Procedural Safety and Device Performance of the Portico™ Valve from Experienced TAVI Centers: 30-Day Outcomes in the Multicenter CONFIDENCE Registry
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Patients
3.2. Procedural Characteristics
3.3. VARC-2 Endpoints
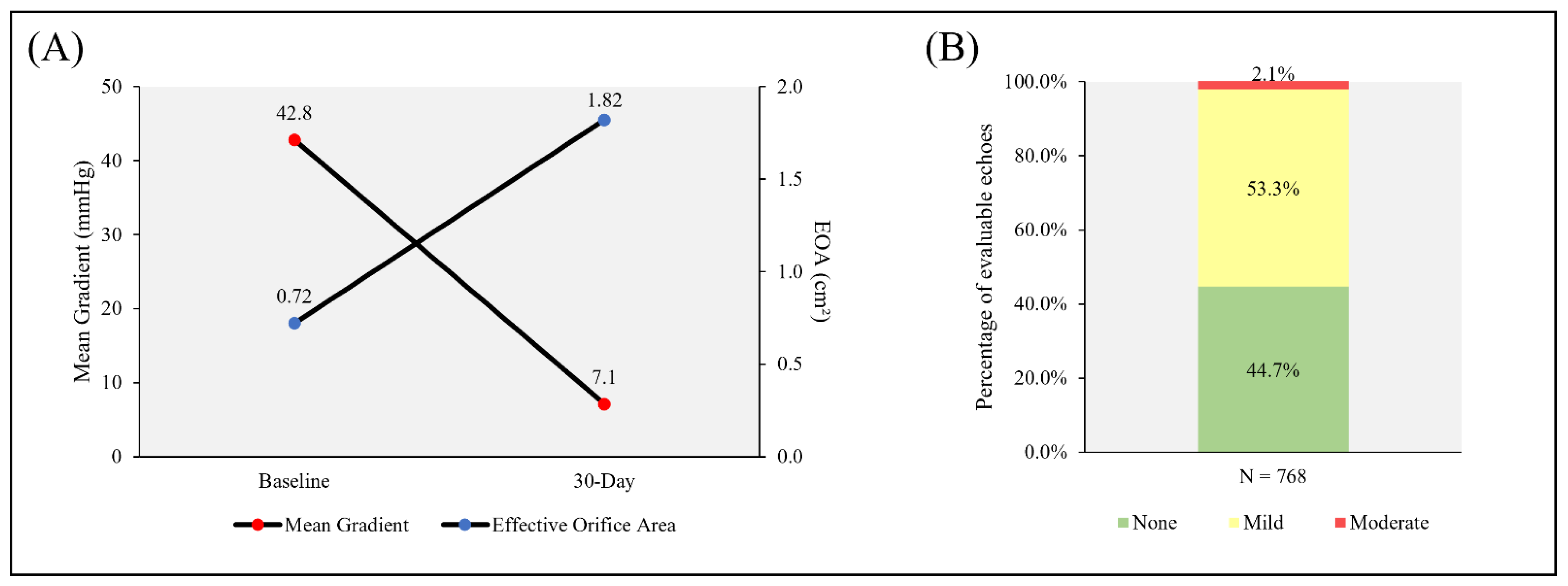
3.4. Hemodynamics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. J. Cardio-Thorac. Surg. 2021, 60, 727–800. [Google Scholar]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F., 3rd; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef] [PubMed]
- Maisano, F.; Worthley, S.; Rodés-Cabau, J.; Linke, A.H.-P.; Fichtlscherer, S.; Schäfer, U.; Makkar, R.R.; Fontana, G.; Asch, F.M.; Søndergaard, L. Early commercial experience from transcatheter aortic valve implantation using the Portico™ bioprosthetic valve: 30-day outcomes in the multicentre PORTICO-1 study. EuroIntervention 2018, 14, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Sondergaard, L.; Rodes-Cabau, J.; Hans-Peter Linke, A.; Fichtlscherer, S.; Schafer, U.; Kuck, K.H.; Kempfert, J.; Arzamendi, D.; Bedogni, F.; Asch, F.M.; et al. Transcatheter Aortic Valve Replacement with a Repositionable Self-Expanding Pros-thesis: The PORTICO-I Trial 1-Year Outcomes. J. Am. Coll. Cardiol. 2018, 72, 2859–2867. [Google Scholar] [CrossRef] [PubMed]
- Kappetein, A.P.; Head, S.J.; Généreux, P.; Piazza, N.; Van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; Van Es, G.-A.; et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document (VARC-2). Eur. J. Cardio-Thoracic Surg. 2012, 42, S45–S60. [Google Scholar] [CrossRef] [PubMed]
- Möllmann, H.; Linke, A.; Holzhey, D.M.; Walther, T.; Manoharan, G.; Schäfer, U.; Heinz-Kuck, K.; Van Boven, A.J.; Redwood, S.R.; Kovac, J.; et al. Implantation and 30-Day Follow-Up on All 4 Valve Sizes within the Portico Transcatheter Aortic Bioprosthetic Family. JACC Cardiovasc. Interv. 2017, 10, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Linke, A.; Holzhey, D.; Möllmann, H.; Manoharan, G.; Schäfer, U.; Frerker, C.; Worthley, S.G.; van Boven, A.J.; Redwood, S.; Kovac, J.; et al. Treatment of Aortic Stenosis with a Self-Expanding, Resheathable Transcatheter Valve: One-Year Results of the International Multicenter Portico Transcatheter Aortic Valve Implantation System Study. Circ. Cardiovasc. Interv. 2018, 11, e005206. [Google Scholar] [CrossRef] [PubMed]
- Fontana, G.P.; Bedogni, F.; Groh, M.; Smith, D.; Chehab, B.M.; Garrett, H.E.; Yong, G.; Worthley, S.; Manoharan, G.; Walton, A.; et al. Safety Profile of an Intra-Annular Self-Expanding Transcatheter Aortic Valve and Next-Generation Low-Profile Delivery System. JACC Cardiovasc. Interv. 2020, 13, 2467–2478. [Google Scholar] [CrossRef] [PubMed]
- Grube, E.; Van Mieghem, N.M.; Bleiziffer, S.; Modine, T.; Bosmans, J.; Manoharan, G.; Linke, A.; Scholtz, W.; Tchétché, D.; Finkelstein, A.; et al. Clinical Outcomes with a Repositionable Self-Expanding Transcatheter Aortic Valve Prosthesis: The International FORWARD Study. J. Am. Coll. Cardiol. 2017, 70, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Wendler, O.; Schymik, G.; Treede, H.; Baumgartner, H.; Dumonteil, N.; Ihlberg, L.; Neumann, F.J.; Tarantini, G.; Zamarano, J.L.; Vahanian, A. SOURCE 3 Registry: Design and 30-Day Results of the European Postapproval Registry of the Latest Generation of the SAPIEN 3 Transcatheter Heart Valve. Circulation 2017, 135, 1123–1132. [Google Scholar] [CrossRef]
- Möllmann, H.; Hengstenberg, C.; Hilker, M.; Kerber, S.; Schäfer, U.; Rudolph, T.; Linke, A.; Franz, N.; Kuntze, T.; Nef, H.; et al. Real-world experience using the ACURATE neo prosthesis: 30-day outcomes of 1000 patients enrolled in the SAVI TF registry. EuroIntervention 2018, 13, e1764–e1770. [Google Scholar] [CrossRef] [PubMed]
- Dvir, D.; Leipsic, J.; Blanke, P.; Ribeiro, H.B.; Kornowski, R.; Pichard, A.; Rodés-Cabau, J.; Wood, D.A.; Stub, D.; Ben-Dor, I.; et al. Coronary obstruction in transcatheter aortic valve-in-valve implantation: Preprocedural evaluation, device selection, protection, and treatment. Circ. Cardiovasc. Interv. 2015, 8, e002079. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, G.; Grube, E.; Van Mieghem, N.M.; Brecker, S.; Fiorina, C.; Kornowski, R.; Danenberg, H.; Ruge, H.; Thiele, H.; Lancellotti, P.; et al. Thirty-day clinical outcomes of the Evolut PRO self-expanding transcatheter aortic valve: The international FORWARD PRO study. EuroIntervention 2020, 16, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Schymik, G.; Bartorelli, A.L.; Rubino, P.; Urban, P.; Walther, T.; Thomas, M.; Lefèvre, T.; Windecker, S.; Baumgartner, H.; Vahanian, A.; et al. European experience with the second-generation Edwards SAPIEN XT transcatheter heart valve in patients with severe aortic stenosis: 1-year outcomes from the SOURCE XT Registry. JACC Cardiovasc. Interv. 2015, 8, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Walther, T.; Manoharan, G.; Linke, A.; Mollmann, H.; Holzhey, D.; Worthley, S.G.; Kim, W.-K.; Schäfer, U. Incidence of new-onset left bundle branch block and predictors of new permanent pacemaker following transcatheter aortic valve replacement with the Portico™ valve. Eur. J. Cardio-Thoracic Surg. 2018, 54, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Bieliauskas, G.; Wong, I.; Bajoras, V.; Wang, X.; Kofoed, K.F.; De Backer, O.; Søndergaard, L. Patient-Specific Implantation Tech-nique to Obtain Neo-Commissural Alignment with Self-Expanding Transcatheter Aortic Valves. JACC Cardiovasc. Interv. 2021, 14, 2097–2108. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-K.; Pellegrini, C.; Ludwig, S.; Möllmann, H.; Leuschner, F.; Makkar, R.; Leick, J.; Amat-Santos, I.J.; Dörr, O.; Breitbart, P.; et al. Feasibility of Coronary Access in Patients with Acute Coronary Syndrome and Previous TAVR. JACC Cardiovasc. Interv. 2021, 14, 1578–1590. [Google Scholar] [CrossRef] [PubMed]
- Kanso, M.; Kibler, M.; Hess, S.; Rischner, J.; Plastaras, P.; Kindo, M.; Hoang, M.; De Poli, F.; Leddet, P.; Petit, H.; et al. Effective Orifice Area of Balloon-Expandable and Self-Expandable Transcatheter Aortic Valve Prostheses: An Echo Doppler Comparative Study. J. Clin. Med. 2021, 10, 186. [Google Scholar] [CrossRef] [PubMed]
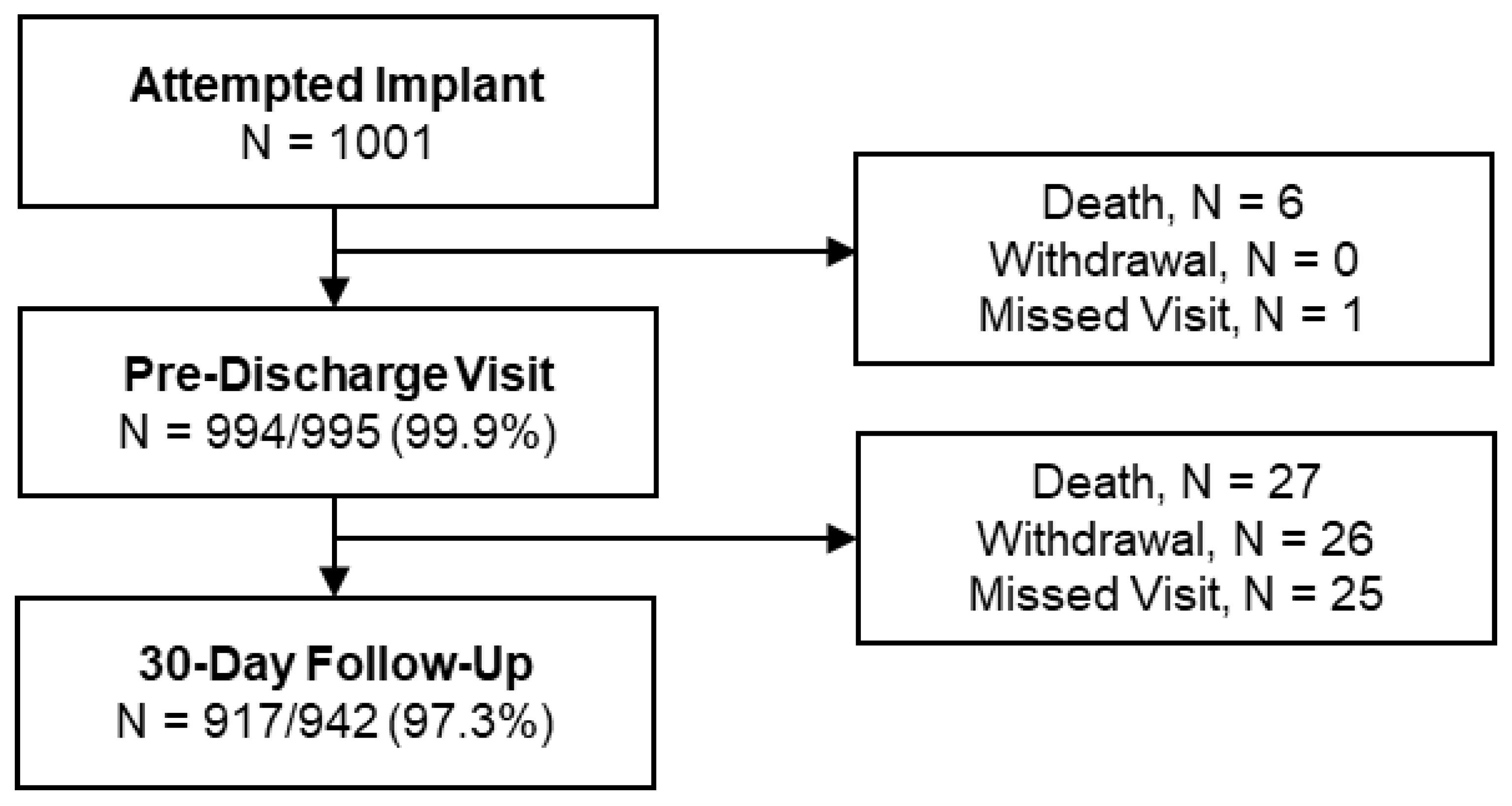
| Characteristic | Portico™ DS 1 % (n) of Subjects (n = 501) | FlexNav™ DS % (n) of Subjects (n = 500) | Total (n = 1001) |
|---|---|---|---|
| Age (Years) | 81.7 ± 5.4 | 82.3 ± 5.3 | 82.0 ± 5.3 |
| Gender (Female) | 63.7% | 61.4% | 62.5% |
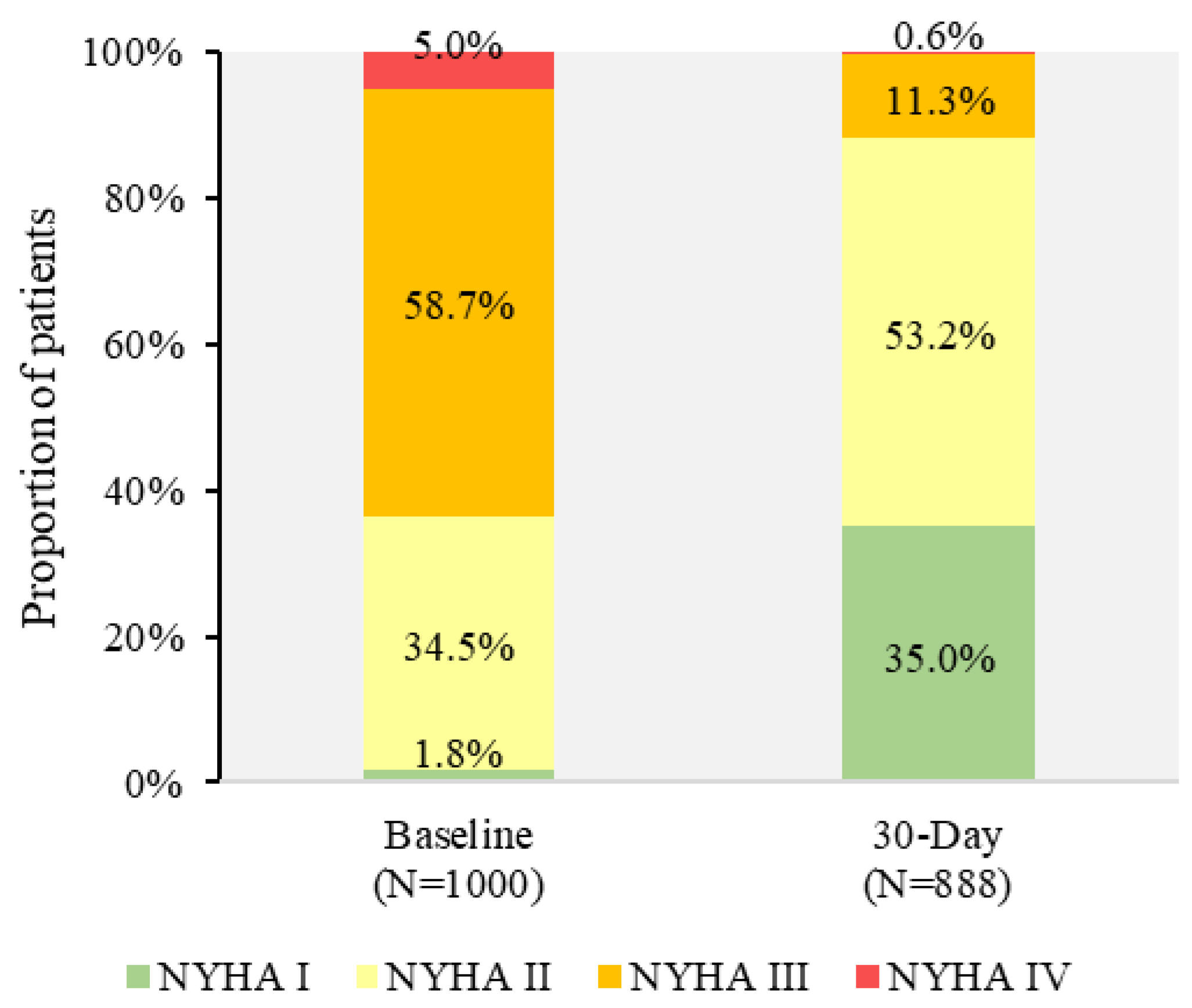
| NYHA Class | |||
| I | 2.8% | 0.8% | 1.8% |
| II | 31.9% | 37.0% | 34.5% |
| III | 58.9% | 58.6% | 58.7% |
| IV | 6.4% | 3.6% | 5.0% |
| EuroSCORE I (%) | 16.4 ± 11.1 | 14.9 ± 10.3 | 15.7 ± 10.8 |
| EuroSCORE II (%) | 4.8 ± 3.8 | 4.7 ± 4.3 | 4.8 ± 4.1 |
| STS Mortality Risk Score (%) | 4.2 ± 2.9 | 4.2 ± 2.7 | 4.2 ± 2.8 |
| Number of frailty factors contributing to the subject’s surgical risk score | |||
| 1 | 23.5% | 19.3% | 21.1% |
| 2 | 14.7% | 11.8% | 13.1% |
| 3 | 9.7% | 10.7% | 10.2% |
| 4 | 0.9% | 1.2% | 1.0% |
| Cardiac arrhythmia | 49.1% | 47.6% | 48.4% |
| Carotid artery disease | 12.6% | 10.6% | 11.6% |
| Chronic kidney disease | 27.7% | 26.0% | 26.9% |
| Dialysis | 2.9% | 1.5% | 2.2% |
| Chronic lung disease | 19.4% | 21.0% | 20.2% |
| Coronary artery disease | 57.9% | 52.4% | 55.1% |
| Diabetes | 35.1% | 36.4% | 35.8% |
| Dyslipidemia | 59.3% | 64.4% | 61.8% |
| Hematologic disorders | 10.4% | 10.8% | 10.6% |
| Hypertension | 87.8% | 86.2% | 87.0% |
| Liver disease or cirrhosis | 3.4% | 3.0% | 3.2% |
| Mitral valve disease | 61.7% | 61.0% | 61.3% |
| Myocardial infarction | 13.6% | 12.2% | 12.9% |
| Peripheral artery disease | 12.0% | 11.8% | 11.9% |
| Prior permanent pacemaker | 9.4% | 11.2% | 10.3% |
| Prior CABG | 7.4% | 8.8% | 8.1% |
| Prior PCI | 31.7% | 31.4% | 31.6% |
| Prior stroke | 10.6% | 7.6% | 9.1% |
| Prior TIA | 4.4% | 5.0% | 4.7% |
| Mean aortic valve gradient (mmHg) | 43.4 ± 14.5 | 42.2 ± 15.0 | 42.8 ± 14.7 |
| Aortic valve area (cm2) | 0.71 ± 0.2 | 0.72 ± 0.2 | 0.72 ± 0.18 |
| Characteristic | Portico™ DS 1 % (n) of Subjects (n = 501) | FlexNav™ DS % (n) of Subjects (n = 500) | p-Value | Total % (n) of Subjects (n = 1001) |
|---|---|---|---|---|
| Portico™ valve implant success | 97.4% (488) | 97.6% (488) | 0.8434 | 97.5% (976) |
| Procedural mortality | 0.2% (1) | 0.0% (0) | 1.0000 | 0.1% (1) |
| Conversion to SAVR | 0.4% (2) | 0.0% (0) | 0.4995 | 0.2% (2) |
| More than 1 valve implanted 2 | 1.8% (9) | 2.0% (10) | 0.8134 | 1.9% (19) |
| No Portico™ valve implanted | 0.2% (1) | 0.4% (2) | 0.6242 | 0.3% (3) |
| Anesthesia | <0.0001 | |||
| General anesthesia | 30.1% (151) | 16.4% (82) | 23.3% (233) | |
| Conscious sedation | 69.9% (350) | 82.6% (413) | 76.2% (763) | |
| Access method | 0.1639 | |||
| Transfemoral | 98.2% (492) | 99.2% (496) | 98.7% (988) | |
| Subclavian/axillary | 1.8% (9) | 0.8% (4) | 1.3% (13) | |
| Vessel diameter (mm) | 7.42 ± 1.44 | 7.09 ± 1.36 | 0.0002 | 7.25 ± 1.41 |
| Valve utilized | ||||
| 23 mm | 5.6% (28) | 9.0% (45) | 0.0380 | 7.3% (73) |
| 25 mm | 25.7% (129) | 31.4% (157) | 0.0478 | 28.6% (286) |
| 27 mm | 39.1% (196) | 32.2% (161) | 0.0223 | 35.7% (357) |
| 29 mm | 29.5% (148) | 27.4% (137) | 0.4530 | 28.5% (285) |
| Valve resheathed | 34.3% (172) | 41.2% (206) | 0.0250 | 37.8% (378) |
| Implant too high | 55.8% (96) | 77.7% (160) | 67.7% (256) | |
| Implant too low | 35.5% (61) | 18.0% (37) | 25.9% (98) | |
| Coronary occlusion | 0.0% (0) | 0.0% (0) | 0.0% (0) | |
| Other | 8.7% (15) | 4.4% (9) | 6.3% (24) | |
| Introducer sheath used | 93.6% (469) | 24.0% (120) | <0.0001 | 58.8% (589) |
| 18F | 32.8% (154) | 25.8% (31) | 31.4% (185) | |
| 19F | 65.7% (308) | 65.0% (78) | 65.5% (386) | |
| 20F | 0.2% (1) | 4.2% (5) | 1.0% (6) | |
| Other | 1.3% (6) | 5.0% (6) | 2.0% (12) | |
| Pre-balloon valvuloplasty | 85.4% (428) | 88.4% (442) | 0.1635 | 86.9% (870) |
| Post-balloon valvuloplasty | 37.7% (189) | 37.4% (187) | 0.9156 | 37.6% (376) |
| Concomitant procedures | 8.4% (42) | 4.8% (24) | 0.0224 | 6.6% (66) |
| Non-coronary cusp (NCC) depth (mm) | 5.1 ± 3.0 | 4.5 ± 3.0 | 0.0043 | 4.8 ± 3.0 |
| Left coronary cusp (LCC) depth (mm) | 5.8 ± 2.9 | 5.5 ± 2.9 | 0.0610 | 5.6 ± 2.9 |
| Total procedure time (first incision to closure, min) | 64.6 ± 38.0 | 73.4 ± 39.8 | 0.0004 | 69.0 ± 39.1 |
| Event Type | Portico™ DS % (n) of Subjects (n = 501) | FlexNav™ DS % (n) of Subjects (n = 500) | p-Value | Total: % (n) of Subjects (n = 1001) |
|---|---|---|---|---|
| All-cause mortality | 3.2% (16) | 2.0% (10) 1 | 0.6376 | 2.6% (26) |
| Cardiovascular mortality 2 | 3.0% (15) | 1.2% (6) | 0.1270 | 2.1% (21) |
| Non-cardiovascular mortality | 0.2% (1) | 0.8% (4) | 0.1516 | 0.5% (5) |
| Myocardial infarction | 0.4% (2) | 0.2% (1) | 1.0000 | 0.3% (3) |
| Acute kidney injury stage | 1.4% (7) | 0.8% (4) | 0.3841 | 1.1% (11) |
| Bleeding events | ||||
| Life-threatening | 3.2% (16) | 3.6% (18) | 0.6755 | 3.4% (34) |
| Major bleeding | 5.2% (26) | 6.6% (33) | 0.3020 | 5.9% (59) |
| Minor bleeding | 6.2% (31) | 4.0% (20) | 0.1903 | 5.1% (51) |
| Stroke | ||||
| Disabling | 1.6% (8) | 2.0% (10) | 0.5989 | 1.8% (18) |
| Non-disabling | 1.0% (5) | 1.2% (6) | 0.7320 | 1.1% (11) |
| Vascular complications | 13.2% (66) | 12.6% (63) | 0.8250 | 12.9% (129) |
| Major vascular complications | 6.4% (32) | 8.2% (41) | 0.2311 | 7.3% (73) |
| Access site complication | 6.0% (30) | 7.4% (37) | 0.3249 | 6.7% (67) |
| TAVI Delivery System site | 4.0% (20) | 5.6% (28) | 0.2054 | 4.8% (48) |
| Non-TAVI Delivery System site | 2.0% (10) | 1.8% (9) | 0.8573 | 1.9% (19) |
| Non-access site complication | 0.4% (2) | 0.8% (4) | 0.4466 | 0.6% (6) |
| Minor vascular complications | 7.2% (36) | 5.0% (25) | 0.1108 | 6.1% (61) |
| Naïve permanent pacemaker insertion 3 | 19.2% (87) | 18.9% (84) | 0.9777 | 19.0% (171) |
| Annular rupture | 0.4% (2) | 0.2% (1) | 1.0000 | 0.3% (3) |
| Coronary obstruction | 0.4% (2) | 0.6% (3) | 0.6833 | 0.5% (5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mollmann, H.; Linke, A.; Nombela-Franco, L.; Sluka, M.; Dominguez, J.F.O.; Montorfano, M.; Kim, W.-K.; Arnold, M.; Vasa-Nicotera, M.; Conradi, L.; et al. Procedural Safety and Device Performance of the Portico™ Valve from Experienced TAVI Centers: 30-Day Outcomes in the Multicenter CONFIDENCE Registry. J. Clin. Med. 2022, 11, 4839. https://doi.org/10.3390/jcm11164839
Mollmann H, Linke A, Nombela-Franco L, Sluka M, Dominguez JFO, Montorfano M, Kim W-K, Arnold M, Vasa-Nicotera M, Conradi L, et al. Procedural Safety and Device Performance of the Portico™ Valve from Experienced TAVI Centers: 30-Day Outcomes in the Multicenter CONFIDENCE Registry. Journal of Clinical Medicine. 2022; 11(16):4839. https://doi.org/10.3390/jcm11164839
Chicago/Turabian StyleMollmann, Helge, Axel Linke, Luis Nombela-Franco, Martin Sluka, Juan Francisco Oteo Dominguez, Matteo Montorfano, Won-Keun Kim, Martin Arnold, Mariuca Vasa-Nicotera, Lenard Conradi, and et al. 2022. "Procedural Safety and Device Performance of the Portico™ Valve from Experienced TAVI Centers: 30-Day Outcomes in the Multicenter CONFIDENCE Registry" Journal of Clinical Medicine 11, no. 16: 4839. https://doi.org/10.3390/jcm11164839
APA StyleMollmann, H., Linke, A., Nombela-Franco, L., Sluka, M., Dominguez, J. F. O., Montorfano, M., Kim, W.-K., Arnold, M., Vasa-Nicotera, M., Conradi, L., Camuglia, A., Bedogni, F., & Manoharan, G. (2022). Procedural Safety and Device Performance of the Portico™ Valve from Experienced TAVI Centers: 30-Day Outcomes in the Multicenter CONFIDENCE Registry. Journal of Clinical Medicine, 11(16), 4839. https://doi.org/10.3390/jcm11164839







