The Combining of Tyrosine Kinase Inhibitors and Immune Checkpoint Inhibitors as First-Line Treatment for Advanced Stage Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Patient Characteristics
3.2. Best Radiological Response
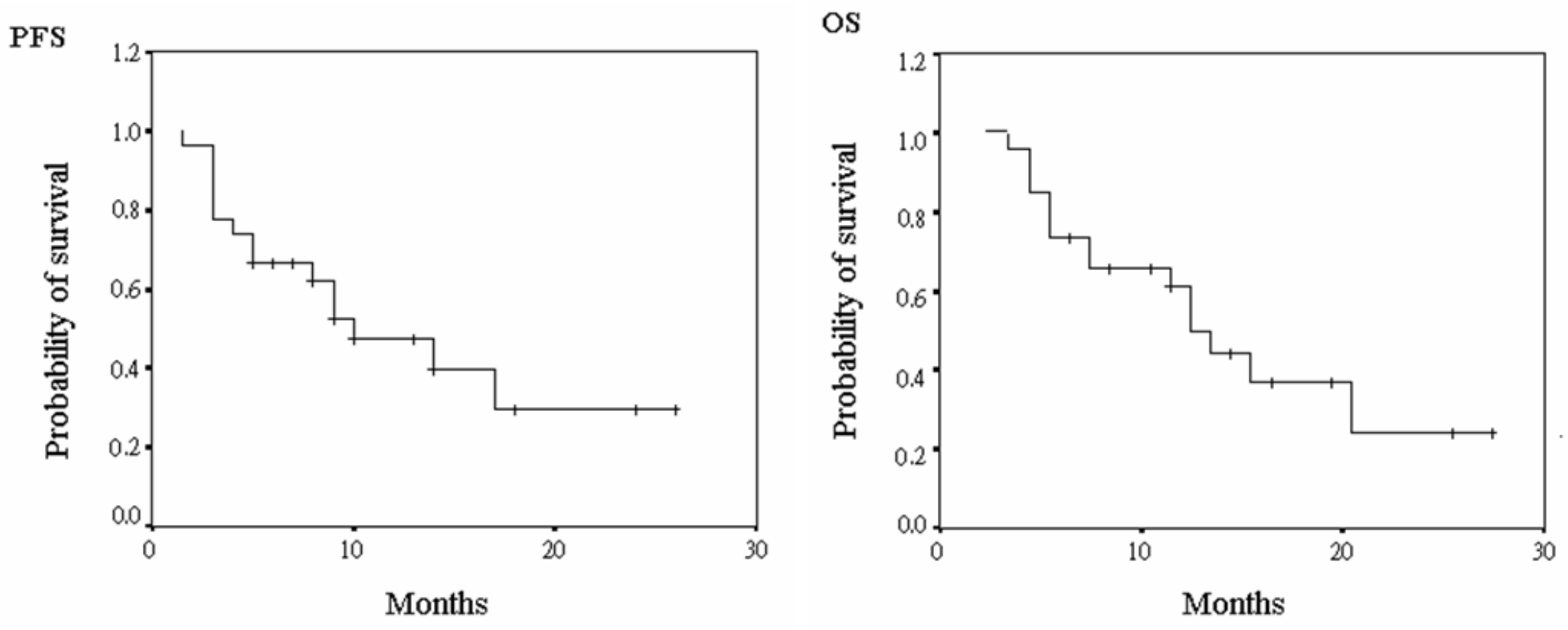
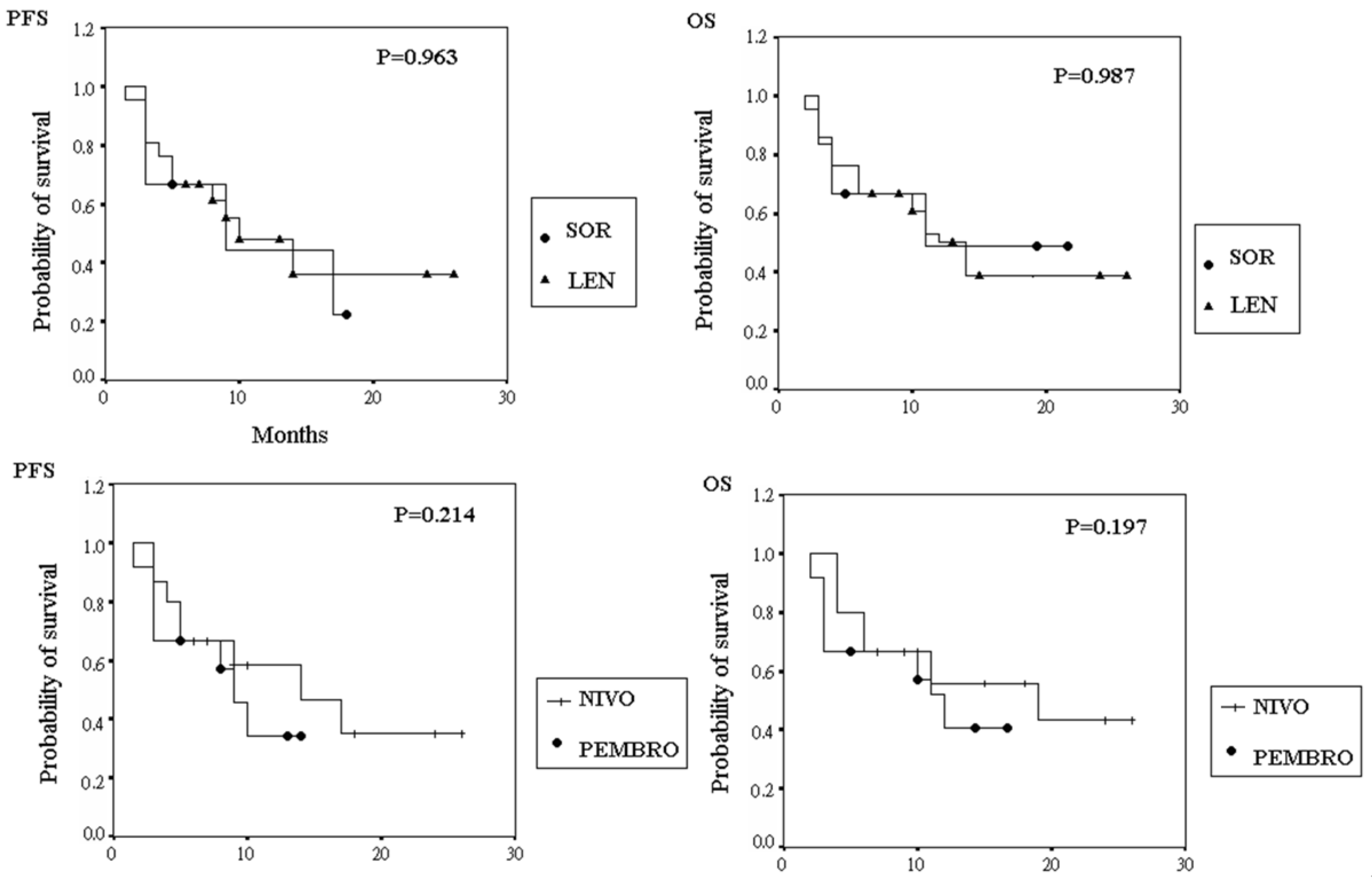
3.3. Progression-Free Survival and Overall Survival
3.4. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A Phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus Sorafenib in First-Line Treatment of Patients with Unresectable Hepatocellular Carcinoma: A Randomised Phase 3 Non-Inferiority Trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Callahan, M.K.; Postow, M.A.; Wolchok, J.D. Targeting T cell co-receptors for cancer therapy. Immunity 2016, 44, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmar, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase III trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Park, J.; Finn, R.; Cheng, A.; Mathurin, P.; Edeline, J.; Kudo, M.; Han, K.; Harding, J.; Merle, P.; et al. LBA-3 CheckMate 459: Long-term (minimum follow-up 33.6 months) survival outcomes with nivolumab versus sorafenib as first-line treatment in patients. Ann. Oncol. 2020, 31, S241–S242. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef]
- Clark, J.W.; Eder, J.P.; Ryan, D.; Lathia, C.; Lenz, H.J. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43-9006, in patients with advanced, refractory solid tumors. Clin. Cancer Res. 2005, 11, 5472–5480. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Lenvatinib in advanced hepatocellular carcinoma. Liver Cancer 2017, 6, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Combination cancer immunotherapy in hepatocellular carcinoma. Liver Cancer 2018, 7, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H.; Liang, Q.; Liu, B.; Mei, X.; Ma, Y. Combinatorial immunotherapy of sorafenib and blockade of programmed death-ligand 1 induces effective natural killer cell responses against hepatocellular carcinoma. Tumor Biol. 2015, 36, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ikeda, M.; Motomura, K.; Okusaka, T.; Kato, N.; Dutcus, C.E.; Hisai, T.; Suzuki, M.; Ikezawa, H.; Iwatam, T.; et al. A phase Ib study of lenvatinib (LEN) plus nivolumab (NIV) in patients (pts) with unresectable hepatocellular carcinoma (uHCC): Study 117. J. Clin. Oncol. 2020, 38, 513. [Google Scholar] [CrossRef]
- Finn, R.S.; Ikeda, M.; Zhu, A.X.; Sung, M.W.; Baron, A.D.; Kudo, M.; Okusaka, T.; Kobayashi, M.; Kumada, H.; Kaneko, S.; et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J. Clin. Oncol. 2020, 38, 2960–2970. [Google Scholar] [CrossRef] [PubMed]
| All (N = 33) | SOR/NIVO (N = 8) | SOR/PEMBRO (N = 4) | LEN/NIVO (N = 11) | LEN/PEMBRO (N = 10) | p-Value | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | (IQR) | N | % | M | (IQR) | N | % | M | (IQR) | N | % | M | (IQR) | N | % | M | (IQR) | N | % | |||
| Age (years) | 66 | (14) | 68 | (9) | 70 | (4) | 60 | (18) | 56 | (15) | 0.090 a | |||||||||||
| Gender (male) | 26 | (78.8%) | 6 | (75.0%) | 2 | (50.0%) | 8 | (72.7%) | 10 | (100%) | 0.173 b | |||||||||||
| Hepatitis infection | HBV | 19 | (57.6%) | 4 | (50.0%) | 0 | 8 | (72.7%) | 7 | (70.0%) | 0.063 b | |||||||||||
| HCV | 8 | (24.2%) | 0 | 4 | (100%) | 2 | (18.2%) | 2 | (25.0%) | 0.062 b | ||||||||||||
| Child-Pugh stage | A | 33 | (100%) | 8 | (100%) | 4 | (100%) | 11 | (100%) | 10 | (100%) | 1.000 b | ||||||||||
| BCLC stage | C | 33 | (100%) | 8 | (100%) | 4 | (100%) | 11 | (100%) | 10 | (100%) | 1.000 b | ||||||||||
| MVI | 16 | (48.2%) | 4 | (50.0%) | 6 | (54.5%) | 6 | (60.0%) | 0.215 b | |||||||||||||
| EHS | 19 | (57.6%) | 4 | (50.0%) | 4 | (100%) | 6 | (54.5%) | 5 | (50.0%) | 0.332 b | |||||||||||
| Bilirubin (U/L) | 0.7 | (0.9) | 1.4 | (1.9) | 0.5 | (0.2) | 0.7 | (0.5) | 0.9 | (0.8) | 0.281 a | |||||||||||
| ALT (U/L) | 36 | (34) | 34 | (25) | 15 | (15) | 51 | (45) | 40 | (19) | 0.206 a | |||||||||||
| Abnormal ALT (male > 50 U/L or female > 35 U/L) | 10 | (30.3%) | 2 | (25.0%) | 0 | 6 | (54.5%) | 2 | (20.0%) | 0.144 b | ||||||||||||
| AFP (ng/mL) | 130 | (3115) | 227 | (3408) | 1036 | (2067) | 132 | (3916) | 34 | (7502) | 0.575 a | |||||||||||
| Abnormal AFP (AFP > 7 ng/mL) | 22 | (66.7%) | 6 | (75.0%) | 2 | (50.0%) | 9 | (81.8%) | 5 | (50.0%) | 0.371 b | |||||||||||
| AFP (ng/mL) | ≥400 | 13 | (39.4%) | 2 | (25.0%) | 2 | (50.0%) | 5 | (45.5%) | 4 | (40.0%) | 0.788 b | ||||||||||
| <400 | 20 | (60.6%) | 6 | (75.0%) | 2 | (50.0%) | 6 | (54.5%) | 6 | (60.0%) | ||||||||||||
| AFP decreased > 10% | 10 | (30.3%) | 4 | (50.0%) | 0 | 4 | (36.4%) | 2 | (20.0%) | 0.272 b | ||||||||||||
| All (N = 33) | SOR/NIVO (N = 8) | SOR/PEMBRO (N = 4) | LEN/NIVO (N = 11) | LEN/PEMBRO (N = 10) | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | |||
| mRECIST | 0.279 | |||||||||||
| CR | 2 | (6.1%) | 0 | 1 | (25.0%) | 1 | (7.4%) | 0 | ||||
| PR | 14 | (42.4%) | 2 | (25.0%) | 2 | (50.0%) | 3 | (44.4%) | 7 | (70.0%) | ||
| SD | 8 | (24.2%) | 2 | (25.0%) | 1 | (25.0%) | 4 | (22.3%) | 1 | (10.0%) | ||
| PD | 9 | (27.3%) | 4 | (50.0%) | 0 | 3 | (25.9%) | 2 | (20.0%) | |||
| ORR | 16 | (48.5%) | 2 | (25.0%) | 3 | (75.0%) | 4 | (51.8%) | 7 | (70.0%) | 0.145 | |
| DCR | 24 | (72.7%) | 4 | (50.0%) | 4 | (100%) | 8 | (74.1%) | 8 | (80.0%) | 0.278 | |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | p-Value | HR | (95% CI) | p-Value | |
| Age (≤65 vs. >65 years) | 1.87 | (0.44–7.85) | 0.395 | 1.07 | (0.13–8.96) | 0.948 |
| Gender (male vs. female) | 8.17 | (0.85–77.97) | 0.068 | 8.69 | (0.66–114.26) | 0.100 |
| HBV (HBsAg + vs. −) | 0.90 | (0.23–3.58) | 0.881 | |||
| HCV (anti-HCV + vs. −) | 4.50 | (0.75–26.93) | 0.099 | |||
| AFP (≤400 vs. >400 ng/mL) | 1.96 | (0.47–8.11) | 0.356 | |||
| AFP decreased > 10% (yes vs. no) | 1.64 | (0.36–7.38) | 0.521 | |||
| MVI (yes vs. no) | 0.42 | (0.10–1.70) | 0.224 | |||
| EHS (yes vs. no) | 0.55 | (0.14–2.20) | 0.394 | |||
| TKI (LEN vs. SOR) | 1.54 | (0.37–6.45) | 0.554 | 1.27 | (0.15–11.09) | 0.826 |
| ICI (PEMBRO vs. NIVO) | 5.42 | (1.19–24.52) | 0.028 | 5.54 | (1.06–28.91) | 0.042 |
| HFRS (yes vs. no) | 1.95 | (0.43–8.82) | 0.386 | |||
| Hypertension (yes vs. no) | 1.29 | (0.10–3.27) | 0.849 | |||
| Diarrhea (yes vs. no) | 1.09 | (0.25–4.81) | 0.908 | |||
| Fatigue (yes vs. no ) | 3.00 | (0.92–23.45) | 0.057 | |||
| All (N = 33) | SOR/NIVO (N = 8) | SOR/PEMBRO (N = 4) | LEN/NIVO (N = 11) | LEN/PEMBRO (N = 10) | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | ||
| HFRS | 10 | (30.3%) | 4 | (50.0%) | 2 | (50.0%) | 1 | (16.7%) | 3 | (30.0%) | 0.208 |
| Hypertension | 6 | (18.2%) | 0 | 0 | 2 | (18.2%) | 4 | (40.0%) | 0.118 | ||
| Diarrhea | 10 | (30.3%) | 2 | (25.0%) | 2 | (50.0%) | 3 | (27.3%) | 3 | (30.0%) | 0.828 |
| Fatigue | 12 | (36.4%) | 2 | (25.0%) | 2 | (50.0%) | 5 | (45.5%) | 3 | (30.0%) | 0.721 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-W.; Yang, S.-S.; Lien, H.-C.; Peng, Y.-C.; Tung, C.-F.; Lee, T.-Y. The Combining of Tyrosine Kinase Inhibitors and Immune Checkpoint Inhibitors as First-Line Treatment for Advanced Stage Hepatocellular Carcinoma. J. Clin. Med. 2022, 11, 4874. https://doi.org/10.3390/jcm11164874
Lee S-W, Yang S-S, Lien H-C, Peng Y-C, Tung C-F, Lee T-Y. The Combining of Tyrosine Kinase Inhibitors and Immune Checkpoint Inhibitors as First-Line Treatment for Advanced Stage Hepatocellular Carcinoma. Journal of Clinical Medicine. 2022; 11(16):4874. https://doi.org/10.3390/jcm11164874
Chicago/Turabian StyleLee, Shou-Wu, Sheng-Shun Yang, Han-Chung Lien, Yen-Chun Peng, Chun-Fang Tung, and Teng-Yu Lee. 2022. "The Combining of Tyrosine Kinase Inhibitors and Immune Checkpoint Inhibitors as First-Line Treatment for Advanced Stage Hepatocellular Carcinoma" Journal of Clinical Medicine 11, no. 16: 4874. https://doi.org/10.3390/jcm11164874
APA StyleLee, S.-W., Yang, S.-S., Lien, H.-C., Peng, Y.-C., Tung, C.-F., & Lee, T.-Y. (2022). The Combining of Tyrosine Kinase Inhibitors and Immune Checkpoint Inhibitors as First-Line Treatment for Advanced Stage Hepatocellular Carcinoma. Journal of Clinical Medicine, 11(16), 4874. https://doi.org/10.3390/jcm11164874






