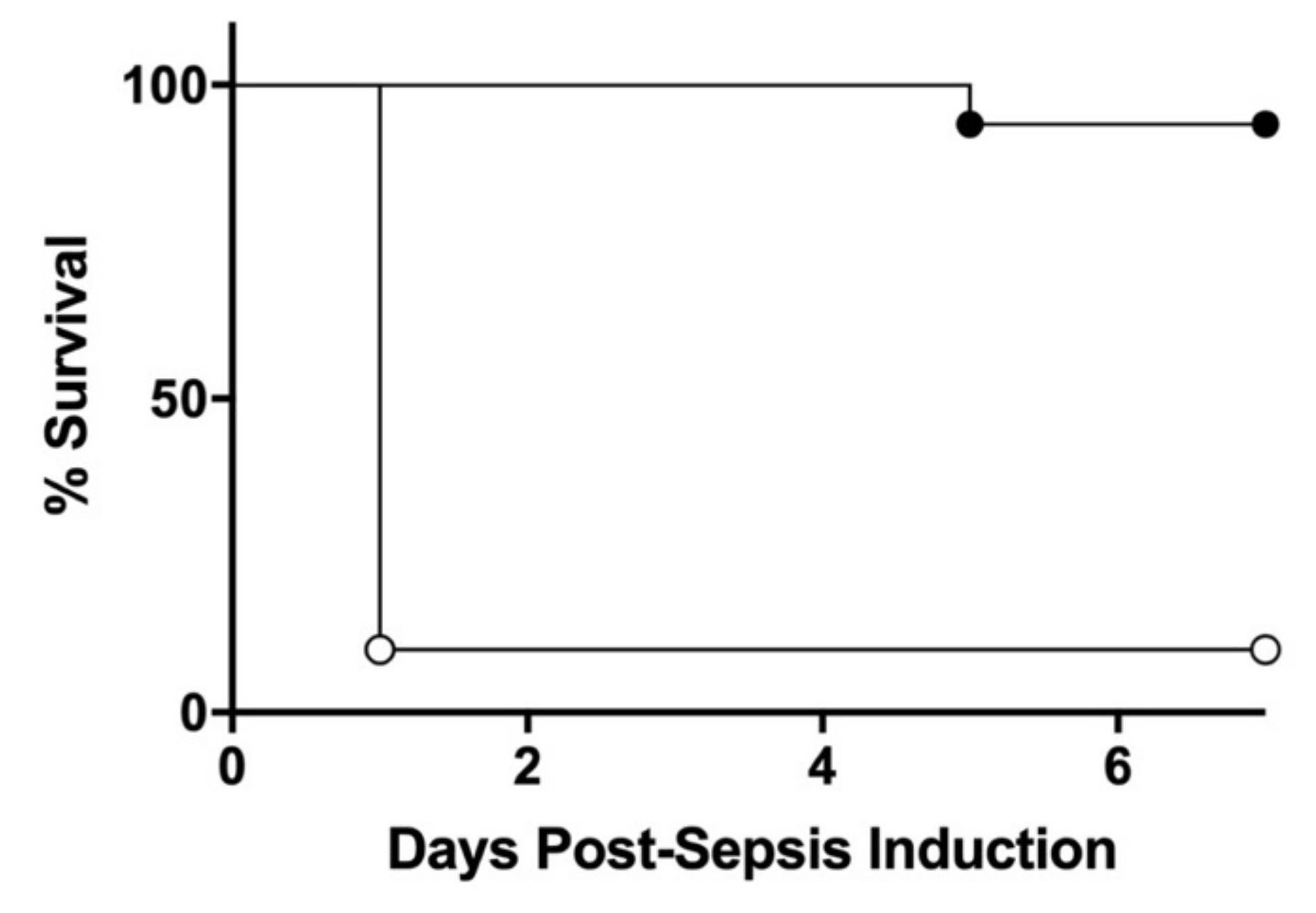
3.1. BW Changes Post-Initial Low Dose Septic Challenge
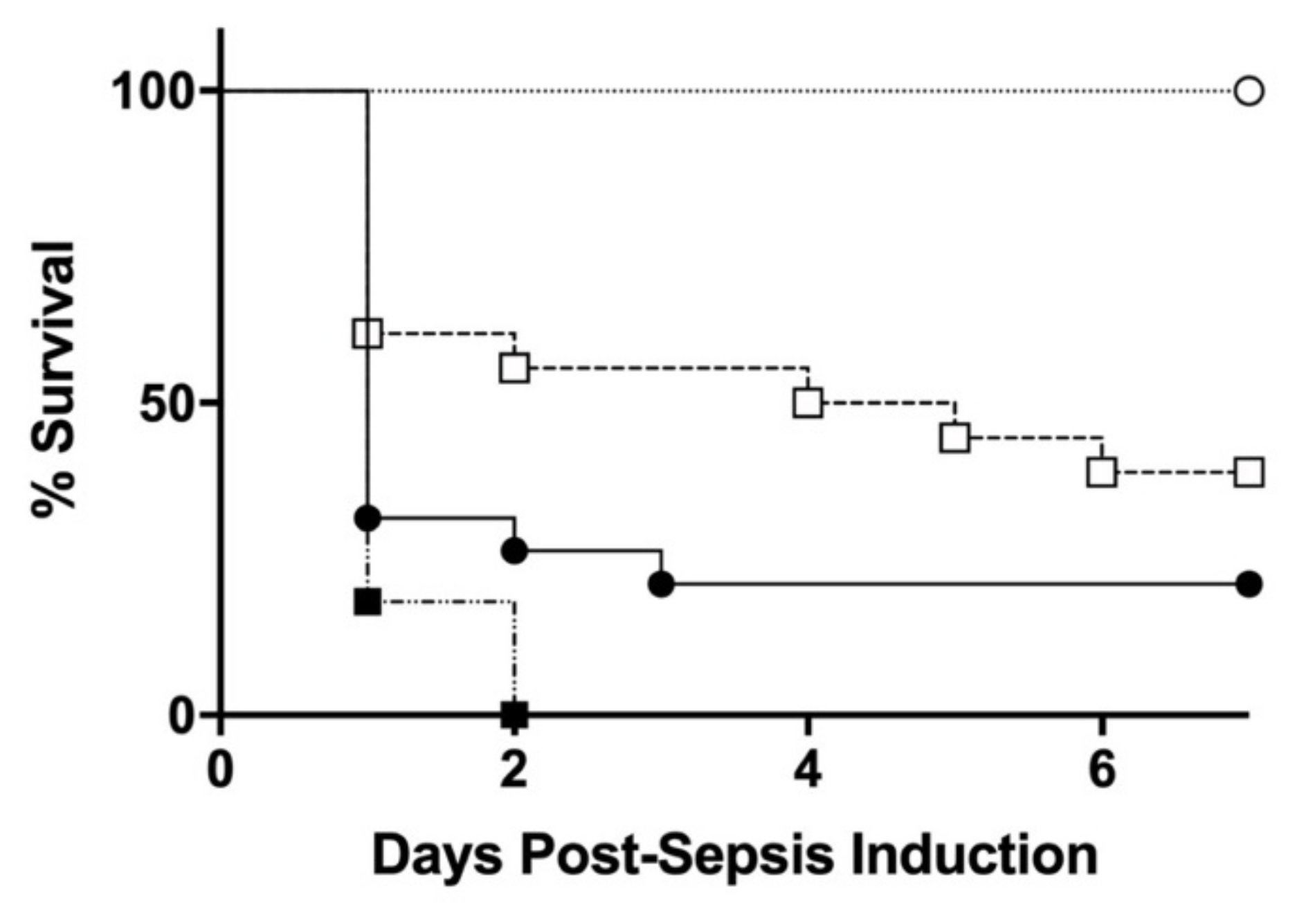
3.2. Effect of Initial Septic Challenge on the Severity of Sepsis Mortality
3.3. Organ Bacterial Colonization
3.4. Effect of Initial Septic Challenge on Expression Profiles of Genes Involved in Innate and Adaptive Immunity Post-Lethal Sepsis Induction
3.5. Effect of Initial Septic Challenge on LM Post-Sepsis Induction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shane, A.L.; Sanchez, P.J.; Stoll, B.J. Neonatal sepsis. Lancet 2017, 390, 1770–1780. [Google Scholar] [CrossRef]
- Liu, L.; Oza, S.; Hogan, D.; Perin, J.; Rudan, I.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of child mortality in 2000–2013, with projections to inform post-2015 priorities: An updated systematic analysis. Lancet 2015, 385, 430–440. [Google Scholar] [CrossRef]
- Fleischmann-Struzek, C.; Goldfarb, D.M.; Schlattmann, P.; Schlapbach, L.J.; Reinhart, K.; Kissoon, N. The global burden of paediatric and neonatal sepsis: A systematic review. Lancet Respir. Med. 2018, 6, 223–230. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Shankaran, S.; Laptook, A.R.; Walsh, M.C.; Hale, E.C.; Newman, N.S.; Schibler, K.; Carlo, W.A.; et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010, 126, 443–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoll, B.J.; Hansen, N.I.; Sanchez, P.J.; Faix, R.G.; Poindexter, B.B.; Van Meurs, K.P.; Bizzarro, M.J.; Goldberg, R.N.; Frantz, I.D., 3rd; Hale, E.C.; et al. Early onset neonatal sepsis: The burden of group B Streptococcal and E. coli disease continues. Pediatrics 2011, 127, 817–826. [Google Scholar]
- Hartman, M.E.; Linde-Zwirble, W.T.; Angus, D.C.; Watson, R.S. Trends in the epidemiology of pediatric severe sepsis. Pediatr. Crit. Care Med. 2013, 14, 686–693. [Google Scholar] [CrossRef]
- Wynn, J.L.; Scumpia, P.O.; Winfield, R.D.; Delano, M.J.; Kelly-Scumpia, K.; Barker, T.; Ungaro, R.; Levy, O.; Moldawer, L.L. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood 2008, 112, 1750–1758. [Google Scholar] [CrossRef] [Green Version]
- Raymond, S.L.; Stortz, J.A.; Mira, J.C.; Larson, S.D.; Wynn, J.L.; Moldawer, L.L. Immunological Defects in Neonatal Sepsis and Potential Therapeutic Approaches. Front. Pediatr. 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rincon, J.C.; Cuenca, A.L.; Raymond, S.L.; Mathias, B.; Nacionales, D.C.; Ungaro, R.; Efron, P.A.; Wynn, J.L.; Moldawer, L.L.; Larson, S.D. Adjuvant pretreatment with alum protects neonatal mice in sepsis through myeloid cell activation. Clin. Exp. Immunol. 2018, 191, 268–278. [Google Scholar] [CrossRef] [Green Version]
- Brook, B.; Harbeson, D.J.; Shannon, C.P.; Cai, B.; He, D.; Ben-Othman, R.; Francis, F.; Huang, J.; Varankovich, N.; Liu, A.; et al. BCG vaccination-induced emergency granulopoiesis provides rapid protection from neonatal sepsis. Sci. Transl. Med. 2020, 12, eaax4517. [Google Scholar] [CrossRef]
- Wynn, J.L.; Neu, J.; Moldawer, L.L.; Levy, O. Potential of immunomodulatory agents for prevention and treatment of neonatal sepsis. J. Perinatol. 2009, 29, 79–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadarangani, M.; Kollmann, T.; Bjornson, G.; Heath, P.; Clarke, E.; Marchant, A.; Levy, O.; Leuridan, E.; Ulloa-Gutierrez, R.; Cutland, C.L.; et al. The Fifth International Neonatal and Maternal Immunization Symposium (INMIS 2019): Securing Protection for the Next Generation. mSphere 2021, 6, e00862-20. [Google Scholar] [CrossRef]
- Netea, M.G.; Dominguez-Andres, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef] [Green Version]
- Bistoni, F.; Vecchiarelli, A.; Cenci, E.; Puccetti, P.; Marconi, P.; Cassone, A. Evidence for macrophage-mediated protection against lethal Candida albicans infection. Infect. Immun. 1986, 51, 668–674. [Google Scholar] [CrossRef] [Green Version]
- Quintin, J.; Saeed, S.; Martens, J.H.A.; Giamarellos-Bourboulis, E.J.; Ifrim, D.C.; Logie, C.; Jacobs, L.; Jansen, T.; Kullberg, B.J.; Wijmenga, C.; et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host. Microb. 2012, 12, 223–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benn, C.S.; Netea, M.G.; Selin, L.K.; Aaby, P. A small jab—A big effect: Nonspecific immunomodulation by vaccines. Trends Immunol. 2013, 34, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef] [Green Version]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef]
- Wynn, J.L.; Hansen, N.I.; Das, A.; Cotten, C.M.; Goldberg, R.N.; Sanchez, P.J.; Bell, E.F.; Van Meurs, K.P.; Carlo, W.A.; Laptook, A.R.; et al. Early sepsis does not increase the risk of late sepsis in very low birth weight neonates. J. Pediatr. 2013, 162, 942–948.e3. [Google Scholar] [CrossRef] [Green Version]
- Wynn, J.L.; Scumpia, P.O.; Delano, M.J.; O’Malley, K.A.; Ungaro, R.; Abouhamze, A.; Moldawer, L.L. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock 2007, 28, 675–683. [Google Scholar] [CrossRef]
- Fujioka, K.; Kalish, F.; Zhao, H.; Lu, S.; Wong, S.; Wong, R.J.; Stevenson, D.K. Induction of Heme Oxygenase-1 Attenuates the Severity of Sepsis in a Non-Surgical Preterm Mouse Model. Shock 2017, 47, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M.; Fujioka, K.; Nishida, K.; Okubo, S.; Ikuta, T.; Shinohara, M.; Iijima, K. Recombinant human thrombomodulin attenuated sepsis severity in a non-surgical preterm mouse model. Sci. Rep. 2020, 10, 333. [Google Scholar] [CrossRef]
- Starr, M.E.; Steele, A.M.; Saito, M.; Hacker, B.J.; Evers, B.M.; Saito, H. A new cecal slurry preparation protocol with improved long-term reproducibility for animal models of sepsis. PLoS ONE 2014, 9, e115705. [Google Scholar] [CrossRef] [Green Version]
- Adkins, B.; Leclerc, C.; Marshall-Clarke, S. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 2004, 4, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Kronforst, K.D.; Mancuso, C.J.; Pettengill, M.; Ninkovic, J.; Power Coombs, M.R.; Stevens, C.; Otto, M.; Mallard, C.; Wang, X.; Goldmann, D.; et al. A neonatal model of intravenous Staphylococcus epidermidis infection in mice <24 h old enables characterization of early innate immune responses. PLoS ONE 2012, 7, e43897. [Google Scholar] [CrossRef]
- Fujioka, K.; Kalish, F.; Zhao, H.; Wong, R.J.; Stevenson, D.K. Heme oxygenase-1 deficiency promotes severity of sepsis in a non-surgical preterm mouse model. Pediatr. Res. 2018, 84, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Colas, R.A.; Shinohara, M.; Dalli, J.; Chiang, N.; Serhan, C.N. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am. J. Physiol. Cell Physiol. 2014, 307, C39–C54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolognese, A.C.; Yang, W.L.; Hansen, L.W.; Sharma, A.; Nicastro, J.M.; Coppa, G.F.; Wang, P. Activation of Invariant Natural Killer T Cells Redirects the Inflammatory Response in Neonatal Sepsis. Front. Immunol. 2018, 9, 833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speer, E.M.; Diago-Navarro, E.; Ozog, L.S.; Raheel, M.; Levy, O.; Fries, B.C. A Neonatal Murine Escherichia coli Sepsis Model Demonstrates That Adjunctive Pentoxifylline Enhances the Ratio of Anti- vs. Pro-inflammatory Cytokines in Blood and Organ Tissues. Front. Immunol. 2020, 11, 577878. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Kim, K.D.; Yang, X.; Auh, S.; Fu, Y.X.; Tang, H. Hyper innate responses in neonates lead to increased morbidity and mortality after infection. Proc. Natl. Acad. Sci. USA 2008, 105, 7528–7533. [Google Scholar] [CrossRef] [Green Version]
- Gentile, L.F.; Cuenca, A.G.; Efron, P.A.; Ang, D.; Bihorac, A.; McKinley, B.A.; Moldawer, L.L.; Moore, F.A. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J. Trauma Acute Care Surg. 2012, 72, 1491–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.L. Sepsis and septic shock. Nat. Rev. Dis. Primers 2016, 2, 16045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biering-Sorensen, S.; Aaby, P.; Lund, N.; Monteiro, I.; Jensen, K.J.; Eriksen, H.B.; Schaltz-Buchholzer, F.; Jorgensen, A.S.P.; Rodrigues, A.; Fisker, A.B.; et al. Early BCG-Denmark and Neonatal Mortality Among Infants Weighing <2500 g: A Randomized Controlled Trial. Clin. Infect. Dis. 2017, 65, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Schaltz-Buchholzer, F.; Biering-Sorensen, S.; Lund, N.; Monteiro, I.; Umbasse, P.; Fisker, A.B.; Andersen, A.; Rodrigues, A.; Aaby, P.; Benn, C.S. Early BCG Vaccination, Hospitalizations, and Hospital Deaths: Analysis of a Secondary Outcome in 3 Randomized Trials from Guinea-Bissau. J. Infect. Dis. 2019, 219, 624–632. [Google Scholar] [CrossRef]

| Veh-CS Treated (Veh) vs. Veh-Veh Treated Control (Cont) | CS-CS Treated (PTx) vs. Veh-Veh Treated Control (Cont) | |||
|---|---|---|---|---|
| Gene Symbol | Fold Change | p | Fold Change | p |
| Cd14 | 1.63 | 0.039 | −58.35 | 0.029 |
| Il10 | 5.38 | 0.002 | −2.15 | 0.623 |
| Il1a | 6.31 | 0.036 | 1.21 | 0.592 |
| Il1b | 8.09 | 0.016 | −1.57 | 0.540 |
| Il1r1 | 1.60 | 0.020 | −7.15 | 0.036 |
| Il6 | 9.90 | 0.002 | 1.43 | 0.518 |
| Mx1 | 15.81 | 0.023 | 1.01 | 0.853 |
| Rorc | 1.87 | 0.005 | 4.87 | 0.015 |
| CS-CS Treated (PTx) over Veh-CS Treated (Veh) | ||
|---|---|---|
| Gene Symbol | Fold Change | p |
| Cd14 | −95.29 | 0.009 |
| Mx1 | −15.64 | 0.024 |
| Il1b | −12.68 | 0.333 |
| Il10 | −11.58 | 0.226 |
| Il1r1 | −11.43 | 0.002 |
| Il6 | −6.94 | 0.096 |
| Il1a | −5.20 | 0.094 |
| Irf7 | −4.55 | 0.020 |
| C5ar1 | −4.4 | 0.006 |
| Rorc | 2.6 | 0.032 |
| LM (pg/mg Tissue). | Post 3 h | Post 6 h | |||
|---|---|---|---|---|---|
| Veh (n = 4) | PTx (n = 4) | Veh (n = 4) | PTx (n = 4) | ||
| EPA | Resolvin E3 | 23.5 ± 6.6 | 15.9 ± 1.1 | 8.2 ± 2.6 | 3.7 ± 0.6 |
| 5-HEPE | 1.7 ± 0.6 | 1.8 ± 0.1 | 0.9 ± 0.2 | 0.4 ± 0.0 | |
| 12-HEPE | 1.5 ± 0.6 | 1.5 ± 0.1 | 1.5 ± 0.3 | 0.6 ± 0.2 * | |
| 15-HEPE | 1.2 ± 0.3 | 1.3 ± 0.2 | 0.6 ± 0.1 | 0.2 ± 0.0 * | |
| 18-HEPE | 2.1 ± 0.6 | 2.3 ± 0.4 | 1.0 ± 0.2 | 0.5 ± 0.0 * | |
| EPA | 149.8 ± 51.9 | 184.1 ± 33.9 | 100.7 ± 12.9 | 60.6 ± 9.2 * | |
| DHA | 4-HDHA | 3.5 ± 0.8 | 3.7 ± 0.5 | 5.2 ± 0.5 | 2.3 ± 0.4 ** |
| 7-HDHA | 1.0 ± 0.2 | 1.3 ± 0.2 | 0.9 ± 0.1 | 0.5 ± 0.1 ** | |
| 14-HDHA | 2.7 ± 0.6 | 3.1 ± 0.4 | 3.4 ± 0.4 | 1.5 ± 0.3 * | |
| 17-HDHA | 5.4 ± 1.4 | 6.2 ± 0.8 | 5.3 ± 0.8 | 2.2 ± 0.2 ** | |
| DHA | 474 ± 109.5 | 620.3 ± 58.2 | 706.6 ± 56.3 | 433.2 ± 94.9 * | |
| AA | PGE2 | 15.7 ± 3.5 | 18.5 ± 2.3 | 27.8 ± 9.2 | 25.1 ± 4.8 |
| PGD2 | 133.8 ± 29.6 | 142.3 ± 56.5 | 212.1 ± 55.0 | 171.3 ± 16.7 | |
| 15deoxy-d12,14 PGJ2 | 147.7 ± 30.3 | 67.3 ± 11.4 * | 119.7 ± 18.0 | 111.1 ± 30.1 | |
| PGF2a | 91.4 ± 29.6 | 34.9 ± 8.5 | 134.9 ± 91.7 | 24.7 ± 3.2 | |
| TxB2 | 30.7 ± 4.2 | 23.2 ± 9.1 | 38.6 ± 11.8 | 40.9 ± 9.5 | |
| 12S-HHT | 9.8 ± 3.7 | 7.2 ± 1.5 | 13.8 ± 6.1 | 5.0 ± 0.9 | |
| LTB4 | 0.1 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.0 | 0.1 ± 0.0 | |
| Lipoxin B4 | 70.7 ± 22.0 | 49.8 ± 13.6 | 31.2 ± 8.8 | 13.0 ± 2.8 | |
| 5-HETE | 4.7 ± 1.3 | 5.3 ± 0.4 | 6.0 ± 0.8 | 2.3 ± 0.3 ** | |
| 12-HETE | 7.1 ± 2.0 | 7.4 ± 0.6 | 13.0 ± 1.5 | 4.8 ± 1.3 ** | |
| 15-HETE | 7.6 ± 2.1 | 9.4 ± 1.3 | 7.6 ± 0.7 | 3.8 ± 0.4 ** | |
| AA | 547.6 ± 156.0 | 598.1 ± 8.9 | 725.1 ± 64.9 | 364.8 ± 57.5 ** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakasone, R.; Ashina, M.; Kido, T.; Miyauchi, H.; Saito, M.; Inoue, S.; Shinohara, M.; Nozu, K.; Fujioka, K. Protective Role of an Initial Low-Dose Septic Challenge against Lethal Sepsis in Neonatal Mice: A Pilot Study. J. Clin. Med. 2021, 10, 5823. https://doi.org/10.3390/jcm10245823
Nakasone R, Ashina M, Kido T, Miyauchi H, Saito M, Inoue S, Shinohara M, Nozu K, Fujioka K. Protective Role of an Initial Low-Dose Septic Challenge against Lethal Sepsis in Neonatal Mice: A Pilot Study. Journal of Clinical Medicine. 2021; 10(24):5823. https://doi.org/10.3390/jcm10245823
Chicago/Turabian StyleNakasone, Ruka, Mariko Ashina, Takumi Kido, Harunori Miyauchi, Masafumi Saito, Shigeaki Inoue, Masakazu Shinohara, Kandai Nozu, and Kazumichi Fujioka. 2021. "Protective Role of an Initial Low-Dose Septic Challenge against Lethal Sepsis in Neonatal Mice: A Pilot Study" Journal of Clinical Medicine 10, no. 24: 5823. https://doi.org/10.3390/jcm10245823
APA StyleNakasone, R., Ashina, M., Kido, T., Miyauchi, H., Saito, M., Inoue, S., Shinohara, M., Nozu, K., & Fujioka, K. (2021). Protective Role of an Initial Low-Dose Septic Challenge against Lethal Sepsis in Neonatal Mice: A Pilot Study. Journal of Clinical Medicine, 10(24), 5823. https://doi.org/10.3390/jcm10245823







