Cardiac Magnetic Resonance Shows Improved Outcomes in Patients with an ST-Segment Elevation Myocardial Infarction and a High Thrombus Burden Treated with Adjuvant Aspiration Thrombectomy
Abstract
:1. Introduction
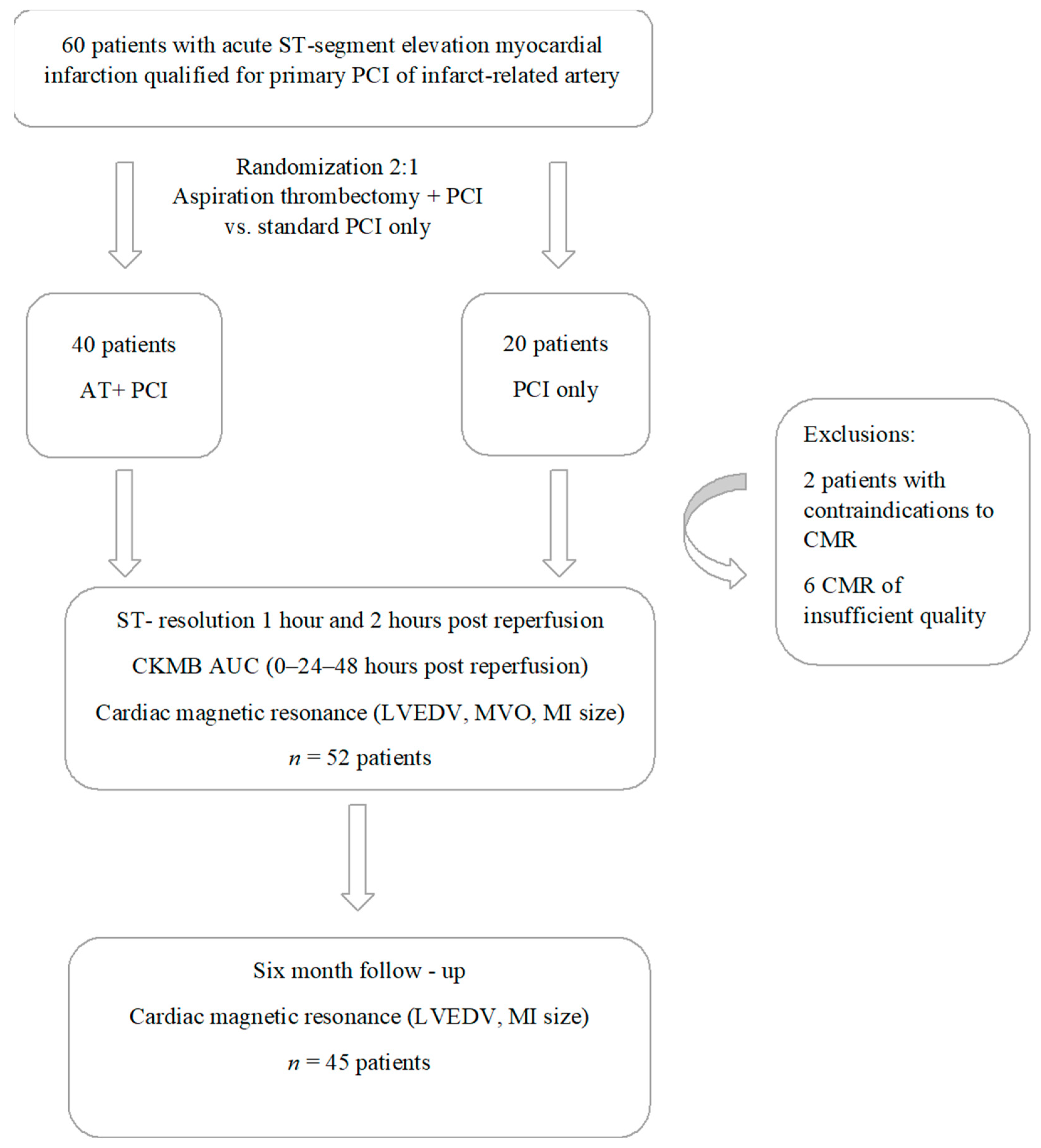
2. Materials and Methods
2.1. Study Population
2.2. Study Protocol
2.3. CKMB Release
2.4. Resolution of ST-Segment Elevation on 12-Lead ECG
2.5. CMR Acquisition
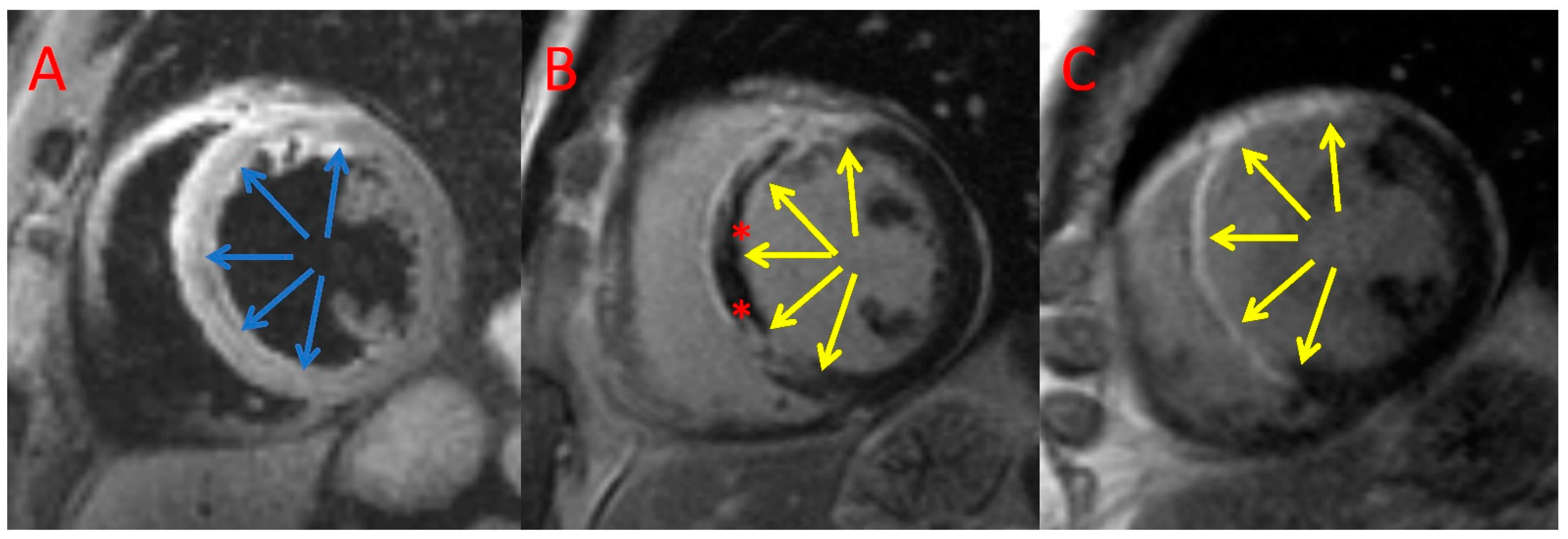
2.6. CMR Analysis
2.7. Statistical Analyses
3. Results
3.1. Patients Characteristics
3.2. Angiography and PCI
3.3. Enzymatic and ECG Assessment
3.4. CMR Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- White, H.D.; Chew, D.P. Acute Myocardial Infarction. Lancet 2008, 372, 570–584. [Google Scholar] [CrossRef] [PubMed]
- Namazi, M.H.; Mazloomi, S.S.; Kalate Aghamohammadi, M. Correlation Between TIMI Risk Score and the Number of Vessels Involved in the Angiographic Study; a Cross-Sectional Study. Arch. Acad. Emerg. Med. 2022, 10, e16. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, P.; Topór-Mądry, R.; Gąsior, M.; Cegłowska, U.; Gierlotka, M.; Kubica, J.; Kalarus, Z.; Lesiak, M.; Wojakowski, W.; Legutko, J.; et al. Management and Predictors of Clinical Events in 75,686 Patients with Acute Myocardial Infarction. Kardiol. Pol. 2022, 80, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation: The Task Force for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2017, 39, 119–177. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Tiroch, K.; Fusaro, M.; Keta, D.; Seyfarth, M.; Byrne, R.A.; Pache, J.; Alger, P.; Mehilli, J.; Schömig, A.; et al. 5-Year Prognostic Value of No-Reflow Phenomenon After Percutaneous Coronary Intervention in Patients with Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2010, 55, 2383–2389. [Google Scholar] [CrossRef]
- Krawczyk, K.; Stepien, K.; Nowak, K.; Nessler, J.; Zalewski, J. ST-Segment Re-Elevation Following Primary Angioplasty in Acute Myocardial Infarction with Patent Infarct-Related Artery: Impact on Left Ventricular Function Recovery and Remodeling. Postepy Kardiol Interwencyjnej 2019, 15, 412–421. [Google Scholar] [CrossRef]
- de Waha, S.; Patel, M.R.; Granger, C.B.; Ohman, E.M.; Maehara, A.; Eitel, I.; Ben-Yehuda, O.; Jenkins, P.; Thiele, H.; Stone, G.W. Relationship between Microvascular Obstruction and Adverse Events Following Primary Percutaneous Coronary Intervention for ST-Segment Elevation Myocardial Infarction: An Individual Patient Data Pooled Analysis from Seven Randomized Trials. Eur. Heart J. 2017, 38, 3502–3510. [Google Scholar] [CrossRef]
- McAlindon, E.; Pufulete, M.; Harris, J.; Lawton, C.; Johnson, T.; Strange, J.; Baumbach, A.; Bucciarelli-Ducci, C. Microvascular Dysfunction Determines Infarct Characteristics in Patients with Reperfused ST-Segment Elevation Myocardial Infarction: The MICROcirculation in Acute Myocardial Infarction (MICRO-AMI) Study. PLoS ONE 2018, 13, e0203750. [Google Scholar] [CrossRef]
- Sia, C.-H.; Tan, S.-H.; Chan, S.-P.; Marchesseau, S.; Sim, H.-W.; Carvalho, L.; Chen, R.; Amin, N.H.M.; Fong, A.Y.-Y.; Richards, A.M.; et al. Enhanced Thrombin Generation Is Associated with Worse Left Ventricular Scarring after ST-Segment Elevation Myocardial Infarction: A Cohort Study. Pharmaceuticals 2022, 15, 718. [Google Scholar] [CrossRef]
- Niccoli, G.; Scalone, G.; Lerman, A.; Crea, F. Coronary Microvascular Obstruction in Acute Myocardial Infarction. Eur. Heart J. 2016, 37, 1024–1033. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Mandry, D.; Chen, B.; Huttin, O.; Hossu, G.; Wang, H.; Beaumont, M.; Girerd, N.; Felblinger, J.; Odille, F. Impact of Microvascular Obstruction on Left Ventricular Local Remodeling after Reperfused Myocardial Infarction. J. Magn. Reason. Imaging 2018, 47, 499–510. [Google Scholar] [CrossRef]
- Reindl, M.; Eitel, I.; Reinstadler, S.J. Role of Cardiac Magnetic Resonance to Improve Risk Prediction Following Acute ST-Elevation Myocardial Infarction. J. Clin. Med. 2020, 9, 1041. [Google Scholar] [CrossRef] [PubMed]
- Lichota, E.; Stępień, K.; Nowak, K.; Nowak, G.; Karcińska, A.; Matrejek, A.; Platschek, M.; del Yika, A.C.; Furczyńska, P.; Nessler, J.; et al. Optical Coherence Tomography-Guided Percutaneous Coronary Intervention in a Myocardial Infarction Patient. One More Argument for a Wider Use of Now Reimbursed Optical Coherence Tomography. Kardiol. Pol. 2022, 80, 616–618. [Google Scholar] [CrossRef]
- Gardner, B.I.; Bingham, S.E.; Allen, M.R.; Blatter, D.D.; Anderson, J.L. Cardiac Magnetic Resonance versus Transthoracic Echocardiography for the Assessment of Cardiac Volumes and Regional Function after Myocardial Infarction: An Intrasubject Comparison Using Simultaneous Intrasubject Recordings. Cardiovasc. Ultrasound 2009, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Bulluck, H.; Go, Y.Y.; Crimi, G.; Ludman, A.J.; Rosmini, S.; Abdel-Gadir, A.; Bhuva, A.N.; Treibel, T.A.; Fontana, M.; Pica, S.; et al. Defining Left Ventricular Remodeling Following Acute ST-Segment Elevation Myocardial Infarction Using Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. 2017, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Dissmann, R.; Linderer, T.; Schröder, R. Estimation of Enzymatic Infarct Size: Direct Comparison of the Marker Enzymes Creatine Kinase and Alpha-Hydroxybutyrate Dehydrogenase. Am. Heart J. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Fagel, N.D.; Amoroso, G.; Vink, M.A.; Slagboom, T.; van der Schaaf, R.J.; Herrman, J.-P.; Patterson, M.S.; Oosterwerff, E.F.J.; Vos, N.S.; Verheugt, F.W.A.; et al. An Immediate or Early Invasive Strategy in Non-ST-Elevation Acute Coronary Syndrome: The OPTIMA-2 Randomized Controlled Trial. Am. Heart J. 2021, 234, 42–50. [Google Scholar] [CrossRef]
- Dizon, J.M.; Brener, S.J.; Maehara, A.; Witzenbichler, B.; Biviano, A.; Godlewski, J.; Parise, H.; Dambrink, J.-H.; Mehran, R.; Gibson, C.M.; et al. Relationship between ST-Segment Resolution and Anterior Infarct Size after Primary Percutaneous Coronary Intervention: Analysis from the INFUSE-AMI Trial. Eur. Heart J. Acute Cardiovasc. Care 2014, 3, 78–83. [Google Scholar] [CrossRef]
- Zasada, W.; Bobrowska, B.; Plens, K.; Dziewierz, A.; Siudak, Z.; Surdacki, A.; Dudek, D.; Bartuś, S. Acute Myocardial Infarction in Young Patients. Kardiol. Pol. 2021, 79, 1093–1098. [Google Scholar] [CrossRef]
- Srikanth, S.; Ambrose, J.A. Pathophysiology of Coronary Thrombus Formation and Adverse Consequences of Thrombus During PCI. Curr. Cardiol. Rev. 2012, 8, 168–176. [Google Scholar] [CrossRef] [Green Version]
- Bellis, A.; Di Gioia, G.; Mauro, C.; Mancusi, C.; Barbato, E.; Izzo, R.; Trimarco, B.; Morisco, C. Reducing Cardiac Injury during ST-Elevation Myocardial Infarction: A Reasoned Approach to a Multitarget Therapeutic Strategy. J. Clin. Med. 2021, 10, 2968. [Google Scholar] [CrossRef] [PubMed]
- Sagris, M.; Theofilis, P.; Antonopoulos, A.S.; Oikonomou, E.; Paschaliori, C.; Galiatsatos, N.; Tsioufis, K.; Tousoulis, D. Inflammation in Coronary Microvascular Dysfunction. Int. J. Mol. Sci. 2021, 22, 13471. [Google Scholar] [CrossRef] [PubMed]
- David, S.W.; Khan, Z.A.; Patel, N.C.; Metzger, D.C.; Wood, F.O.; Wasserman, H.S.; Lotfi, A.S.; Hanson, I.D.; Dixon, S.R.; LaLonde, T.A.; et al. Evaluation of Intracoronary Hyperoxemic Oxygen Therapy in Acute Anterior Myocardial Infarction: The IC-HOT Study. Catheter. Cardiovasc. Interv. 2019, 93, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Scărlătescu, A.I.; Micheu, M.M.; Popa-Fotea, N.-M.; Dorobanțu, M. MicroRNAs in Acute ST Elevation Myocardial Infarction-A New Tool for Diagnosis and Prognosis: Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 4799. [Google Scholar] [CrossRef] [PubMed]
- Badacz, R.; Przewłocki, T.; Gacoń, J.; Stępień, E.; Enguita, F.J.; Karch, I.; Żmudka, K.; Kabłak-Ziembicka, A. Circulating MiRNA Levels Differ with Respect to Carotid Plaque Characteristics and Symptom Occurrence in Patients with Carotid Artery Stenosis and Provide Information on Future Cardiovascular Events. Postepy Kardiol. Interwencyjnej 2018, 14, 75–84. [Google Scholar] [CrossRef]
- Musialek, P.; Nizankowski, R.; Hopkins, L.N.; Micari, A.; Alvarez, C.A.; Nikas, D.N.; Ruzsa, Z.; Kühn, A.L.; Petrov, I.; Politi, M.; et al. Interdisciplinary Management of Acute Ischaemic Stroke—Current Evidence on Training Requirements for Endovascular Stroke Treatment. Position Paper from the ESC Council on Stroke and the European Association for Percutaneous Cardiovascular Interventions with the Support of the European Board of Neurointervention: A Step Forward. Adv. Interv. Cardiol. 2021, 17, 245–250. [Google Scholar] [CrossRef]
- Dohi, T.; Maehara, A.; Brener, S.J.; Généreux, P.; Gershlick, A.H.; Mehran, R.; Gibson, C.M.; Mintz, G.S.; Stone, G.W. Utility of Peak Creatine Kinase-MB Measurements in Predicting Myocardial Infarct Size, Left Ventricular Dysfunction, and Outcome after First Anterior Wall Acute Myocardial Infarction (from the INFUSE-AMI Trial). Am. J. Cardiol. 2015, 115, 563–570. [Google Scholar] [CrossRef]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized Cardiovascular Magnetic Resonance Imaging (CMR) Protocols: 2020 Update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef]
- Tymińska, A.; Ozierański, K.; Caforio, A.L.P.; Marcolongo, R.; Marchel, M.; Kapłon-Cieślicka, A.; Baritussio, A.; Filipiak, K.J.; Opolski, G.; Grabowski, M. Myocarditis and Inflammatory Cardiomyopathy in 2021: An Update. Pol Arch. Intern. Med. 2021, 131, 594–606. [Google Scholar] [CrossRef]
- Meier, D.; Fournier, S.; Masci, P.G.; Eeckhout, E.; Antiochos, P.; Tzimas, G.; Stoyanov, N.; Muenkaew, M.; Monney, P.; Schwitter, J.; et al. Impact of Manual Thrombectomy on Microvascular Obstruction in STEMI Patients. Catheter. Cardiovasc. Interv. 2021, 97, 1141–1148. [Google Scholar] [CrossRef]
- An, Y.; Kaji, S.; Kim, K.; Yamamuro, A.; Kinoshita, M.; Ehara, N.; Kobori, A.; Kitai, T.; Tani, T.; Kita, T.; et al. Successful Thrombus Aspiration during Primary Percutaneous Coronary Intervention Reduces Infarct Size and Preserves Myocardial Viability: A Cardiac Magnetic Resonance Imaging Study. J. Invasive Cardiol. 2011, 23, 172–176. [Google Scholar] [PubMed]
- Haeck, J.D.; Kuijt, W.J.; Koch, K.T.; Bilodeau, L.; Henriques, J.P.; Rohling, W.J.; Baan, J.; Vis, M.M.; Nijveldt, R.; van Geloven, N.; et al. Infarct Size and Left Ventricular Function in the PRoximal Embolic Protection in Acute Myocardial Infarction and Resolution of ST-Segment Elevation (PREPARE) Trial: Ancillary Cardiovascular Magnetic Resonance Study. Heart 2010, 96, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Zia, M.I.; Ghugre, N.R.; Connelly, K.A.; Joshi, S.B.; Strauss, B.H.; Cohen, E.A.; Wright, G.A.; Dick, A.J. Thrombus Aspiration during Primary Percutaneous Coronary Intervention Is Associated with Reduced Myocardial Edema, Hemorrhage, Microvascular Obstruction and Left Ventricular Remodeling. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2012, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Jenča, D.; Melenovský, V.; Stehlik, J.; Staněk, V.; Kettner, J.; Kautzner, J.; Adámková, V.; Wohlfahrt, P. Heart Failure after Myocardial Infarction: Incidence and Predictors. ESC Heart Fail. 2020, 8, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Bhar-Amato, J.; Davies, W.; Agarwal, S. Ventricular Arrhythmia after Acute Myocardial Infarction: ‘The Perfect Storm’. Arrhythm. Electrophysiol. Rev. 2017, 6, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Sardella, G.; Mancone, M.; Bucciarelli-Ducci, C.; Agati, L.; Scardala, R.; Carbone, I.; Francone, M.; Di Roma, A.; Benedetti, G.; Conti, G.; et al. Thrombus Aspiration during Primary Percutaneous Coronary Intervention Improves Myocardial Reperfusion and Reduces Infarct Size: The EXPIRA (Thrombectomy with Export Catheter in Infarct-Related Artery during Primary Percutaneous Coronary Intervention) Prospective, Randomized Trial. J. Am. Coll. Cardiol. 2009, 53, 309–315. [Google Scholar] [CrossRef]
- Jolly, S.S.; James, S.; Džavík, V.; Cairns, J.A.; Mahmoud, K.D.; Zijlstra, F.; Yusuf, S.; Olivecrona, G.K.; Renlund, H.; Gao, P.; et al. Thrombus Aspiration in ST-Segment-Elevation Myocardial Infarction: An Individual Patient Meta-Analysis: Thrombectomy Trialists Collaboration. Circulation 2017, 135, 143–152. [Google Scholar] [CrossRef]
- Stone, G.W.; Maehara, A.; Witzenbichler, B.; Godlewski, J.; Parise, H.; Dambrink, J.-H.E.; Ochala, A.; Carlton, T.W.; Cristea, E.; Wolff, S.D.; et al. Intracoronary Abciximab and Aspiration Thrombectomy in Patients with Large Anterior Myocardial Infarction: The INFUSE-AMI Randomized Trial. JAMA 2012, 307, 1817–1826. [Google Scholar] [CrossRef]
- De Carlo, M.; Aquaro, G.D.; Palmieri, C.; Guerra, E.; Misuraca, L.; Giannini, C.; Lombardi, M.; Berti, S.; Petronio, A.S. A Prospective Randomized Trial of Thrombectomy versus No Thrombectomy in Patients with ST-Segment Elevation Myocardial Infarction and Thrombus-Rich Lesions: MUS℡A (MUltidevice Thrombectomy in Acute ST-Segment ELevation Acute Myocardial Infarction) Trial. JACC Cardiovasc. Interv. 2012, 5, 1223–1230. [Google Scholar] [CrossRef]

| AT + PCI (n = 35) | PCI Only (n = 17) | p Value | |
|---|---|---|---|
| Age (years) | 55 (16.5) | 57 (20.0) | 1.00 |
| Body mass index (kg/m2) | 27.8 (3.4) | 28.4 (3.9) | 0.67 |
| Sex: male (n, %) | 23 (65.7%) | 14 (82.4%) | 0.80 |
| Hypertension (n, %) | 26 (74.3%) | 6 (35.3%) | 0.23 |
| Diabetes (n, %) | 8 (22.9%) | 3 (17.6%) | 0.43 |
| Dyslipidaemia (n, %) | 26 (74.3%) | 12 (70.6%) | 0.42 |
| Smoking (n, %) | 23 (65.7%) | 8 (47.1%) | 0.30 |
| Parameters on admission | |||
| Total ischemia time (min) | 240 (110) | 300 (250) | 0.37 |
| Systolic blood pressure (mmHg) | 125 (32) | 130 (30) | 0.55 |
| Heart rate (beats per minute) | 80 (15) | 75 (30) | 0.96 |
| Killip Class 0–1 (n, %) | 34 (97.1%) | 16 (94.1%) | 0.26 |
| Previous myocardial infarction (n, %) | 3 (8.6%) | 1 (5.9%) | 0.85 |
| Anterior myocardial infarction (n, %) | 14 (40.0%) | 10 (58.8%) | 0.37 |
| AT + PCI (n = 35) | PCI Only (n = 17) | p Value | |
|---|---|---|---|
| Multivessel disease (n, %) | 23 (65.7%) | 12 (70.6%) | 0.60 |
| IRA reference diameter (mm) | 3.12 (0.38) | 3.02 (0.56) | 0.40 |
| IRA location (n, %) | 0.41 | ||
| LAD | 16 (45.7%) | 10 (58.8%) | |
| LCx | 3 (8.6%) | 0 (0%) | |
| RCA | 16 (45.7%) | 7 (41.2%) | |
| Rentrop Grade (n, %) | 0.08 | ||
| 2 | 10 (28.6%) | 3 (17.6%) | |
| 1 | 14 (40.0%) | 3 (17.6%) | |
| 0 | 11 (31.4%) | 11 (64.7%) | |
| TIMI Thrombus Grade (n, %) | 0.40 | ||
| 5 | 29 (82.9%) | 13 (76.5%) | |
| 4 | 5 (14.2%) | 2 (11.8%) | |
| 3 or less | 1 (2.6%) | 2 (11.8%) | |
| Final TMPG (n, %) | 0.26 | ||
| 2 or more | 30 (85.7%) | 10 (58.8%) | |
| 1 or less | 5 (14.3%) | 7 (41.2%) | |
| Embolization (n, %) | 9 (25.7%) | 4 (23.5%) | 0.86 |
| Direct stenting (n, %) | 15 (42.9%) | 1 (5.9%) | 0.014 |
| AT + PCI (n = 28) | PCI Only (n = 17) | p Value | |
|---|---|---|---|
| CKMB AUC (U/l) | 10,787 (12,815) | 6337 (5597) | 0.09 |
| ST segment elevation | 18 (12.0) | 15 (10.0) | 0.32 |
| on admission | |||
| ST segment elevation post PCI | |||
| Directly post PCI | 7 (5.8) | 7 (8.0) | 0.47 |
| 1 h post PCI | 4 (4.5) | 4 (4.0) | 0.72 |
| 2 h post PCI | 3 (3.0) | 3 (3.0) | 0.27 |
| LVEDV (mL) | |||
| Index | 142.6 (40.1) | 127.8 (45.5) | 0.54 |
| Follow-up | 149.2 (54.8) | 158.0 (75.0) | 0.26 |
| ∆LVEDV | 8.7 (19.3) | 20.0 (20.5) | 0.004 |
| LV remodelling | |||
| ∆LVEDV >12% (n, %) | 3 (8.6%) | 9 (52.9%) | 0.019 |
| LVEF (%) | |||
| Index | 37.8 (16.7) | 45.7 (14.7) | 0.96 |
| Follow-up | 44.2 (19.4) | 43.5 (11.4) | 0.98 |
| ∆LVEF | 5.8 (10.1) | 4.0 (6.3) | 0.41 |
| MI size (g) | |||
| Index | 28.0 (21.5) | 25.0 (15.0) | 0.39 |
| Follow-up | 19.8 (13.0) | 16.9 (12.2) | 0.55 |
| MI size reduction | −7.8 (10.8) | −4.5 (4.8) | 0.03 |
| MI size/LV mass (%) | |||
| Index | 18.7 (11.3) | 15.8 (8.5) | 0.23 |
| Follow-up | 14.0 (11.7) | 15.4 (9.8) | 0.89 |
| MVO (g) | 4.0 (5.4) | 6.0 (6.1) | 0.26 |
| MVO/MI (%) | 11.7 (9.0) | 22.2 (26.9) | 0.009 |
| MSI (%) | |||
| Index | 0.5 (0.3) | 0.4 (0.3) | 0.42 |
| Follow-up | 0.7 (0.2) | 0.6 (0.2) | 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zajdel, W.; Miszalski-Jamka, T.; Zalewski, J.; Legutko, J.; Żmudka, K.; Paszek, E. Cardiac Magnetic Resonance Shows Improved Outcomes in Patients with an ST-Segment Elevation Myocardial Infarction and a High Thrombus Burden Treated with Adjuvant Aspiration Thrombectomy. J. Clin. Med. 2022, 11, 5000. https://doi.org/10.3390/jcm11175000
Zajdel W, Miszalski-Jamka T, Zalewski J, Legutko J, Żmudka K, Paszek E. Cardiac Magnetic Resonance Shows Improved Outcomes in Patients with an ST-Segment Elevation Myocardial Infarction and a High Thrombus Burden Treated with Adjuvant Aspiration Thrombectomy. Journal of Clinical Medicine. 2022; 11(17):5000. https://doi.org/10.3390/jcm11175000
Chicago/Turabian StyleZajdel, Wojciech, Tomasz Miszalski-Jamka, Jarosław Zalewski, Jacek Legutko, Krzysztof Żmudka, and Elżbieta Paszek. 2022. "Cardiac Magnetic Resonance Shows Improved Outcomes in Patients with an ST-Segment Elevation Myocardial Infarction and a High Thrombus Burden Treated with Adjuvant Aspiration Thrombectomy" Journal of Clinical Medicine 11, no. 17: 5000. https://doi.org/10.3390/jcm11175000
APA StyleZajdel, W., Miszalski-Jamka, T., Zalewski, J., Legutko, J., Żmudka, K., & Paszek, E. (2022). Cardiac Magnetic Resonance Shows Improved Outcomes in Patients with an ST-Segment Elevation Myocardial Infarction and a High Thrombus Burden Treated with Adjuvant Aspiration Thrombectomy. Journal of Clinical Medicine, 11(17), 5000. https://doi.org/10.3390/jcm11175000






