A Comparison of the Catheter-Based Transapical and Surgical Treatment Modalities for Mitral Paravalvular Leak
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Data Analysis
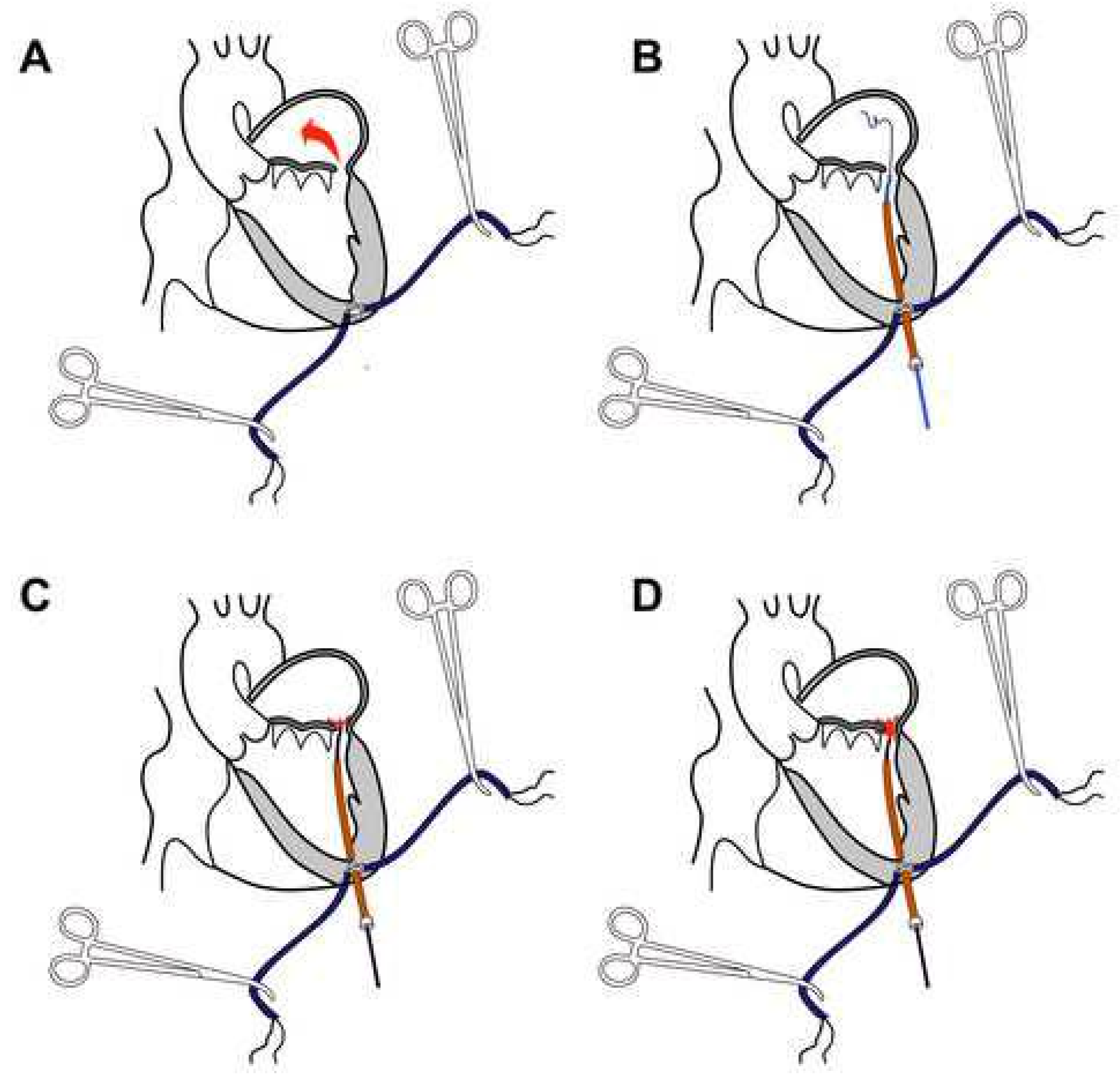
2.3. Transapical Catheter-Based Mitral PVL Closure Procedure
3. Results
3.1. Preoperative Characteristics
3.2. Early Postoperative Data and Complications
3.3. Results of Mitral PVL Treatment at Discharge from Hospital
3.4. Results of Mitral PVL Treatment at Follow-Up
3.5. Technical Success in the “Catheter” Group
3.6. Device Success in the “Catheter” Group
3.7. Procedural Success in the “Catheter” Group
3.8. Individual Patient Success in the “Catheter” Group
4. Discussion
Research Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Echevarria, J.; Bernal, J.; Rabasa, J.; Morales, D.; Revilla, Y.; Revuelta, J. Reoperation for bioprosthetic valve dysfunction. A decade of clinical experience. Eur. J. Cardio-Thorac. Surg. 1991, 5, 523–527. [Google Scholar] [CrossRef]
- Emery, R.W.; Krogh, C.C.; Mc Adams, S.; Emery, A.M.; Holter, A.R. Long-term follow-up of patients undergoing reoperative surgery with aortic or mitral valve replacement using a St. Jude Medical prosthesis. J. Heart Valve Dis. 2010, 19, 473–484. [Google Scholar]
- Thourani, V.H.; Smith, C.M.; Guyton, R.A.; Block, P.; Liff, D.; Willis, P.; Lerakis, S.; Arepalli, C.D.; Howell, S.; Boulton, B.J.; et al. Repair of Prosthetic Mitral Valve Paravalvular Leak Using an Off-Pump Transapical Approach. Ann. Thorac. Surg. 2012, 94, 275–278. [Google Scholar] [CrossRef]
- Calvert, P.A.; Northridge, D.B.; Malik, I.S.; Shapiro, L.; Ludman, P.; Qureshi, S.A.; Mullen, M.; Henderson, R.; Turner, M.; Been, M.; et al. Percutaneous de- vice closure of paravalvular leak: Combined experience from the United Kingdom and Ireland. Circulation 2016, 134, 934–944. [Google Scholar] [CrossRef]
- Wells, J.A.; Condado, J.F.; Kamioka, N.; Dong, A.; Ritter, A.; Lerakis, S.; Clements, S.; Stewart, J.; Leshnower, B.; Guyton, R.; et al. Outcomes after paravalvular leak closure: Transcatheter versus surgical approaches. JACC Cardiovasc. Interv. 2017, 10, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Smolka, G.; Pysz, P.; Jasinski, M.; Gocoł, R.; Domaradzki, W.; Hudziak, D.; Deja, M.; Ochała, A.; Gasior, Z. Transapical closure of mitral paravalvu- lar leaks with use of Amplatzer Vascular Plug III. J. Invasive Cardiol. 2013, 25, 497–501. [Google Scholar] [PubMed]
- Taramasso, M.; Maisano, F.; Denti, P.; Guidotti, A.; Sticchi, A.; Pozzoli, A.; Buzzatti, N.; De Bonis, M.; La Canna, G.; Alfieri, O. Surgical treatment of paravalvular leak: Long-term results in a single-center experience (up to 14 years). J. Thorac. Cardiovasc. Surg. 2015, 149, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, C.E.; Hahn, R.T.; Berrebi, A.; Borer, J.S.; Cutlip, D.E.; Fontana, G.; Gerosa, G.; Ibrahim, R.; Jelnin, V.; Jilaihawi, H.; et al. Paravalvular Leak Academic Research Consortium. Clinical Trial Principles and Endpoint Definitions for Paravalvular Leaks in Surgical Prosthesis. Eur. Heart J. 2018, 39, 1224–1245. [Google Scholar] [CrossRef] [PubMed]
- Millán, X.; Bouhout, I.; Nozza, A.; Samman, K.; Stevens, L.M.; Lamarche, Y.; Serra, A.; Asgar, A.W.; El-Hamamsy, I.; Cartier, R.; et al. Surgery Versus Transcatheter Interventions for Significant Paravalvular Prosthetic Leaks. JACC Cardiovasc. Interv. 2017, 10, 1959–1969. [Google Scholar] [CrossRef] [PubMed]
- Busu, T.; Alqahtani, F.; Badhwar, V.; Cook, C.C.; Rihal, C.S.; Alkhouli, M. Meta-analysis Comparing Transcatheter and Surgical Treatments of Paravalvular Leaks. Am. J. Cardiol. 2018, 122, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Zorinas, A.; Janušauskas, V.; Davidavičius, G.; Šimakauskas, R.; Puodžiukaitė, L.; Zakarkaitė, D.; Bilkis, V.; Čypienė, R.J.; Samalavičius, R.S.; Onorato, E.M.; et al. Retrospective analysis of single-center early and midterm results of transapical catheter-based mitral paravalvular leak closure with a purpose-specific device. Postep. Kardiol. Interwencyjnej 2018, 14, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Guler, A.; Tavlasoglu, M.; Kadan, M.; Barcin, C. Transapical closure of mitral paravalvular leakage. Eur. J. Cardio-Thorac. Surg. 2013, 43, 861–863. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nietlispach, F.; Maisano, F.; Sorajja, P.; Leon, M.B.; Rihal, C.; Feldman, T. Percutaneous paravalvular leak closure: Chasing the chameleon. Eur. Heart J. 2016, 37, 3495–3502. [Google Scholar] [CrossRef] [PubMed]
- Nijenhuis, V.J.; Swaans, M.J.; Post, M.C.; Heijmen, R.H.; de Kroon, T.L.; Ten Berg, J.M. Open transapical approach to transcatheter paravalvular leakage closure: A preliminary experience. Circ. Cardiovasc. Interv. 2014, 7, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Jelnin, V.; Dudiy, Y.; Einhorn, B.N.; Kronzon, I.; Cohen, H.A.; Ruiz, C.E. Clinical experience with percutaneous left ventricular transapical access for interventions in structural heart defects a safe access and secure exit. JACC Cardiovasc. Interv. 2011, 4, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, M.; Rihal, C.S.; Zack, C.J.; Eleid, M.F.; Maor, E.; Sarraf, M.; Cabalka, A.K.; Reeder, G.S.; Hagler, D.J.; Maalouf, J.F.; et al. Transcatheter and surgical management of mitral paravalvular leak: Long-term outcomes. JACC Cardiovasc. Interv. 2017, 10, 1946–1956. [Google Scholar] [CrossRef] [PubMed]
- Angulo-Llanos, R.; Sarnago-Cebada, F.; Rivera, A.R.; Elízaga Corrales, J.; Cuerpo, G.; Solis, J.; Gutierrez-Ibañes, E.; Sanz-Ruiz, R.; Vázquez Álvarez, M.E.; Fernandez-Avilés, F. Two-Year Follow-up After Surgical Versus Percutaneous Paravalvular Leak Closure: A Non-Randomized Analysis. Catheter. Cardiovasc. Interv. 2016, 88, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.P.; Rezek, D.; Paiva Costa, E.; de Carvalho, E.S.L.; Brito Moscoso, F.A.; Chavez Taborga, P.R.; Jeronimo, A.D.; Cunha Abizaid, A.A.; de Oliveira Ramos, A.I. Paravalvular Regurgitation: Clinical Outcomes in Surgical and Percutaneous Treatments. Arq. Bras. Cardiol. 2016, 107, 55–62. [Google Scholar] [CrossRef] [PubMed]
| Clinical Variables | “Catheter” N (%)/Median [Q1–Q3] | “Surgical” N (%)/Median [Q1–Q3] | p Value |
|---|---|---|---|
| Number of patients | 27 (%) | 49 (%) | |
| Age, years | 67 (61–70) | 64 (57–67) | 0.027 |
| Gender, male | 16 (59%) | 22 (45%) | 0.231 |
| Time form MVR, months | 34 (10–147) | 60 (14–179) | 0.431 |
| Previous PVL surgery | 4 (15%) | 5 (10%) | 0.552 |
| NYHA | |||
| II | 4 (15%) | 2 (4%) | 0.097 |
| III | 21 (78%) | 35 (71%) | 0.547 |
| IV | 2 (7%) | 12 (25%) | 0.066 |
| EuroSCORE II, % | 6 (4–10) | 8 (6–11) | 0.03 |
| STS risk of mortality, % | 2 (1.3–2.6) | 2 (1.4–1.2) | 0.789 |
| Coronary artery disease | 3 (11%) | 9 (18%) | 0.406 |
| Hemolysis | 12 (44%) | 15 (31%) | 0.228 |
| Anemia Hb < 100g/L | 9 (33%) | 15 (31%) | 0.06 |
| Creatinine concentration, μmol/L | 90 (74–107) | 88 (77–115) | 0.607 |
| Left ventricle function | |||
| Severe (LVEF < 30%) | 1 (4%) | 2 (4%) | 0.935 |
| Moderate (LVEF 31–44%) | 10 (37%) | 15 (31%) | 0.568 |
| Mild LVEF 45–54%) | 6 (22%) | 22 (45%) | 0.05 |
| Good (LVEF ≥ 55%) | 10 (37%) | 10 (20%) | 0.115 |
| PAP > 55 mmHg | 12 (56%) | 16 (33%) | 0.308 |
| Prosthetic valve type | |||
| Bioprosthesis | 9 (33%) | 4 (8%) | 0.005 |
| Mechanical | 18 (%) | 45 (%) | 0.005 |
| Indications for PVL closure | |||
| Hemolytic anemia only | 2 (7%) | 1 (2%) | 0.25 |
| Heart failure only | 15 (56%) | 34 (69%) | 0.228 |
| Both | 10 (37%) | 14 (%) | 0.447 |
| Number of PVL per patient | 1 (1–1) | 1 (1–1) | |
| 1 defect | 14 (52%) | 43 (88%) | 0.001 |
| 2 defects | 9 (33%) | 5 (10%) | 0.013 |
| 3 defects | 2 (7%) | 1 (1%) | 0.25 |
| >3 defects | 2 (7%) | 0 (0%) | 0.054 |
| Degree of PVL regurgitation | |||
| Moderate | 7 (26%) | 17 (35%) | 0.431 |
| Severe | 20 (74%) | 32 (65%) | 0.431 |
| Variables | “Catheter” N (%)/Median (Q1–Q3) | “Surgical” N (%)/Median (Q1–Q3) | p Value |
|---|---|---|---|
| Number of patients | 27 (33%) | 49 (67%) | |
| Immediate mortality (≤72 h) | 0 (0%) | 4 (8%) | 0.127 |
| Mortality (≤30 days/in-hospital) | 0 (0%) | 9 (18%) | 0.039 |
| MI (≤72 h after procedure) | 0 (0%) | 8 (16%) | 0.026 |
| MI (≤30 days or in-hospital) | 0 (0%) | 9 (18%) | 0.018 |
| Stroke (≤30 days or in-hospital) | 0 (0%) | 3 (6%) | 0.19 |
| Bleeding according to BARC | |||
| Life-threatening | 0 (0%) | 9 (18%) | 0.018 |
| Major bleeding | 2 (7%) | 1 (2%) | 0.250 |
| Minor bleeding | 1 (4%) | 4 (8%) | 0.453 |
| Major access site complications | 1 (4%) | 11 (22%) | 0.032 |
| Sepsis | (0%) | 7 (14%) | 0.039 |
| Drainage, mL/24 h | 150 (100–250) | 675 (600–1550) | 0.001 |
| Hospital stay, days | 9 (6–13) | 15 (12–21) | 0.001 |
| ITU stay, days | 1 (1–1) | 3 (2–8) | 0.001 |
| Clinical Variables | “Catheter PLD” N (%)/Median (Q1–Q3) | “Surgical” N (%)/Median (Q1–Q3) | p Value | |
|---|---|---|---|---|
| Patients at discharge | 27 (100%) | 40 | (82%) | 0.018 |
| Degree of residual paravalvular regurgitation | ||||
| None/Trivial | 22 (81%) | 36 | (90%) | 0.316 |
| Mild | 4 (15%) | 1 | (3%) | 0.060 |
| Moderate | 0 (0%) | 1 | (3%) | 0.408 |
| Severe | 1 (4%) | 2 | (5%) | 0.801 |
| Clinical Variables | “CatheterPLD” N (%)/Median (Q1–Q3) | “Surgical” N (%)/Median (Q1–Q3) | p Value |
|---|---|---|---|
| Follow-up available, years | 2.45 (0.96–3.15) | 6.3 (3.3–10.1) | 0.001 |
| Overall mortality | 2 (7%) | 19 (39%) | 0.003 |
| PVL > Mild | 2 (7%) | 5 (13%) | 0.504 |
| Moderate | 1 (4%) | 1 (3%) | 0.408 |
| Severe | 1 (4%) | 4 (10%) | 0.520 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorinas, A.; Janušauskas, V.; Austys, D.; Davidavičius, G.; Puodžiukaitė, L.; Zakarkaitė, D.; Samalavičius, R.S.; Urbonas, K.; Kramena, R.; Onorato, E.M.; et al. A Comparison of the Catheter-Based Transapical and Surgical Treatment Modalities for Mitral Paravalvular Leak. J. Clin. Med. 2022, 11, 4999. https://doi.org/10.3390/jcm11174999
Zorinas A, Janušauskas V, Austys D, Davidavičius G, Puodžiukaitė L, Zakarkaitė D, Samalavičius RS, Urbonas K, Kramena R, Onorato EM, et al. A Comparison of the Catheter-Based Transapical and Surgical Treatment Modalities for Mitral Paravalvular Leak. Journal of Clinical Medicine. 2022; 11(17):4999. https://doi.org/10.3390/jcm11174999
Chicago/Turabian StyleZorinas, Aleksejus, Vilius Janušauskas, Donatas Austys, Giedrius Davidavičius, Lina Puodžiukaitė, Diana Zakarkaitė, Robertas Stasys Samalavičius, Karolis Urbonas, Rita Kramena, Eustaquio Maria Onorato, and et al. 2022. "A Comparison of the Catheter-Based Transapical and Surgical Treatment Modalities for Mitral Paravalvular Leak" Journal of Clinical Medicine 11, no. 17: 4999. https://doi.org/10.3390/jcm11174999
APA StyleZorinas, A., Janušauskas, V., Austys, D., Davidavičius, G., Puodžiukaitė, L., Zakarkaitė, D., Samalavičius, R. S., Urbonas, K., Kramena, R., Onorato, E. M., & Ručinskas, K. (2022). A Comparison of the Catheter-Based Transapical and Surgical Treatment Modalities for Mitral Paravalvular Leak. Journal of Clinical Medicine, 11(17), 4999. https://doi.org/10.3390/jcm11174999







